Back to Journals » Drug Design, Development and Therapy » Volume 16
Comparative Dose-Response Study of Phenylephrine Bolus for the Treatment of the First Episode of Spinal Anesthesia-Induced Hypotension for Cesarean Delivery in Severe Preeclamptic versus Normotensive Parturients
Authors Hu LJ, Mei Z , Shen YP, Sun HT, Sheng ZM , Chen XZ, Qian XW
Received 1 April 2022
Accepted for publication 28 June 2022
Published 8 July 2022 Volume 2022:16 Pages 2189—2198
DOI https://doi.org/10.2147/DDDT.S368480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Li-Juan Hu,1 Zhong Mei,1,2 Yan-Ping Shen,1 Hao-Tian Sun,1 Zhi-Min Sheng,1 Xin-Zhong Chen,1 Xiao-Wei Qian1
1Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Anesthesiology, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, People’s Republic of China
Correspondence: Xiao-Wei Qian, Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Xueshi Road 1, Hangzhou, 310006, People’s Republic of China, Tel +86-571-87061501, Fax +86 571 87061878, Email [email protected]
Background: It is well-known that severe preeclamptic parturients have less vasopressor requirements than normotensive parturients; however, the exact dose difference is poorly documented. This study aimed to determine and compare the ED50 and ED90 of a single bolus phenylephrine for the treatment of spinal anesthesia-induced hypotension in parturients with severe preeclampsia and parturients with normotension.
Methods: Seventy-five parturients with severe preeclampsia scheduled for cesarean delivery under combined spinal-epidural anesthesia were enrolled and randomly allocated to receive a single bolus of phenylephrine at five different doses (40, 50, 60, 70, and 80 μg), whereas 75 parturients with normotension were randomized to receive a single bolus of phenylephrine at five different doses (70, 80, 90, 100, and 110 μg) for the treatment of the first episode of hypotension. Phenylephrine dose values were log-transformed, the proportions of the successful interventions at each dose were converted to probits, and regression analysis was performed.
Results: The ED50 and ED90 (95% CI) of bolus phenylephrine were 72.1 (61.7 to 79.9) μg and 107 (95.9– 128.6) μg in parturients with normotension. The ED50 and ED90 values in parturients with severe preeclampsia were 47.6 (41.3– 52.7) μg and 70.7 (62.9– 86.7) μg. The relative median potency was 1.51 (1.16– 2.61).
Conclusion: Under this study conditions, severe preeclamptic parturients required a 34% reduction of ED50 of phenylephrine dose compared with normotensive parturients.
Keywords: cesarean delivery, spinal anesthesia, hypotension, phenylephrine, preeclampsia
Introduction
Phenylephrine has been advocated as a first-line vasopressor agent for the treatment of maternal hypotension after spinal anesthesia for cesarean delivery;1,2 however, the exact dose difference is poorly documented, especially in parturients with severe preeclampsia compared to parturients with normotension.3 Previous studies have indicated that a bolus of 50 to 100 μg phenylephrine could effectively treat spinal anesthesia-induced hypotension during cesarean delivery in severe preeclampsia parturients.4–6 Studies have shown that compared with parturients with normotension, parturients with severe preeclampsia have greater endogenous vasoactive mediators and are more sensitive to exogenous vasopressors.7–9 Therefore, we hypothesized that the optimal dose of bolus phenylephrine for the treatment of spinal anesthesia-induced hypotension for cesarean delivery in parturients with severe preeclampsia would be lower than that in parturients with normotension.
Using probit regression model, the optimal dose of phenylephrine for the treatment of the first episode of spinal anesthesia-induced hypotension were calculated. The difference in phenylephrine dose requirement between parturients with severe preeclampsia and parturients with normotension was assessed by calculating the relative median potency.
We aimed to determine the dose response of phenylephrine for the treatment of the first episode of spinal anesthesia-induced hypotension in parturients with severe preeclampsia and parturients with normotension.
