Back to Journals » Journal of Pain Research » Volume 16
Clinical Observation on Auricular Acupressure for Primary Dysmenorrhea: A Study Protocol for a Randomized Clinical Trial
Authors Liu L , Hu J , Lu J, Yang J
Received 14 May 2023
Accepted for publication 12 August 2023
Published 21 September 2023 Volume 2023:16 Pages 3217—3225
DOI https://doi.org/10.2147/JPR.S414416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Lumin Liu,1,* Junwei Hu,1,* Junjie Lu,2 Jiaxin Yang3
1Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Social and Behavioral Sciences, Harvard University T.H. Chan School of Public Health, Boston, MA, USA; 3Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiaxin Yang, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, 110 Ganhe Road, Hongkou District, Shanghai, 200437, People’s Republic of China, Email [email protected]
Purpose: The objective of this study is to evaluate the immediate and time-dependent effects of AA in treating PD and assess its safety.
Methods/Design: This study is a randomized, single-blinded, controlled trial that will enroll 92 patients in a 1:1 allocation ratio. Patients will be assigned to either the treatment group (n=46) or the control group (n=46). During the first menstrual period, the treatment group will receive AA treatment, while the control group will receive sham AA treatment for 7 days. The second menstrual period will serve as the follow-up period. The primary outcome measure is the Visual Analog Scale (VAS) score 30 min after the first treatment. Secondary outcome measures include the VAS score immediately after the first treatment, onset time of analgesic effect, duration of pain, extra dosing rate of ibuprofen, and change of the Menstrual Distress Questionnaire (MDQ) score. The outcomes will be assessed at baseline, during the intervention period, and during the follow-up period.
Conclusion: The study results will provide evidence on the efficacy and safety of AA in managing PD by analyzing its immediate effect, time-effect relationship, and reduction of painkiller use.
Trial Registration: Chinese Clinical Trial Registry (ChiCTR2300069741).
Keywords: acupressure, dysmenorrhea, menstrual pain, ear
Introduction
Dysmenorrhea, a common gynecological condition, is characterized by lower abdominal pain, swelling, and discomfort before, during, or after menstruation, often accompanied by low back pain.1 The prevalence of dysmenorrhea in young females exceeds 50%.2 Dysmenorrhea can be classified into primary dysmenorrhea (PD) and secondary dysmenorrhea based on its pathophysiology.3 PD is characterized by periodic menstrual pain in the absence of other pelvic diseases and accounts for more than 90% of dysmenorrhea cases.1
PD has a significant impact on women’s quality of life, academic performance, and mental health. In the European Union, PD leads to a loss of 3.6 million quality-adjusted life years annually, which is equivalent to the impact of chronic diseases such as type 1 diabetes, asthma, or chronic migraine.4 Recurrent episodes of PD negatively affect women’s physical activity, schoolwork, and overall well-being.3 Globally, 10–46% of young women miss one or more days of school each month due to PD,5 with the majority struggling with mental health issues and sleep disturbances.6
The treatment of PD is primarily based on drug therapy, including non-steroidal anti-inflammatory drugs and compound oral contraceptives.7 However, medication effectiveness can vary significantly among individuals, and adverse reactions can cause discomfort for patients. In a study on self-perceived medication effectiveness for PD, a significant proportion of women reported that common medications for PD were ineffective and bothered them with adverse effects.8
Given the limited efficacy and adverse effects of conventional drug therapies for PD, lifestyle changes9 and complementary and alternative medicine (CAM) approaches, such as exercise, massage, acupuncture, auricular acupressure (AA),10 have emerged as potential treatment options. While previous studies have largely focused on the analgesic effect of acupuncture, we propose that AA is a more practical and convenient alternative for managing PD while ensuring efficacy.
AA involves the use of vaccaria or magnetic beads to stimulate patients’ auricular points, and evidence suggests that AA can significantly and immediately relieve both acute and chronic visceral pain.11,12 AA is easy to operate, safe, and effective, making it a promising option for self-treatment of PD. Despite this, few studies have investigated the clinical efficacy of AA in treating PD. AA used alone was found to be superior to analgesics and non-intervention in terms of cured rate, total effective rate, visual analogue scale (VAS), and symptom scores, but the quality of the study is not high enough to provide sufficient evidence. Similarly, when AA was used as an adjunctive therapy to other treatments, it demonstrated the same superior outcomes. However, AA alone showed limited effectiveness when compared to other therapies such as Chinese herb, acupuncture, health education, etc.13 To establish a more definitive conclusion, we design this single-blinded, randomized, controlled trial to explore the effectiveness and safety of AA in managing PD.
