Back to Journals » International Journal of General Medicine » Volume 17
Comparison of Two Norepinephrine Rescue Bolus Doses for Management of Severe Post-Spinal Hypotension During Elective Caesarean Delivery: A Randomized, Controlled Trial
Authors Amin SM , Hasanin A , Ghanem NT, Mostafa M, Elzayat N, Elsherbiny M, Abdelwahab Y
Received 22 October 2023
Accepted for publication 12 January 2024
Published 19 January 2024 Volume 2024:17 Pages 153—160
DOI https://doi.org/10.2147/IJGM.S446021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Sarah M Amin, Ahmed Hasanin, Nashwa Talaat Ghanem, Maha Mostafa, Nashwa Elzayat, Mona Elsherbiny, Yaser Abdelwahab
Department of Anesthesia and Critical Care Medicine, Cairo University, Cairo, Egypt
Correspondence: Ahmed Hasanin, Department of Anesthesia and Critical Care Medicine, Cairo University, Faculty of Medicine, 01 Elsarayah Street, Elmanyal, Cairo, 11559, Egypt, Fax +20224168736, Email [email protected]
Background: Post-spinal hypotension is associated with maternal and neonatal complications; therefore, prompt control maternal blood pressure is necessary. In this study, we aimed to compare the efficacy and safety of two norepinephrine bolus doses in the rescue management of severe maternal hypotension during elective Cesarean delivery.
Methods: We included full-term pregnant women scheduled for Cesarean delivery under spinal anesthesia. Patients were randomized to receive either 5-mcg norepinephrine (n=79) or 10-mcg norepinephrine (n=79) for treatment of severe postspinal hypotension (systolic blood pressure ≤ 60% of baseline reading). The management of the hypotensive episode was considered successful if systolic blood pressure was > 80% of the baseline within 2 mins of the bolus. The primary outcome was the incidence of successful management of severe post-spinal hypotension. Secondary outcomes included the incidence of reactive bradycardia, reactive hypertension, umbilical blood gases, and neonatal Apgar score at 5-min post-delivery.
Results: We included 73 patients in the 5-mcg group and 76 patients in the 10-mcg group into the final analysis. The incidence of successful management of severe hypotensive episodes was comparable between the two groups (43/73 [59%] and 46/76 [60%] in the 5-and 10-mcg group, respectively, P=0.917). The incidence of reactive hypertension, bradycardia, and neonatal outcomes were comparable between the two groups.
Conclusion: In mothers undergoing Cesarean delivery under spinal anesthesia, 10-mcg norepinephrine bolus was not superior to the 5-mcg bolus in the management of severe hypotension. Furthermore, the incidence of reactive bradycardia and hypertension was comparable in the two doses.
Clinical Trial Registration: NCT05290740, URL: https://clinicaltrials.gov/ct2/show/NCT05290740.
Keywords: cesarean delivery, spinal anesthesia, severe hypotension, norepinephrine
Introduction
Maternal hypotension after spinal block is associated with maternal and neonatal complications; therefore, it is recommended to use vasopressors, prophylactically and interactively, for prompt control of maternal blood pressure during Cesarean delivery.1,2 Despite the presence of various preventive regimens (fluid loading, maternal positioning, and vasopressors), many mothers develop postspinal hypotension that requires the use of a vasopressor bolus.3,4 Severe maternal hypotension, defined as systolic blood pressure ≤60% of the baseline, is a special and critical complication whose incidence reached 7–20%, even while using various protocols for prophylaxis.3,4 For being a serious and life-threatening condition, it should be promptly managed using an appropriate rescue vasopressor bolus targeting rapid restoration of maternal blood pressure.
Norepinephrine is an alpha-adrenergic agonist with weak beta-adrenergic agonistic activity and is increasingly used in obstetric anesthesia with acceptable maternal and neonatal outcomes.5 Norepinephrine bolus could be used for rapid correction of maternal blood pressure; however, there is no consensus, till date, for the optimum dose.6–8 Furthermore, no studies focused on the best dose for the management of severe hypotension. A recent report compared 6- and 10-mcg norepinephrine boluses in the management of maternal hypotension and found that both doses had the same success rate (≈90%); however, most of the participants in the mentioned study had non-severe hypotension.3 Therefore, we hypothesize that severe hypotension should be separately investigated for the possible superiority of the higher over the lower dose of norepinephrine bolus.
In this study, we aimed to compare the efficacy and safety of two norepinephrine bolus doses in the rescue management of severe maternal hypotension during elective Cesarean delivery.