Materials and Methods
Design and Study Subjects
This randomized, double-blinded study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (Approval No. 20180165), and was registered prior to patient enrollment at the Chinese Clinical Trials (www.chictr.org.cn, Registration No. ChiCTR1900023108). Written informed consent was obtained from all participants. The study was performed in the operating room of our hospital from May 13, 2019, to February 29, 2020. Parturients scheduled for cesarean delivery under spinal anesthesia were enrolled in the study. The experimental group (group P) included 75 patients with severe preeclampsia. Severe preeclampsia was defined as systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 110 mmHg on ≥ 2 separate occasions, or evidence of end organ injury (headache, visual disturbance, and epigastric pain) with ≥ 3 + proteinuria.10 Inclusion criteria were parturients with severe preeclampsia, singleton pregnancy, and American Society of Anesthesiologists physical status (ASA) of 3 or 4. The control group (group N) included 75 patients with normotension. The inclusion criteria were normotension, singleton pregnancy, and ASA score 2. The exclusion criteria were thrombocytopenia, coagulopathy, or any other medical contraindication to spinal anesthesia, umbilical cord prolapse, active labor, placenta previa or other placental abnormalities, known fetal abnormalities, known cardiovascular or cerebrovascular disease, known allergy to phenylephrine, less than 28 weeks gestation, weight less than 50 kg or greater than 100 kg, height less than 140 cm or greater than 180 cm, inability or refusal to give informed consent, age less than 18 years or more than 40 years, and patient refusal.
Study Protocol
Randomization was conducted by a statistician using MedCalc (Version 18.2.1 BV, Ostend, Belgium) to create a separate randomization code sequence for each group. The codes were placed into serially numbered sealed opaque envelopes. An assistant who was not involved in the subsequent study opened the envelope and prepared the study drug. According to the randomization codes, parturients with severe preeclampsia received a single IV bolus of phenylephrine at doses of 40, 50, 60, 70, or 80 μg, whereas those in the normotension group received a single IV bolus of phenylephrine at doses of 70, 80, 90, 100, or 110 μg. All of the phenylephrine doses were labeled “study drug” in identical 10-mL syringes and were diluted to a total volume of 10 mL.
According to the established protocol of our hospital, antepartum treatment of severe preeclampsia is as follows: patients receive 4 g magnesium sulfate intravenously as a loading dose, followed by 1 g hourly, for acute seizure prophylaxis. IV bolus of urapidil 50 mg or labetalol 50 mg every 10–30 minutes to reduce systolic blood pressure to < 160 mmHg in the acute admission ward. After the parturients were transferred to the operating room, routine monitoring with electrocardiography, blood pressure (BP), heart rate (HR), and pulse oximetry were performed. An IV line was established with an 18-gauge IV cannula in the lower forearm, and lactated Ringer’s solution was started at a minimal rate to keep the vein open. Before spinal anesthesia, mean arterial pressure (MAP) was measured at rest in the supine position. Baselined MAP was determined by averaging three consecutive noninvasive blood pressure readings taken at 1-min interval with a difference of less than 10%. Combined spinal-epidural anesthesia was performed by the same senior anesthesiologist who was not involved in data recording and study drug preparation. Epidural anesthesia was administered at the L1-2 interspace with the patient in the left lateral position, and no local anesthetic was administered. Spinal anesthesia was then administered using a 25-gauge spinal needle inserted at the L3-4 interspace. After confirming free outflow of cerebrospinal fluid, 3 mL hyperbaric ropivacaine 0.5% w/v (15 mg) was injected intrathecally over 15s. The patient was then restored to the left 15 degree supine position until the newborn was delivered. A loss of cold sensation up to the T6 dermatome or above was considered adequate for surgery. After intrathecal drug injection, the sensory block was measured every 2 min in the first 10 min and then at 10 min intervals. Patients were excluded if adequate block was not obtained. All patients received oxygen at 3 L/min via a facemask.