Materials and Methods
Study Design
This randomized, single-blinded, controlled trial has been registered in the Chinese Clinical Trial Registry (ChiCTR) under registration number ChiCTR2300069741. The protocol for this study was based on the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) 2013, as outlined in Supplementary File 1. Ethics approval was obtained from the Medical Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine (Approval number 2023-011). Any modifications to the protocol will be reported to both the ChiCTR and the Medical Ethics Committee. The study flowchart is presented in Figure 1, and the schedule of enrolment, interventions, and assessments can be found in Table 1.
 |
Table 1 Schedule of Enrolment, interventions, and Assessment |
 |
Figure 1 Flow chart. |
Participants
A total of 92 female patients with primary dysmenorrhea (PD) will be recruited from both outpatient clinics at Yueyang Hospital of Integrated Traditional Chinese and Western Medicine and posters on hospital websites. After undergoing eligibility assessments based on predetermined inclusion and exclusion criteria, patients who meet the criteria will sign an informed consent form and be randomly allocated to the treatment group or control group in a 1:1 ratio. Patients will have the right to withdraw from the trial at any time, and personal data will be used only for medical research purposes.
Inclusion Criteria
- Patients who meet the diagnostic criteria for PD:14 PD is a condition marked by crampy, suprapubic pain occurring a few hours before or after menstrual bleeding starts. Symptoms peak during the heaviest flow and may last for a few days. The condition is generally consistent from one menstrual period to another and may be accompanied by various symptoms such as diarrhea, nausea, vomiting, fatigue, light-headedness, headache, dizziness, and, in rare cases, syncope and fever.
- Between the ages of 18 and 30;
- With regular menstrual cycle of 28±7 days, menstrual period of 3–7 days, and the pain starting on the first day of the cycle;
- Have not received treatment for PD within a month prior to enrolment;
- VAS score ≥4;
- Voluntarily participate in the study and sign the informed consent.
Exclusion Criteria
- With serious cardiovascular, liver, kidney, hematopoietic system disease or malignant tumors;
- Other pelvic diseases such as uterine fibroids, endometrial polyps, endometriosis, pelvic inflammation, and abnormal development of reproductive systems;
- Previously received AA therapy;
- Auricle skin damage, tape allergy;
- Pregnancy or lactation;
- Individuals who engage in bad habits such as smoking tobacco, consuming alcohol, or following a specific diet.
Interventions
The specific auricular points to be treated are: shenmen (TF4), lumbosacral vertebrae (AH9), and pelvis (TF5),15 as indicated in Figure 2 and Table 2. During the treatment, the acupuncturist will use a metal probe to identify the auricular points and ask the patients if they experience “deqi” sensations such as heat, numbness, distension, or pain. Once the auricular points have been confirmed, the ear will be disinfected using a 75% ethanol solution and dried using a sterile dry cotton ball. The acupuncturist will then hold the ear in place with their left hand while using their right hand to manipulate a tweezer and apply tape (0.5 x 0.5 cm) with or without vaccaria (Shanghai Taicheng Technology Development Co., LTD., Shanghai, China) to the selected auricular point.
 |
Table 2 The Locations of Auricular Points |
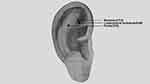 |
Figure 2 The locations of auricular points. |
The first menstrual cycle serves as the treatment period, beginning on the first day of menstruation in the morning. The right auricular acupoints will be treated first and the tapes will be kept in place for 3 days. On the fourth day, the tapes on the right ear will be removed and new tapes will be applied to the left ear. The purpose of replacing tapes is to reduce the adverse events that may be caused by long-term stimulation unilaterally. The treatment will last for 7 days, and the follow-up will be done at the second menstrual cycle.
Treatment Group
The treatment group will receive the tapes with vaccaria. After the tapes have been attached to the auricular points, the acupuncturist will apply pressure to the vaccaria using their fingertips to induce the “deqi” sensation in the patient. Each point will be pressed for 1 min. Besides, the patients will be instructed to apply pressure to each point for 1 min, 4 times per day.16
Control Group
The manipulation of the sham AA refers to previous research.17,18 The acupuncturist will place the same skin-colored adhesive tapes without vaccaria on the auricular points, but these tapes will not be pressed during treatment.
Rescue Medication
If the patients still have unbearable pain related to PD after treatment, they are allowed to take Ibuprofen Sustained Release Tablets (Tianjin Smith Kline & French Laboratories Ltd, Tianjin, China), which is 300mg/tablet, 30 min after the first treatment under the doctor’s guidance. The dosage can be adjusted according to the situation, but the daily maximum dose is no more than 600mg according to drug instructions according to drug instructions. Both patients and researchers are required to keep detailed records of the drug dosage, date of administration, and any reactions after medication, to ensure that the patients receive safe and effective treatment. This information will also be useful for evaluating the effectiveness of the treatment.
