Back to Journals » Clinical Interventions in Aging » Volume 17
Combined Vision and Hearing Impairment is Associated with Frailty in Older Adults: Results from the West China Health and Aging Trend Study
Authors Zhao Y , Ding Q , Lin T, Shu X, Xie D, Gao L, Yue J
Received 12 February 2022
Accepted for publication 26 April 2022
Published 2 May 2022 Volume 2022:17 Pages 675—683
DOI https://doi.org/10.2147/CIA.S362191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Yanli Zhao, Qunfang Ding, Taiping Lin, Xiaoyu Shu, Dongmei Xie, Langli Gao, Jirong Yue
Department of Geriatrics and National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Qunfang Ding, Department of Geriatrics and National Clinical Research Center for Geriatrics West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Tel +86 18980601353, Email [email protected]
Objective: Hearing and vision loss have been independently associated with frailty in older adults, but the relationship between concurrent hearing and visual impairment (dual sensory impairment) and frailty is not well understood. Therefore, we aimed to examine whether dual sensory impairment is associated with frailty in older adults.
Methods: This cross-sectional study was based on the data from the West China Health and Aging Trend (WCHAT) study of community-dwelling individuals aged 60 years and older. Frailty status was evaluated by the FRAIL scale and categorized as robust, prefrail and frail. Hearing and vision functions were based on self-report. We used multinomial regression models to explore the association between dual sensory impairment and frailty.
Results: Of 3985 participants, 1655 (41.5%) were male and the median age was 66 years (interquartile range: 61– 68). Overall, 7.6% of participants reported hearing impairment only, 32.7% reported vision impairment only, and 28.6% reported dual sensory impairment. The prevalence of prefrailty and frailty was 60.7% and 6.1%, respectively. After adjustment for confounding variables, results from the multinomial regression analysis showed that dual sensory impairment was significantly associated with greater odds of becoming frail (OR = 2.17, 95% CI = 1.40– 3.38) compared with no impairment. When stratified by gender, dual sensory impairment was significantly associated with frailty in women (OR = 2.42, 95% CI = 1.40– 4.20) but not in men (OR = 1.30, 95% CI = 0.58– 2.91).
Conclusion: Older adults with dual sensory impairment are more likely to be frail than those with no impairment, suggesting that interventions to improve sensory function may potentially help reduce the risk of frailty in older adults.
Keywords: frailty, sensory impairment, vision impairment, hearing impairment
Introduction
Hearing and vision impairments are common age-related conditions in older people and often occur concurrently.1 The prevalence of concurrent hearing and vision loss, defined as dual sensory impairment, estimates ranging from 10% to 34% among older adults of long-term care facilities, and 13–25% in home care, respectively.2 With the rapid population aging, the prevalence of age-related sensory impairment is gradually increasing, which becomes an important public health concern.1 Dual sensory impairment limits the ability to obtain information, communication, daily activities, and social interactions.3,4 Evidence has shown that dual sensory impairment is significantly associated with cognitive impairment, function decline, anxiety, depression, and mortality.5–7
Frailty is another age-related clinical condition in older adults that is associated with an increased risk of mortality, hospitalization, falls, and disability.8 It is defined as a syndrome of increased vulnerability to stressors due to multisystem impairments and decreased physiologic reserve.9 Sensory impairments have been postulated as a potential marker for frailty because of their sharing of some common risk factors, such as cognitive impairment, depression, poor physical function, and disability.10 To date, a number of studies have only focused on the impact of a single sensory impairment (eg, hearing or vision impairment) on frailty. Previous cross-sectional studies found an association between self-reported hearing/vision impairment and frailty in older adults.11,12 Longitudinal analyses of community-dwelling older adults have also showed an association between hearing/vision impairment and incident frailty.13–16 However, the association between dual sensory impairment and frailty in older adults has not been examined.
In the present study, we aimed to explore the relationship between dual sensory impairment and frailty in a large cohort of community-dwelling older adults. We hypothesized that dual sensory impairment would be associated with greater odds of being frail compared with no sensory impairment.
Methods
Study Design and Population
The cross-sectional study used data from the baseline survey of the West China Health and Aging Trend (WCHAT), a population-based longitudinal study conducted in western China.17 The study aimed to explore the determinants of healthy aging among community-dwelling adults aged 50 years and older from 18 ethnic groups in Sichuan, Yunnan, Guizhou and Xinjiang province. All the baseline data were collected from July 2018 to November 2018. The study was approved by the Ethics Committee of West China Hospital, Sichuan University and was performed according to the Declaration of Helsinki. All participants (or legal proxies) gave written informed consent.