Another assistant who had not participated in the study recorded the data. MAP, heart rate, and pulse oximetry were measured at 1-min intervals for 10 min after intrathecal injection until delivery, followed by 3-min intervals until the end of the operation. Hypotension was defined as a > 20% decrease in MAP below the baseline in both groups during the period from induction of spinal anesthesia to the delivery of the fetus.11 A 10 mL bolus containing the assigned phenylephrine dose was administered by the investigator. A dose was considered successful in treating the first episode of spinal anesthesia-induced hypotension when the assigned dose of bolus phenylephrine could restore the MAP at or above 80% of baseline within 60s. If MAP was not restored to 80% of the baseline within 60s, phenylephrine (50–100 μg) or ephedrine (5–10 mg) was administered as alternative management. The end of the study period was defined as the time the fetus was delivered. If no hypotension occurred during the period from induction of spinal anesthesia to the delivery of the fetus, the parturient was excluded and the next parturient was assigned the same phenylephrine dose. If bradycardia (defined as heart rate < 50 beats per min) associated with hypotension was observed, ephedrine (5–10 mg) was administered. Atropine 0.5 mg was administered for persistent bradycardia. If a maximum dose of either phenylephrine (300 μg) or ephedrine (45 mg) was used, an alternative vasopressor (norepinephrine) was administered. If hypertension (defined as a MAP > 120% of the baseline value) occurred, a vasodilator (urapidil) was administered The treatment after completion of the study period was at the discretion of the senior anesthesiologist. After delivery of the fetus, a segment of the umbilical cord was obtained for blood gas assessment in the umbilical artery and vein.
The primary outcome of the study was the success rates for the different doses of phenylephrine bolus to restore the MAP at or above 80% of the baseline value to treat the first episode of spinal anesthesia-induced hypotension. Secondary outcomes included the incidence of hypotension, reactive hypertension, nausea or vomiting, bradycardia, tachycardia, dizziness, dyspnea, umbilical blood gases, and Apgar score.
Statistical Analysis
IBM SPSS Statistics for Windows version 25 (IBM Corp., Armonk, NY, USA) was used for analysis. Normally distributed data were assessed using the Kolmogorov–Smirnov test. Normally distributed data were analyzed using the independent-samples t-test, and non-normally distributed data were analyzed using the Mann–Whitney U-test. Fisher’s exact test was used to analyze incidence data. Statistical significance was set at P < 0.05.
The Cochran-Armitage test for trends in the proportions of PASS 11 (NSCC, LCC, Kaysville, UT, USA) was used to calculate the sample size. The sample size was based on data from our preliminary research, in which the proportions of single IV bolus phenylephrine for the effective treatment of the first episode of post-spinal hypotension in parturients with preeclampsia who received 40, 50, 60, 70, and 80 μg phenylephrine, were 0.3, 0.5, 0.7, 0.8, and 0.9, respectively. Eleven patients per dosage group were required using the Z test with continuity correction (significance level of 0.05, power of 80%). To achieve narrower confidence intervals (CIs), we increased the sample size to 75 (15 per dosage group).
Probit regression was used for the dose-response analysis. After logarithmic transformation of dose values of phenylephrine, the proportions of the successful interventions at each dose were converted to probits, and regression analysis was performed. ED50 and ED90 values for a single IV phenylephrine bolus in each group with 95% CI were derived by interpolation. By calculating the relative median potency, the difference in phenylephrine dose requirement between parturients with severe preeclampsia and parturients with normotension was assessed.
Results
The parturient recruitment is summarized in Figure 1. A total of 75 parturients in each group completed the study and were included in the final data analysis. The demographic data are shown in Table 1. There were differences in age, weight, gestation, gestational age < 37 weeks, and block height at 5-min (P < 0.05). The neonatal outcomes are shown in Table 2. Umbilical arterial and venous pH values and Apgar scores were similar between the two groups, but birth weight was lower in Group P (P < 0.001).
 |
Table 1 Demographic Data |
 |
Table 2 Neonatal Outcome |
 |
Figure 1 Consolidated Standards of Reporting Trials showing flow of parturients in the study. |
The dose values of phenylephrine were log-transformed, and the success rates were converted into probabilities for analysis. The probit regression curves are shown in Figure 2. The dose-response curves for a single IV phenylephrine bolus for the two groups are shown in Figure 3. The calculated mean (95% CI) values for ED50 and ED90 of a single IV bolus phenylephrine for the treatment of spinal anesthesia-induced hypotension in parturients with severe preeclampsia were 47.6 μg (95% CI, 41.3–52.7) and 70.7 μg (95% CI, 62.9–86.7), respectively. The ED50 and ED90 values in parturients with normotension were 72.1 μg (95% CI, 61.7 to 79.9) and 107 μg (95% CI, 95.9–128.6), respectively. The relative median potency for phenylephrine between normotensive and preeclampsia pregnancies was 0.66 (95% CI, 0.38–0.86), indicating that the dose requirement for a single IV bolus of phenylephrine in parturients with severe preeclampsia is lower than that in normotensive parturients.