Primary Outcome Measure
The patients will be asked to rate their pain intensity using a 10-mm pain Visual Analogue Scale (VAS) 30 min after the first treatment. This scale ranges from 0 to 100, where 0 represents “no pain” and 100 represents “worst imaginable pain”.19 The results of the VAS will provide an assessment of the immediate analgesic effect of AA in treating PD.
Secondary Outcome Measures
- 10-mm pain VAS immediately after the first treatment and at follow-up;
- Onset time of analgesic effect: the time it takes from the first treatment to the beginning of pain relief in first menstrual cycle;
- Duration of pain in first menstrual cycle and at follow-up;
- Extra dosing rate of ibuprofen in first menstrual cycle and at follow-up;
- The change of Menstrual Distress Questionnaire (MDQ)20,21 score in first menstrual cycle and at follow-up: MDQ was originally designed by Moos in 1968. In this trail, a Chinese version with good reliability and validity will be used to observe patients’ menstrual concomitant symptoms. It can be divided into 6 sub-scales, with 5 items for pain, 3 items for concentration, 5 items for behavioral change, 9 items for autonomic reactions, 2 items for water retention, and 6 items for negative affect. These items are checked on a 6-point scale, with 0 indicating “normal” and 5 indicating “severe”. In total, the MDQ score will be calculated between 0–150, wherein higher scores indicate more discomfort in a patient.
Safety Evaluation
Researchers will record the adverse events (AEs) including date of onset and recovery, severity, its relevance to the treatment and how the event is resolved (or not) in the case report form (CRF). AEs associated with auricular acupressure covers itching, swelling, unbearable pain, wound, infection or other discomforts. If any AEs occur, the research team will provide appropriate treatment and compensation and report any severe events to the data and safety monitoring board.
Sample Size Calculation
The sample size calculation was performed using PASS software (version 15.0.5, NCSS, LLC, USA) with an alpha of 0.05 (two-tailed), a power of 90%, and a 1:1 ratio. The calculation was based on the change in the VAS 30 min after the first treatment. According to our preliminary results, the mean ± standard deviation for the treatment group and control group were 1.72±1.06 and 0.69±1.39, respectively. Taking into account a 15% dropout rate, the sample size was determined to be 46 patients per group, resulting in a total of 96 patients.
Randomization and Blinding
This study employs randomization and blinding to reduce potential bias. Randomization will be performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA) to generate random numbers, which will be used by personnel to assign patients to the treatment or control group. The allocation will be concealed in opaque envelopes, opened in sequence matching the order of hospital visits. To ensure blinding, all researchers will receive training and follow the department’s separation principle. Except for the acupuncturist, patients, outcome assessors, and data analysts will be blinded. After treatment, a blinding assessment will be conducted to determine whether patients correctly identified their intervention. Patients will be asked the following question: “Which intervention do you think you have received? Auricular acupressure, sham auricular acupressure, or uncertain.”
Statistical Methods
Statistical analysis will be conducted using SAS, with intention-to-treat as the primary method of analysis. Per-protocol analysis will also be performed. Continuous variables will be reported as mean±standard deviation if normally distributed, and median (interquartile range) if not. The t-test or Mann–Whitney U-test will be used to analyze continuous variables. Categorical variables will be presented as numbers (percentages) and analyzed with chi-square or Fisher’s exact tests. Furthermore, trends over time and time-by-treatment interactions will be examined using a generalized estimating equation or mixed effect model. The confidence interval will be set at 95%, with a significance level of 5% (P < 0.05).
Quality Control, Data Management and Monitoring
To ensure the validity of this clinical research, standardized training will be provided to all researchers on the protocol, Standard Operating Procedure, and scale evaluation before the study commences. An acupuncturist with at least five years of clinical expertise will receive training on point location, technique criteria, and masking application for the trial. A detailed data management strategy will be implemented, including data collection, entry, and management. Data will be collected using CRF. To ensure accuracy, two independent researchers will enter the data, and then the entries will be confirmed and used. Research assistants will regularly monitor data quality and research progress.
Discussion
PD is a highly prevalent condition among young women that is often undertreated. This not only impacts their quality of life but also exacerbates various mental health issues, such as trait empathy, anxiety, depression, and others, in a subtle manner.6 Therefore, there is a critical need to address this condition in young women to improve their overall well-being.
Drug therapy is a common approach to alleviate the symptoms of dysmenorrhea. However, limitations such as periodic continued drug use and potential adverse reactions have limited the effectiveness of this approach.22 A questionnaire survey conducted among Chinese female college students revealed that many of them have a conservative attitude towards medication and lack knowledge about which drugs can be used to manage PD.23 These findings may be associated with the wider acceptance of CAM as a treatment for PD among Chinese women. Therefore, there is a need to explore alternative management strategies for PD to improve the overall treatment outcomes for affected women.