For the purpose of the present study, we limited our study population to older adults aged 60 and older (n = 4514). Participants with missing data on frailty, vision function or hearing function (n = 529) were excluded, resulting in an analytic sample of 3985 (Figure 1).
 |
Figure 1 Flow chart of study inclusion. |
Data Collection
Data were collected through face-to-face interviews with trained interviewers. Information regarding age, sex, education level, ethnicity (Han, Qiang, Tibetan, Yi, others), marital status, smoking status, and drinking status were collected. A number of chronic diseases were collected based on self-report diagnosis of hypertension, diabetes, heart diseases, cerebrovascular diseases, lung diseases, digestive diseases, osteoarthritis and tumor. Sleep disturbance was defined as having trouble falling asleep or staying asleep in the past month. Cognitive function was measured using the Short Portable Mental Status Questionnaire (SPMSQ), which is composed of 10 items and adjusts for education level.18 Higher SPMSQ scores indicate weaker cognitive function. Depression was considered when the score of the 15-item Geriatric Depression Scale (GDS-15) was 5 or more.19 The basic activities of daily living (ADL) were determined by the Barthel Index score,20 in which a total score of 95 or less indicated functional impairment. Nutritional status was measured by the short form of the Mini Nutritional Assessment (MNA-SF),21 and participants were categorized as malnourished (0–7 points), at risk of malnutrition (8–11 points), and well-nourished (12–14 points).
Assessment of Vision and Hearing Impairment
Vision and hearing functions were assessed based on self-report at baseline. Participants were categorized into four groups: no sensory impairment, vision impairment only, hearing impairment only, and dual sensory impairment (presence of both vision and hearing impairment).
Vision function was measured by asking participants whether their eyesight was good, fair, poor, very poor or blind despite wearing corrective lenses. Vision impairment was defined as reporting fair, poor, very poor or blind eyesight. Hearing function was evaluated by asking participants to rate their hearing as good, fair, poor, or deaf despite using a hearing aid. Reporting fair, poor, or deaf was classified as having hearing impairment. Self-reported vision and hearing impairment is a valid method, which has been widely used in previous studies.14,16 There is evidence suggesting good agreement between self-reported vision and hearing impairment and objective measurements.22
Assessment of Frailty
Frailty was evaluated with the FRAIL scale,23 which has been validated for the assessment of frailty status in older community-dwellers. The scale consists of five items: fatigue (feeling tired all or most of the time in the past month), resistance (inability to climb a flight of stairs without rest and aids), ambulation (inability to walk 100 meters alone without aids), illness (having ≥5 of the following illnesses: hypertension, diabetes, cancer, chronic lung disease, heart attack, congestive heart failure, angina, asthma, arthritis, stroke, kidney disease), and loss of weight (unintentional weight loss of ≥5% over the past year). One point is attributed to each item and the total score ranges from 0 to 5 points. The individuals were divided into the following three groups based on the cut-offs: robust (0 points), prefrail (1–2 points), and frail (3–5 points).
Statistical Analysis
All categorical variables were presented as count and percentage, and the non-normally distributed continuous variables as medians and interquartile range (IQR). Comparisons of differences among groups were tested by Kruskal–Wallis for non-normally distributed continuous variables, and chi-square test for categorical variables. Multinomial logistic regression models were used to determine the association between sensory impairment as compared to no sensory impairment with frailty. Four sets of models were conducted: Model 1 was unadjusted; Model 2 adjusted for age, sex, education, ethnicity and marital status; Model 3 adjusted for smoker, alcohol abuse, number of chronic diseases, cognitive impairment, and variables included in Model 2; Model 4 adjusted for depression, ADL impairment, sleep condition, malnutrition status, and variables included in Model 3. In addition, we analyzed the association of sensory impairment with frailty by gender. All statistical analyses were conducted using SPSS version 21.0 (IBM Corp., Armonk, NY). P < 0.05 was considered statistically significant.