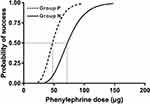 |
Figure 3 Dose-response curves for a single IV phenylephrine bolus for the two groups derived from probit analysis (Group P, dashed curve; Group N, solid curve). |
The hemodynamic changes are shown in Table 3. Baseline MAP, MAP at the first episode of hypotension, percentage of MAP decrease, MAP after using phenylephrine and total dose of phenylephrine were different between the groups (P < 0.05). There was no statistical difference in the time to the first episode of hypotension, total number of hypotensive episodes, total number of vasopressor boluses and total dose of ephedrine between the two groups.
 |
Table 3 Hemodynamic Changes |
The success rates of a single IV bolus of different doses of phenylephrine are shown in Table 4. The side effects are listed in Table 5. The incidence of tachycardia differed between the groups (P = 0.001), and the incidences of other side effects were similar.
 |
Table 4 Success Rates of Single IV Bolus of Different Doses of Phenylephrine |
 |
Table 5 Side Effects |
Discussion
In this study, the ED50 and ED90 (95% CI) of bolus phenylephrine were 72.1 (61.7 to 79.9) μg and 107 (95.9–128.6) μg in parturients with normotension, whereas the ED50 and ED90 values in parturients with severe preeclampsia were 47.6 (41.3–52.7) μg and 70.7 (62.9–86.7) μg, respectively; the primary finding was that the dose requirement of a single IV bolus of phenylephrine for the treatment of the first episode of spinal anesthesia-induced hypotension in parturients with severe preeclampsia would be 34% significantly lower than that in parturients with normotension.
It is well-known that severe preeclamptic parturients have less vasopressor requirements than normotensive parturients for the prevention or treatment of spinal anesthesia-induced hypotension, however, the exact dose difference is poorly documented.3 By determining the comparative ED50 and ED90 of phenylephrine, we therefore reduce the related risks that the excessive use of vasopressors during hypotension can be particularly harmful in severe preeclamptic parturients by exposing them to the risk of intracranial bleeding.
Our results are consistent to those of previous studies, indicating that severe preeclamptic parturients might require less vasopressor than normotensive parturients.7–9,12–16 The reasons for the lower dose of phenylephrine required for parturients with severe preeclampsia may be as follows: First, compared with normotensive parturients, those with severe preeclampsia have greater endogenous vasoactive mediators and are more sensitive to exogenous vasopressors; therefore, smaller doses of phenylephrine can easily restore MAP to baseline.7–9 Second, in clinical practice, parturients with severe preeclampsia tend to have a lower average gestational age and a higher number of preterm births. Results from our study show that compared with the normotensive group, parturients in the severe preeclampsia group had a lower gestational age, the proportion of preterm births was significantly higher, and the birth weights were lower, which resulted in less compression of the inferior vena cava by the gravid uterus, thus leading to a lower cephalad spread of spinal anesthesia.7 Therefore, it has less influence on the hemodynamic fluctuations. This will result in a lower incidence of hypotension and lower vasopressor requirements. However, it has been proposed that the risk of hypotension after subarachnoid block in severe preeclampsia is associated with other severe preeclampsia-related factors rather than uterine size.8 Third, in severe preeclampsia parturients, the damaged vascular endothelium produces higher levels of endogenous vasopressor-like thromboxane and endothelin, resulting in persistent high vascular tone, which is not altered by the sympathetic block from spinal anesthesia, resulting in fewer hemodynamic changes. However, normotensive parturients are more sensitive to spinal anesthesia because of the increased dependence on the sympathetic nerve, which breaks the balance of vascular tone, weakens the reaction to angiotensin II, and increases the synthesis of prostaglandin and nitric oxide.14,17 Fourth, all parturients received lactated Ringer’s solution with a minimal rate to keep the vein open before induction of spinal anesthesia. We limited the volume of fluids in parturients with severe preeclampsia, considering the risk of pulmonary edema.18 Vasodilation and maternal relative volume insufficiency induced by sympathetic blockade can cause severe hypotension in healthy parturients.9 However, severe preeclampsia can still maintain the vascular tone resulting in a limited decrease in blood pressure due to the exaggerated vasoconstriction.19
Although MAP was decreased more in healthy parturients, no differences in Apgar scores and fetal acidosis were observed between the groups, which was consistent with previous studies.20–23 Prematurity linked to the preeclamptic group would be much more likely to affect difference in neonatal outcomes rather than these small differences in MAP decrease.