Patients and Methods
This randomized controlled, double-blinded study was conducted in Cairo University Hospital after institutional ethics committee approval (MS-657-2021). This study was performed in line with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patient before the enrolment. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT05290740, Date of registration: 11/03/2022, first posted 22/03/2022).
We included full-term singleton pregnant women aged between 18 and 40 years, scheduled for elective Cesarean delivery under spinal anesthesia.
Patients with American society of anesthesiologists-physical status of >II, uncontrolled cardiac morbidities (severe valvular lesion, impaired contractility with ejection fraction <50%, heart block, and arrhythmias), hypertensive disorders of pregnancy, peripartum bleeding, coagulation disorders or any contraindication to regional anesthesia, and patients with baseline systolic blood pressure <100 mmHg were excluded from the study.
Computer-generated randomization table was used to randomly assign patients in to two equal groups. The assigned drug’s preparation instructions were included inside sequentially numbered concealed envelopes. A research assistant was responsible for opening the envelope and drug preparation without any further involvement in the study. The assigned dose (5 or 10 mcg) was diluted with normal saline in a 10 mL syringe. The attending anesthetist and the patient were blinded to the dose of the drug. If the enrolled patient did not develop severe hypotension, the research assistant was informed, and the randomization card was returned to the envelope to be reused in the following enrolled patient.
Upon arrival to the operating room, routine monitoring was applied (electrocardiography, pulse oximetry, and non-invasive blood pressure monitor) and intravenous access was obtained. All patients received 10-mg metoclopramide. Baseline heart rate and systolic blood pressure were recorded as the average of three consecutive readings with 2-minute interval, while the patient was in supine position with left uterine displacement.
Lactated Ringer’s solution was administered at rate of 15 mL/Kg over 10 minutes as a co-load; spinal anesthesia was done by injecting 10 mg of hyperbaric bupivacaine and 25 mcg fentanyl into the subarachnoid space at L3-L4 or L4-L5 interspace using 25-G spinal needle.
After subarachnoid block, mothers were returned to the supine position with left-lateral tilt. The decision whether to give vasopressors prophylactic or interactively for management of hypotension was according to the attending anesthetist preferences. Block success was confirmed if T4 sensory block level was achieved, five minutes after local anesthetic injection.
All patients received norepinephrine bolus (5 mcg) if their systolic blood pressure was ≤80% of the baseline reading according to the local protocols. The patient was included in the study only if she developed severe hypotension (defined as systolic blood pressure ≤60% of the baseline reading) as her first hypotensive episode or after 10 minutes from the last successfully managed hypotensive episode and before the delivery. The management of the hypotensive episode was deemed successful if the systolic blood pressure was >80% of the baseline within 2 min of the bolus. If the bolus failed, another 5-mcg bolus was given every 2 min until restoring the maternal blood pressure. All norepinephrine boluses were given slowly over 5 s.
Intraoperative bradycardia (defined as heart rate less than 55 bpm) was managed by IV atropine bolus (0.5 mg).
Fluid administration was resumed up to a maximum of 1.5 liters. An oxytocin bolus of 0.5 IU was administered over five seconds after delivery then infused at a rate of 2.5 IU/hour.
Systolic blood pressure and heart rate was recorded at baseline, at 2-min intervals for 30 min after intrathecal injection then at 5-min intervals until the end of the operation.
Study Outcomes
The primary outcome was the incidence of successful management of severe post-spinal hypotension (from completion of spinal anesthesia until baby delivery). Secondary outcomes included time to severe hypotensive episode, incidence of reactive bradycardia (heart rate <55 bpm), incidence of reactive hypertension (systolic blood pressure >120% of baseline), heart rate and systolic blood pressure at baseline and every 2-min during management of the severe episode and for 6 minutes, postdelivery hypotension, incidence of intraoperative nausea and vomiting.
Neonatal outcomes included umbilical blood gases (pH, PCO2, PO2, and HCO3), and Apgar score for the neonate at 5 min post-delivery. Mother’s age and weight as well as time to delivery were recorded.
Sample Size
The sample size was calculated using MedCalc Software version 14 (MedCalc Software bvba, Ostend, Belgium). Our primary outcome was the rate of successful management of severe maternal hypotension. We performed a pilot study in which we reported a rate of successful management of severe maternal hypotension of 70% with 5-mcg bolus. An absolute improvement of 20% in the rate of successful management of maternal hypotension (aiming 90% success rate) was planned for sample size calculation. One-hundred and forty-four mothers (72 per group) at least were estimated to have a study power of 80% and an alpha error of 0.05. This number was increased to 158 mothers (79 per group) to compensate for possible dropouts.