Results
A total of 3985 participants were enrolled in our studies. The median age of the participants was 66 years (interquartile range: 63–72) and 1655 (41.5%) were male. Among these participants, 2417 (60.7%) were prefrail and 245 (6.1%) were frail. A total of 303 (7.6%) reported hearing impairment, 1305 (32.7%) reported vision impairment, and 1140 (28.6%) reported dual sensory impairment. In addition, the prevalence of malignancy, chronic obstructive pulmonary disease and congestive heart failure were 0.6%, 1.8% and 3.9%, respectively.
Table 1 presents the characteristics of all participants according to type of sensory impairment. There were significant differences in age, gender, education level, ethnicity, marital status, alcohol use, cognitive impairment, ADL impairment, depression, malnutrition, and frailty status among different sensory impairment groups. Table 2 shows the characteristics of all participants stratified by the degree of frailty status. Significant differences were found among different frailty status groups with regard to age, gender, education level, ethnicity, marital status, smoking, alcohol use, number of chronic diseases, cognitive impairment, ADL impairment, depression, sleep disturbance, and malnutrition.
 |
Table 1 Characteristics of Participants According to Sensory Impairment Status |
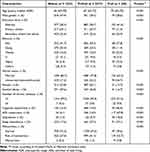 |
Table 2 Characteristics of Participants According to Frailty Status |
Table 3 displays the results of the multinomial logistic regression analysis on the association between sensory impairment and frailty. Compared with participants with no sensory impairment, higher odds of being prefrail and frail were found in those with vision impairment only and dual sensory impairment but not in hearing impairment only in the unadjusted model. After adjustment for age, sex, education, ethnicity, marital status, smoking, alcohol abuse, number of chronic diseases, cognitive impairment, depression, ADL impairment, sleep condition and malnutrition status, the association of dual sensory and vision impairment with prefrailty and frailty remained statistically significant. When stratified by gender, vision impairment only and dual sensory impairment were significantly associated with prefrailty in both genders. Moreover, women with dual sensory impairment had higher odds of being frail than those with no sensory impairment, but no significant association was observed in men (Table 4).
 |
Table 3 Association Between Sensory Impairment and Frailty According to Multinomial Logistic Regression Analyses |
 |
Table 4 Sex Differences in the Association Between Sensory Impairment and Frailty According to Adjusted Multinomial Logistic Regression Models |
Discussion
Results from the present study showed that dual sensory impairment was significantly associated with higher odds of being frail in a large sample of older adults (aged ≥60 years). The association remained unchanged after adjustment for potential confounding covariates. Moreover, we particularly found that dual sensory impairment was significantly associated with frailty only in women, but not among men. Together, our results suggest that frailty in older adults with dual sensory impairment, especially among women, warrants greater attention by clinicians.
In the present study, the prevalence of frailty among community-dwelling older adults was 6.1%. The result was in line with previous study conducted in China, where the prevalence of frailty evaluated by the FRAIL scale in community-dwelling older adults was 6.6%.24 The prevalence of vision impairment in this study was 32.7%, which was in consistent with the prevalence of vision loss reported for adults aged 60 years and older in the United States (16.1–50%).25 However, our study showed a considerably lower prevalence of hearing impairment than the high estimate of >45% reported by Cruickshanks et al.26 One explanation is the difference in the definitions and measures of hearing impairment. Ours defined hearing impairment based on self-report, but Cruickshanks et al used objective measures. Another potential explanation is that older adults are more likely to report better than the fact, leading to a lower identification of participants with hearing impairment.
Existing studies have demonstrated an independent association of hearing impairment with frailty. A population-based cohort study of older adults based on the English Longitudinal Study of Ageing identified that hearing loss was associated with greater odds of frailty.14 A cross-sectional study by Kamil et al11 demonstrated that self-reported hearing impairment was associated with frailty in women but not in men. Meanwhile, their subsequent longitudinal study using objective hearing assessment reported a higher risk of frailty in both men and women with hearing impairment.13 In contrast, our study failed to observe any association between hearing impairment and frailty after adjusting for potential confounders. The fact might potentially be due to the fact that a relatively small number of participants with hearing impairment could diminish their statistical power to detect significant associations.