Previously, using the biased coin up-and-down method (BCUD), we found the ED90 value in parturients with severe preeclampsia was 62.00 µg, because the BCUD method required fewer subjects to obtain the ED90 value.5 As a follow-up study, although more subjects were required, we chose the random dose grouping method to obtain more accurate ED90 values and to compare the difference in phenylephrine dose requirement between parturients with severe preeclampsia and parturients with normotension.
The present study has several important limitations that need to be considered. First, compared with the normotensive group, parturients with severe preeclampsia had a lower average gestational age and a higher number of preterm births, which may result in a lower likelihood of observing a difference in phenylephrine dose requirements between groups. Parturients with full-term severe preeclampsia pregnancies would make the differences (in phenylephrine requirements) even more important and significant. However, it is relatively common for parturients with severe preeclampsia to present for elective cesarean delivery with preterm gestation in clinical circumstances. Aya et al have reported that compared with normotensive parturients with preterm birth, the magnitude of hypotension in severe preeclampsia parturients did not appear to decrease when hypotension occurred, suggesting that fetal weight and compression of the inferior vena cava may also play a role.8 Second, according to the established guidelines of our hospital, prenatal treatment for severe preeclampsia such as MgSO4, urapidil, or labetalol may bias the results of our present studies on the therapeutic dose for hypotension. However, at present, the international consensus is that appropriate reduction of MAP and alleviation of neuromuscular hyperexcitability before anesthesia are beneficial for hemodynamic stability during the operation in parturients with very severe preeclampsia. Third, this study utilized a bolus dose of phenylephrine not adjusted for patient body weight. Considering that the weight of parturients varies widely among different populations, it may be more appropriate to adjust the bolus dose of phenylephrine according to body weight. Fourth, only intermittent non-invasive BP and HR were measured as the key indicators of maternal hemodynamic stability and CO was not monitored. Therefore, we were unable to determine the effect of phenylephrine infusion dose on this parameter.24 Fifth, the highest dose group of the grouping method may experience reactive hypertension, and we observed several such cases; therefore other methods such as coin-based sequencing may be more suitable for the dose-response study. Sixth, using ropivacaine (instead of bupivacaine), particularly without an opioid (such as fentanyl or sufentanil) intrathecally for cesarean delivery is a common practice in China,25,26 but it is quite an uncommon practice in the obstetric anesthesia literature. This constitutes also a study limitation regarding external validity. Seventh, we used “two point method” for neuraxial anesthesia, instead of conventional combined spinal-epidural anesthesia (CSE) performed at one lumbar space. Although we routinely performed this technique in our institute, it is not that popular all over the world, which in turn further restrict the generality of this research. Eighth, the differences in maternal age, weight, and block height at 5-min between the two groups may impact the generality of the results. Ninth, we routinely keep intravenous fluids at a minimal rate for severe preeclamptic parturients in our hospital to prevent heart failure. However, this may not be a common practice in other medical centers, which may also affect the generality of our study. Tenth, phenylephrine range 50–100 μg or ephedrine 5–10 mg were at the discretion of the attending anesthesiologist dependent on the degree of hypotension when hypotension reoccurred. This is the deficiency of our study that there was no standardized treatment after the first dose of phenylephrine.
Conclusions
In conclusion, the ED50 and ED90 of a single IV bolus of phenylephrine for the treatment of the first episode of spinal anesthesia-induced hypotension in parturients with severe preeclampsia were both 34% significantly lower than that in parturients with normotension. This information is clinically relevant for the treatment of spinal-induced hypotension during cesarean delivery in parturients with severe preeclampsia with phenylephrine, and may help avoiding to overtreat these high-risk parturients. However, more studies should be conducted to determine the optimal dose of phenylephrine, and/or norepinephrine as an alternative,27,28 for the prevention or treatment of spinal anesthesia-induced hypotension.