Statistical Analysis
Analysis of data was performed using Statistical Package for Social Science (SPSS) software, version 26 for Microsoft Windows (IBM Corp., Armonk, NY). Categorical data were presented as frequency (%) and were analyzed using the chi-square test. Continuous data were checked for normality using Kolmogorov–Smirnov test. Normally distributed data were presented as means ±standard deviations and were analyzed using the Student’s t-test. Skewed data were expressed as medians (quartiles) and were analyzed using the Mann–Whitney U-test. The repeated measures analysis of variance was used to analyzed repeated measured data (heart rate and systolic blood pressure). Bonferroni test was used to adjust for multiple comparisons. P-value <0.05 was considered significant.
Results
Six-hundred and nineteen patients fulfilling the inclusion criteria were enrolled in this study. Four-hundred and sixty-one patients were not included into the study for either not developing hypotension or developed non-severe hypotension. One-hundred and fifty-eight patients developed severe hypotension and were randomized to receive either 5- or 10-mcg norepinephrine bolus to treat the hypotension. (Figure 1) The included patients did not receive any prophylactic vasopressors.
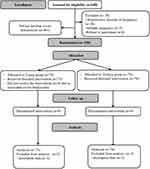 |
Figure 1 CONSORT flowchart. Notes: Figure adapted from Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251.9 Copyright: © 2010 Schulz et al. Creative Commons Attribution License. |
Patients’ demographic and baseline hemodynamic data were comparable between the two groups (Table 1).
 |
Table 1 Demographic Data and Baseline Hemodynamic Characteristics. Data Presented as Mean ±Standard Deviation, Median (Quartiles) and Frequency (%) |
The incidence of successfully managed episode was comparable between the two groups. The systolic blood pressure and heart rate readings post drug injection as well as the incidence of reactive hypertension and bradycardia were comparable between the two groups (Table 2) (Figures 2 and 3).
 |
Table 2 Maternal Outcomes. Data Presented as Median (Quartiles) and Frequency (%) |
The incidence of nausea and vomiting was comparable between the two groups (Table 2).
The 5-minutes Apgar score and umbilical blood gases were comparable between the two groups. (Table 3).
 |
Table 3 Fetal Outcomes. Data Presented as Median (Quartiles) |
Discussion
We compared two boluses of norepinephrine in the management of severe hypotension during Cesarean delivery under spinal anesthesia and found that the 10-mcg bolus was not superior to the 5-mcg bolus. The success rate of norepinephrine bolus in restoring maternal blood pressure was nearly 60% in the two groups. The blood pressure and heart rate readings were comparable in the two doses. Furthermore, the incidence of side effects, namely hypertension and bradycardia, was also comparable between the two doses. The non-superiority of the 10 mcg over 5 mcg bolus could be explained by that there might be a ceiling effect and that there is no value from additional doses over 5 mcg. Another explanation is that severe hypotension might need to be managed by a higher dose than the 10 mcg (eg, 15 mcg); this might be tested in future studies.
The use of norepinephrine boluses for management of maternal hypotension had been reported in several studies; however, all previous studies were restricted to non-severe hypotensive episodes. This is the first study, to the best of our knowledge, which evaluated norepinephrine bolus in the management of severe hypotension.
In a probit analysis in 50 mothers, Mohta et al reported that the ED95 for norepinephrine was 3.7 mcg in non-severe hypotensive episodes,6 while in another graded-dose response study, Ngan Kee reported that the ED50 was 10 mcg. The two studies did not use prophylactic vasopressors; however, they had two different end points. The end-point for Ngan Kee study was restoration of the baseline blood pressure measurement,8 while the end-point for our study and Mohta et al’s study6 was to restore the blood pressure to ≥80% of the baseline measurement. Our study differed from the two mentioned studies in the larger sample size (149 mothers) and in the severity of hypotension (SBP < 60% of the baseline reading).
In a previous report, our group compared 6 mcg and 10 mcg in the management of hypotension and did not find superiority with the larger dose; however, most of the hypotensive episodes in our previous study were not severe;3 thus, we hypothesized that severe hypotension requires specific evaluation as its management might require the higher dose (10 mcg) and thus, we designed the current study to answer this question. The success rate of norepinephrine bolus in restoring maternal blood pressure in the current study is lower than our previous study (60% vs 90%).3 This difference is probably because the current study strictly included severe hypotensive episodes which was not the case with our previous study.3
Maternal hypotension, specially the severe one, is a serious complication during Caesarean delivery and is associated with poor maternal and neonatal outcomes.10 Therefore, effective, and aggressive management for hypotension using a safe and effective dose represents a priority for all obstetric anesthesiologists. The current guidelines insist on the abrupt management of hypotension.1 Norepinephrine had gained uprising evidence in the last few years in prophylaxis against hypotension; however, its use as bolus for interactive management of hypotension has not been adequately investigated. Our results provide two important implications for future practice and research: 1 - The use of 10 mcg norepinephrine bolus does not add benefit in the management of severe maternal hypotension over 5 mcg. 2 - The success rate of the two doses in restoring maternal blood pressure is still not satisfactory (60%); thus, we suggest that future research should evaluate higher does (eg, 15 mcg) to reach the best approach for management of this critical complication, especially with the fact that there were no serious complications with the current two doses.