There are several studies showing that vision impairment has an effect on frailty. For example, a cross-sectional study of US older adults from the National Health and Nutrition Examination Survey observed that individuals with near vision impairment were more likely to be frail than those without sensory impairment.12 Liljas et al,16 using data from the English Longitudinal Study of Ageing, also reported that poor vision was associated with an increased risk of being frail over 4 years. Additionally, a longitudinal study found that individuals with vision impairment had greater odds of frailty.15 However, in our study, the result of the association between vision impairment and frailty was not significant. This observation may be explained by the fact that we were not adequately powered to detect meaningful differences due to the imbalance in the groups based on degree of frailty status. Furthermore, vision loss is not considered as a component of the FRAIL scale, which mainly includes functional and biological factors. Different results might be observed if our study uses the Frailty Risk Index, which incorporates vision impairment, illness, nutritional parameters, and biochemical indexes.27
The primary finding of this study showed that dual sensory impairment was associated with frailty, which is inconsistent with research conducted in Brazil that failed to find a significant association between dual sensory impairment and frailty.28 The discrepancy may be partly due to differences in the definition of frailty and sensory impairment. Additionally, the sample size in their study is relatively small (107 subjects). There are several plausible explanations for the association of dual sensory impairment with frailty. First, dual sensory impairment has been revealed to be independently associated with slow walking speed,29,30 which may have an important impact on resistance and ambulation, the components of the FRAIL scale. Second, compared to single sensory impairment, dual sensory loss leads to greater risk of worse health-related outcomes including depression, cognitive impairment, functional limitation, social isolation, and falls,6,7,31,32 which are known as risk factors for frailty. Third, there are shared comorbidities such as cardiovascular disease and diabetes, which could contribute to both dual sensory impairment and frailty.33–35 Finally, sensory impairment and frailty may share a similar pathological pathway such as systemic inflammation.36–38 Our findings extend the discussion in the literature on the relationship between sensory impairment and frailty in older adults.
Interestingly, we also found that dual sensory impairment was significantly associated with frailty only among older women but not men. This may be because a higher rate of frailty among older women can be identified by the FRAIL scale, with a good known-group divergent validity.24 And there is evidence that frailty is more common in older women compared to men.9 In addition, differences in social, cultural, and economic may increase the risk of illnesses and limit access to services for women.39 Finally, lower muscle mass, strength and androgen may expose women to a greater risk of frailty.40 Thus, it is not surprising that dual impairment has a significant relationship with frailty among women.
Moreover, we also found that sensory impairment was associated with cognitive impairment and depression. This association can be explained by several hypotheses. The first is the sensory deprivation hypothesis: sensory impairment can result in neuroplastic changes, depression and social isolation, consequently leading to cognitive decline.41 The second is the common cause hypothesis: sensory impairment as well as cognitive decline and depression may share common pathological processes, such as vascular disease or inflammation.42
Strengths of the present study include a large sample of older adults, and a comprehensive baseline survey, which enabled us to adjust for numerous relevant confounders. However, our study has several limitations. First, frail was based on self-report, which may be subject to recall bias. Likewise, sensory impairment was also assessed by self-report rather than objective measures, and hence participants may under- or over-report their impairment. Second, cognitive impairment and depression may effect the self-reported assessment of sensory function. However, we excluded individuals who were unable to complete hearing or vision assessment because of severe depression or severe cognitive impairment. Third, we did not investigate the role of assistive devices (eg, hearing aids or glasses) in sensory-impaired participants because the use of assistive devices was included in the definition of sensory impairment. Fourth, the cross-sectional design limits the ability to explore the causal relationship between sensory impairment and frailty among older adults. Longitudinal studies with objective measures of sensory impairment are needed to further prospectively examine this relationship.
Conclusion
In conclusion, our results showed that older adults with dual sensory impairment, particularly in women, were more likely to be frail than those without sensory impairment. Thus, assessment of sensory function in older adults should be as a part of routine assessments to help identify older adults at increased risk of frailty. As the population of sensory impaired older adults increases, future studies are needed to determine whether prevention and treatment strategies could reduce the progression of frailty among sensory impaired older adults.
Abbreviations
ADL, activities of daily living; SPMSQ, Short Portable Mental Status Questionnaire; GDS-15, 15-item Geriatric Depression Scale; MNA-SF, short form of the Mini Nutritional Assessment.
Data Sharing Statement
There are no linked research data sets for this paper. The data are confidential, and the authors do not have permission to share the data.
Ethical Approval and Informed Consent
This study was performed according to the Helsinki Declaration and was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017-445). Each participant provided written informed consent. In participants with cognitive impairment, written informed consent was also obtained from a valid surrogate.