Highlights
- Phenylephrine dose has not been fully determined in parturients with preeclampsia.
- Randomized, double-blind, comparative dose-finding study.
- Bolus phenylephrine dose was significantly lower in severe preeclamptic parturients.
Severe preeclamptic parturients required a 34% reduction of ED50 of phenylephrine dose.
Abbreviations
ED50, the dose effective in 50% of patients; ED90, the dose effective in 90% of patients; IV, intravenous; CI, confidence interval; ASA, American Society of Anesthesiologists; BP, blood pressure; HR, heart rate; MAP, mean arterial pressure; SA, spinal anesthesia; CO, cardiac output; BCUD, the biased coin up-and-down method; CSE, combined spinal-epidural anesthesia.
Data Sharing Statement
The data supporting the study findings are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This randomized, double-blinded study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (Approval No. 20180165), and was registered prior to patient enrollment at the Chinese Clinical Trials (Registration No. ChiCTR1900023108, http://www.chictr.org.cn/showprojen.aspx?proj=35119). Written informed consent was obtained from all participants. We confirm our study complies with the Declaration of Helsinki.
Consent for Publication
All authors have read and approved the manuscript, and agree to submit to your journal.
Acknowledgments
The authors thank staff of Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Disclosure
The authors declare no conflicts of interest.
References
1. Fitzgerald JP, Fedoruk KA, Jadin SM, Carvalho B, Halpern SH. Prevention of hypotension after spinal anaesthesia for caesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. 2020;75:109–121. doi:10.1111/anae.14841
2. Campbell JP, Stocks GM. Management of hypotension with vasopressors at caesarean section under spinal anaesthesia-have we found the Holy Grail of obstetric anaesthesia? Anaesthesia. 2018;73:3–6. doi:10.1111/anae.14114
3. Dusitkasem S, Herndon BH, Somjit M, Stahl DL, Bitticker E, Coffman JC. Comparison of phenylephrine and ephedrine in treatment of spinal-induced hypotension in high-risk pregnancies: a narrative review. Front Med. 2017;4:2. doi:10.3389/fmed.2017.00002
4. Mohta M, Duggal S, Chilkoti GT. Randomised double-blind comparison of bolus phenylephrine or ephedrine for treatment of hypotension in women with pre-eclampsia undergoing caesarean section. Anaesthesia. 2018;73:839–846. doi:10.1111/anae.14268
5. Dyer RA, Daniels A, Vorster A, et al. Maternal cardiac output response to colloid preload and vasopressor therapy during spinal anaesthesia for caesarean section in patients with severe pre-eclampsia: a randomised, controlled trial. Anaesthesia. 2018;73:23–31. doi:10.1111/anae.14040
6. Liu JP, Pan ZB, Zhu M, et al. Determination of the 90% effective dose of phenylephrine boluses to treat spinal anesthesia-induced hypotension in patients with severe preeclampsia during cesarean delivery: a pilot study. Drug Des Devel Ther. 2021;15:3765–3772. doi:10.2147/DDDT.S323715
7. Aya AG, Mangin R, Vialles N, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg. 2003;97:867–872. doi:10.1213/01.ane.0000073610.23885.f2
8. Aya AG, Vialles N, Tanoubi I, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–875. doi:10.1213/01.ANE.0000175229.98493.2B
9. Clark VA, Sharwood-Smith GH, Stewart AV. Ephedrine requirements are reduced during spinal anaesthesia for caesarean section in preeclampsia. Int J Obstet Anesth. 2005;14:9–13. doi:10.1016/j.ijoa.2004.08.002
10. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi:10.1016/S0140-6736(15)00070-7
11. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71–92. doi:10.1111/anae.14080
12. Nikooseresht M, Rabiei MAS, Hajian P, Dastaran R, Alipour N. Comparing the hemodynamic effects of spinal anesthesia in preeclamptic and healthy parturients during cesarean section. Anesthesiol Pain Med. 2016;6(3):e11519. doi:10.5812/aapm.11519