This study has some limitations such as being performed in single center and including mothers without cardiac morbidities. Future studies are warranted to evaluate the efficacy and safety of such doses in mothers with cardiac diseases. The choice of the vasopressor regimen, prophylactic versus interactive management of hypotension, was left to the preference of the attending anesthesiologist. Upon completing the study, we found that all patients who developed severe hypotension and were included into the study did not receive any prophylactic vasopressor. We used similar protocol to previous dose–response studies6,8 which included mothers who did not receive prophylactic vasopressors. The absence of background vasopressor infusion could avoid the possible interaction between the bolus and infusion. Our previous studies3,4 included mothers who received prophylactic norepinephrine infusion, and we reported that severe hypotension was still present and not totally eliminated, and therefore, it is essential to reach the best dose for its management. A recent survey revealed a significant lack of adherence to the current guidelines among participant anesthesiologists in United Kingdom with only 50% having a treatment target.11 Moreover, the survey reported a wide variation in practice between consultants in different centers.11 Therefore, severe hypotension is still expected and requires a definitive evidence-based dose to be managed. We only analyzed severe hypotensive episode if it was the first episode or if it occurred 10 min after the last successfully managed episode. This allowed us to assess the hemodynamic effect of the study vasopressor bolus and to mitigate any residual hemodynamic effect of previously administered vasopressor. We used a fixed-dose regimen instead of a weight-based regimen for ease of calculation and to be applicable to the daily practice. This is also in line with previous literature in the same population.3,6,8
In conclusion, in mothers undergoing Cesarean delivery under spinal anesthesia, the use of 10-mcg norepinephrine bolus was not superior to the 5-mcg bolus in the management of severe hypotension.; furthermore, the incidence of reactive bradycardia and hypertension was comparable in the two doses.
Abbreviation
SPSS, Statistical Package for Social Science.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This randomized controlled, double-blinded study was conducted in Cairo University Hospital after institutional ethics committee approval (MS-657-2021). This study was performed in line with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patient before the enrolment.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71–92. doi:10.1111/anae.14080
2. Hasanin A, Mokhtar AM, Badawy AA, Fouad R. Post-spinal anesthesia hypotension during cesarean delivery, a review article. Egypt J Anaesth. 2017;33(2):189–193. doi:10.1016/j.egja.2017.03.003
3. Hassabelnaby YS, Hasanin AM, Adly N, et al. Comparison of two norepinephrine rescue bolus for management of post-spinal hypotension during cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2020;20(1):84. doi:10.1186/s12871-020-01004-y
4. Hasanin AM, Amin SM, Agiza NA, et al. Norepinephrine infusion for preventing postspinal anesthesia hypotension during cesarean delivery. Anesthesiology. 2019;130(1):55–62. doi:10.1097/ALN.0000000000002483
5. Ngan Kee WD, Lee SWY, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122(4):736–745. doi:10.1097/ALN.0000000000000601
6. Mohta M, Dubey M, Malhotra RK, Tyagi A. Comparison of the potency of phenylephrine and norepinephrine bolus doses used to treat post-spinal hypotension during elective caesarean section. Int J Obstet Anesth. 2019;38:25–31. doi:10.1016/j.ijoa.2018.12.002
7. Onwochei DN, Ngan Kee WD, Fung L, Downey K, Ye XY, Carvalho JCA. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2017;125(1):212–218. doi:10.1213/ANE.0000000000001846
8. Ngan Kee WD. A random-allocation graded dose–response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology. 2017;127(6):934–941. doi:10.1097/ALN.0000000000001880
9. Schulz KF, Altman DG, Moher DC Statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251.
10. Bligard KH, Cameo T, McCallum KN, et al. The association of fetal acidemia with adverse neonatal outcomes at time of scheduled cesarean delivery. Am J Obstet Gynecol. 2022;227(2):265.e1–265.e8. doi:10.1016/j.ajog.2022.04.037
11. Jarvis MS, Blackburn J, Hailstone C, et al. A survey in the West Midlands of the United Kingdom of current practice in managing hypotension in lower segment caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2023;55:103899. doi:10.1016/j.ijoa.2023.103899
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