Acknowledgments
We would like to thank the staff of the Department of Geriatrics Medicine, West China Hospital and all participants for their great contribution.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Chinese National Science and Technology Pillar Program (2020YFC2005600); Sichuan Science and Technology Program (2021YFS0136); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21005); 1.3.5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (19HXFH012); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20191003).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Schneider JM, Gopinath B, McMahon CM, et al. Dual sensory impairment in older age. J Aging Health. 2011;23(8):1309–1324. doi:10.1177/0898264311408418
2. Guthrie DM, Declercq A, Finne-Soveri H, et al. The health and well-being of older adults with Dual Sensory Impairment (DSI) in four countries. PLoS One. 2016;11(5):e0155073. doi:10.1371/journal.pone.0155073
3. Wittich W, Watanabe DH, Gagné JP. Sensory and demographic characteristics of deafblindness rehabilitation clients in Montréal, Canada. Ophthalmic Physiol Opt. 2012;32(3):242–251. doi:10.1111/j.1475-1313.2012.00897.x
4. Jaiswal A, Aldersey H, Wittich W, et al. Participation experiences of people with deafblindness or dual sensory loss: a scoping review of global deafblind literature. PLoS One. 2018;13(9):e0203772. doi:10.1371/journal.pone.0203772
5. Schubert CR, Fischer ME, Pinto AA, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(5):710–715. doi:10.1093/gerona/glw036
6. Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52(12):1996–2002. doi:10.1111/j.1532-5415.2004.52554.x
7. Simning A, Fox ML, Barnett SL, et al. Depressive and anxiety symptoms in older adults with auditory, vision, and dual sensory impairment. J Aging Health. 2019;31(8):1353–1375. doi:10.1177/0898264318781123
8. Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. doi:10.1093/gerona/gls119
9. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi:10.1093/gerona/56.3.m146
10. Tan BKJ, Man REK, Gan ATL, et al. Is sensory loss an understudied risk factor for frailty? A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2020;75(12):2461–2470. doi:10.1093/gerona/glaa171
11. Kamil RJ, Li L, Lin FR. Association between hearing impairment and frailty in older adults. J Am Geriatr Soc. 2014;62(6):1186–1188. doi:10.1111/jgs.12860
12. Varadaraj V, Lee MJ, Tian J, et al. Near vision impairment and frailty: evidence of an association. Am J Ophthalmol. 2019;208:234–241. doi:10.1016/j.ajo.2019.08.009
13. Kamil RJ, Betz J, Powers BB, et al. Association of hearing impairment with incident frailty and falls in older adults. J Aging Health. 2016;28(4):644–660. doi:10.1177/0898264315608730
14. Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported hearing impairment and incident frailty in English community-dwelling older adults: a 4-year follow-up study. J Am Geriatr Soc. 2017;65(5):958–965. doi:10.1111/jgs.14687
15. Swenor BK, Lee MJ, Tian J, et al. Visual impairment and frailty: examining an understudied relationship. J Gerontol A Biol Sci Med Sci. 2020;75(3):596–602. doi:10.1093/gerona/glz182
16. Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71(11):1053–1058. doi:10.1136/jech-2017-209207
17. Hou L, Liu X, Zhang Y, et al. Cohort profile: West China Health and Aging Trend (WCHAT). J Nutr Health Aging. 2021;25(3):302–310. doi:10.1007/s12603-020-1530-1
18. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi:10.1111/j.1532-5415.1975.tb00927.x
19. Nyunt MS, Fones C, Niti M, et al. Criterion-based validity and reliability of the geriatric depression screening scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health. 2009;13(3):376–382. doi:10.1080/13607860902861027
20. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65.