13. Alemayehu TY, Berhe YW, Getnet H, Molallign M. Hemodynamic changes after spinal anesthesia in preeclamptic patients undergoing cesarean section at a tertiary referral center in Ethiopia: a prospective cohort study. Patient Saf Surg. 2020;14:9. doi:10.1186/s13037-020-00234-w
14. Sivevski A, Ivanov E, Karadjova D, Slaninka-Miceska M, Kikerkov I. Spinal-induced hypotension in preeclamptic and healthy parturients undergoing cesarean section. Open Access Maced J Med Sci. 2019;7(6):996–1000. doi:10.3889/oamjms.2019.230
15. Saha D, Ghosh S, Bhattacharyya S, et al. Comparison of hemodynamic response and vasopressor requirement following spinal anaesthesia between normotensive and severe preeclamptic women undergoing caesarean section: a prospective study. J Obstet Anaesth Critical Care. 2013;3(1):23. doi:10.4103/2249-4472.114286
16. Henke VG, Bateman BT, Leffert LR. Spinal anesthesia in severe preeclampsia. Anesth Analg. 2013;117:686–693. doi:10.1213/ANE.0b013e31829eeef5
17. Sharwood–Smith G, Drummond GB. Hypotension in obstetric spinal anaesthesia: a lesson from preeclampsia. Br J Anaesth. 2009;102:291–4. doi:10.1093/bja/aep003
18. Pretorius T, van Rensburg G, Dyer RA, Biccard BM. The influence of fluid management on outcomes in preeclampsia: a systematic review and meta-analysis. Int J Obstet Anesth. 2018;34:85–95. doi:10.1016/j.ijoa.2017.12.004
19. Anthony J, Schoeman LK. Fluid management in pre-eclampsia. Obstet Med. 2013;6:100–104. doi:10.1177/1753495X13486896
20. Ituk US, Cooter M, Habib AS. Retrospective comparison of ephedrine and phenylephrine for the treatment of spinal anesthesia induced hypotension in pre-eclamptic patients. Curr Med Res Opin. 2016;32:1083–1086. doi:10.1185/03007995.2016.1159953
21. Cooper DW, Sharma S, Orakkan P, Gurung S. Retrospective study of association between choice of vasopressor given during spinal anaesthesia for high risk caesarean delivery and fetal pH. Int J Obstet Anesth. 2010;19:44–49. doi:10.1016/j.ijoa.2009.06.002
22. Dyer RA, Emmanuel A, Adams SC, et al. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth. 2018;33:23–31. doi:10.1016/j.ijoa.2017.08.001
23. Heesen M, Rijs K, Hilber N, et al. Ephedrine versus phenylephrine as a vasopressor for spinal anaesthesia-induced hypotension in parturients undergoing high-risk caesarean section: meta-analysis, meta-regression and trial sequential analysis. Int J Obstet Anesth. 2019;37:16–28. doi:10.1016/j.ijoa.2018.10.006
24. Mon W, Stewart A, Fernando R, et al. Cardiac output changes with phenylephrine and ephedrine infusions during spinal anesthesia for cesarean section: a randomized, double-blind trial. J Clin Anesth. 2017;37:43–48. doi:10.1016/j.jclinane.2016.11.001
25. Mei Z, Ngan Kee WD, Sheng ZM, et al. Comparative dose-response study of hyperbaric ropivacaine for spinal anesthesia for cesarean delivery in singleton versus twin pregnancies. J Clin Anesth. 2020;67:110068. doi:10.1016/j.jclinane.2020.110068
26. Khaw KS, Ngan Kee WD, Wong EL, Liu JY, Chung R. Spinal ropivacaine for cesarean section: a dose-finding study. Anesthesiology. 2001;95(6):1346–1350. doi:10.1097/00000542-200112000-00011
27. Mercier FJ, Soued M, Morau E, Ngan Kee WD. Noradrenaline for haemodynamic control in obstetric anaesthesia: is it now a suitable alternative to phenylephrine? Anaesth Crit Care Pain Med. 2019;38(6):591–593. doi:10.1016/j.accpm.2019.10.006
28. Wang X, Mao M, Liu S, Xu S, Yang J. Norepinephrine, phenylephrine, and ephedrine for the treatment of maternal hypotension in parturients with preeclampsia during cesarean delivery under spinal anesthesia. Med Sci Monit. 2019;25:1093–1101. doi:10.12659/MSM.914143
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