21. Rubenstein LZ, Harker JO, Salvà A, et al. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. 2001;56(6):M366–72. doi:10.1093/gerona/56.6.m366
22. Cavazzana A, Röhrborn A, Garthus-Niegel S, et al. Sensory-specific impairment among older people. An investigation using both sensory thresholds and subjective measures across the five senses. PLoS One. 2018;13(8):e0202969. doi:10.1371/journal.pone.0202969
23. Aprahamian I, Cezar NOC, Izbicki R, et al. Screening for frailty with the FRAIL Scale: a comparison with the phenotype criteria. J Am Med Dir Assoc. 2017;18(7):592–596. doi:10.1016/j.jamda.2017.01.009
24. Dong L, Qiao X, Tian X, et al. Cross-cultural adaptation and validation of the FRAIL scale in Chinese community-dwelling older adults. J Am Med Dir Assoc. 2018;19(1):12–17. doi:10.1016/j.jamda.2017.06.011
25. Varma R, Vajaranant TS, Burkemper B, et al. Visual impairment and blindness in adults in the United States: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016;134(7):802–809. doi:10.1001/jamaophthalmol.2016.1284
26. Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res. 2010;264(1–2):3–9. doi:10.1016/j.heares.2009.10.008
27. Ng TP, Feng L, Nyunt MS, et al. Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc. 2014;15(9):635–642. doi:10.1016/j.jamda.2014.03.008
28. Garcia A, Wittich W, Momensohn-Santos T. Association between frailty and dual sensory loss: hearing and vision. Rev Kairós Gerontologia. 2021;24(1):329–349. doi:10.23925/2176-901X.2021v24i1p329-349
29. Swenor BK, Simonsick EM, Ferrucci L, et al. Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. 2015;63(1):46–54. doi:10.1111/jgs.13183
30. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38(1):25–29. doi:10.1016/j.gaitpost.2012.10.006
31. Lopez D, McCaul KA, Hankey GJ, et al. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment–is there a gender difference? Maturitas. 2011;69(4):359–364. doi:10.1016/j.maturitas.2011.05.006
32. Mick P, Parfyonov M, Wittich W, et al. Associations between sensory loss and social networks, participation, support, and loneliness: analysis of the Canadian longitudinal study on aging. Can Fam Physician. 2018;64(1):e33–e41.
33. Mitnitski AB, Graham JE, Mogilner AJ, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi:10.1186/1471-2318-2-1
34. Nadruz W
35. Wattamwar K, Qian ZJ, Otter J, et al. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngol Head Neck Surg. 2018;144(7):623–629. doi:10.1001/jamaoto.2018.0643
36. Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ study. Mech Ageing Dev. 2012;133(6):456–466. doi:10.1016/j.mad.2012.05.005
37. Verschuur CA, Dowell A, Syddall HE, et al. Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire ageing study. Age Ageing. 2012;41(1):92–97. doi:10.1093/ageing/afr140
38. Kauppinen A, Paterno JJ, Blasiak J, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi:10.1007/s00018-016-2147-8
39. Courtright P, Lewallen S. Why are we addressing gender issues in vision loss? Community Eye Health. 2009;22(70):17–19.
40. Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinol Metab Clin North Am. 2013;42(2):391–405. doi:10.1016/j.ecl.2013.02.006
41. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154–166. doi:10.1016/j.arr.2015.06.002
42. Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res. 2001;127(1–2):137–158. doi:10.1016/s0166-4328(01)00361-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Experiences of Being Significant Others to Older Adults with Frailty Living Alone in Rural Arctic Norway: A Qualitative Study
Bjerkmo L, Helgesen AK, Blix BH
Risk Management and Healthcare Policy 2022, 15:1283-1292
Published Date: 1 July 2022
Intrinsic Capacity Declines with Elevated Homocysteine in Community-Dwelling Chinese Older Adults
Lin S, Wang F, Zheng J, Yuan Y, Huang F, Zhu P
Clinical Interventions in Aging 2022, 17:1057-1068
Published Date: 7 July 2022
An Easy-to-Implement Clinical-Trial Frailty Index Based on Accumulation of Deficits: Validation in Zoster Vaccine Clinical Trials

Andrew MK, Matthews S, Kim JH, Riley ME, Curran D
Clinical Interventions in Aging 2022, 17:1261-1274
Published Date: 19 August 2022
Coexisting Frailty and Cognitive Impairment as a Predictor of Adverse Outcomes in Older Inpatients After Discharge: Results from a One-Year Follow-Up Study
Zeng XK, Shen SS, Guan HL, Chen LY, Chen XJ
Clinical Interventions in Aging 2022, 17:1697-1706
Published Date: 29 November 2022
Recognition and Management of Hospital-Acquired Sepsis Among Older General Medical Inpatients: A Multi-Site Retrospective Study
Barker N, Scott IA, Seaton R, Mehta N, Kalke VR, Redpath L
International Journal of General Medicine 2023, 16:1039-1046
Published Date: 21 March 2023