CAM has been widely acknowledged for its analgesic effects and minimal adverse reactions.24,25 One such approach is AA, which involves stimulating specific auricular points in the ear to alleviate physical and mental dysfunction. According to classic Chinese Medicine literature, the ears, meridians, and viscera are interrelated, and auricular points can be used for diagnosis and treatment. Western medicine has also discovered a somatotopic organization of the body represented on the human auricle, leading to the concept of an inverted fetus map on the external ear.26 In recent years, research has confirmed the efficacy of AA and explored its mechanism in pain management.27 AA activates nerve reflexes, which lead to the release of neurotransmitters and endogenous opioids, such as endorphins, helping to relieve pain and inflammation.28
AA has great clinical value in the treatment of PD, with its efficacy confirmed by several studies.29–31 However, some of these studies did not include control groups, while others used AA as part of combination therapy, which interfered with the evaluation of AA’s efficacy in PD treatment. What’s more, it is important to note that different placebo treatments were used in existing studies comparing AA with placebo controls, and the benefit of AA compared to placebo for patients with dysmenorrhea remains inconclusive.13 To address these issues, we designed a randomized, single-blind, controlled study. It is noteworthy that the immediate analgesic effect and its timeliness are crucial for PD patients, but limited research has focused on this topic. Therefore, we will also observe the time-effect relationship, which will provide further insight into the efficacy of AA and guide the treatment frequency in future clinical practice.
To ensure the validity of this study, we will use sham AA to eliminate the comforting effect.17 The consistent appearance of the tapes used in both groups will contribute to blinding. In addition, taking painkillers will be allowed when patients experience unbearable pain during treatment. The time and dosage of painkillers will be recorded in detail to reflect the analgesic effect of AA, allowing us to evaluate efficacy more ethically and realistically. These measures are taken to obtain an objective and truthful result in this study.
Despite the efforts to enhance the validity of this study, there are still some limitations. The trial was not designed as a multi-center study, which may limit the generalizability of our findings to other populations. Additionally, because of the characteristics of AA, it is not possible to implement a double-blind approach, which may introduce some bias in our results. Nevertheless, we will try to minimize these limitations and report our findings objectively and transparently.
Conclusion
This study will provide comprehensive insights into the curative effect of AA on PD from multiple perspectives, including immediate effect, time-effect relationship, and the potential for reducing the use of painkillers. The findings of this study can serve as a basis for developing an alternative therapy for clinical application in the treatment of PD, which has the potential to improve the quality of life for affected women.
Abbreviations
PD, Primary Dysmenorrhea; AA, Auricular Acupressure; VAS, Visual Analogue Scale; MDQ, Menstrual Distress Questionnaire; CAM, Complementary and Alternative Medicine; ChiCTR, Chinese Clinical Trial Registry; SPIRIT, Standard Protocol Items: Recommendations for Intervention Trials; AEs, Adverse Events; CRF, Case Report Form.
Data Sharing Statement
Data requested for public use will be available after publication.
Ethics and Dissemination
This study has been approved by Medical Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine (Approval number 2023-011), and will be carried out in conformity with the Declaration of Helsinki. Prior to signing the informed consent, patients will receive complete information about the trail. Results of the trial will be published in peer-reviewed journals.
Acknowledgments
The trial was registered at China Clinical Trials Registry on March 24, 2023 (ChiCTR2300069741). The date of the intended trial period is from 1 April 2023 to 1 December 2023. Patient recruitment began on 7 April 2023 and is currently ongoing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferries-Rowe E, Corey E, Archer JS. Primary dysmenorrhea: diagnosis and therapy. Obstet Gynecol. 2020;136(5):1047–1058. doi:10.1097/AOG.0000000000004096
2. Kho KA, Shields JK. Diagnosis and management of primary dysmenorrhea. JAMA. 2020;323(3):268–269. doi:10.1001/jama.2019.16921
3. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi:10.1093/humupd/dmv039
4. Rencz F, Pentek M, Stalmeier PFM, et al. Bleeding out the quality-adjusted life years: evaluating the burden of primary dysmenorrhea using time trade-off and willingness-to-pay methods. Pain. 2017;158(11):2259–2267. doi:10.1097/j.pain.0000000000001028
5. O’Connell K, Davis AR, Westhoff C. Self-treatment patterns among adolescent girls with dysmenorrhea. J Pediatr Adolesc Gynecol. 2006;19(4):285–289. doi:10.1016/j.jpag.2006.05.004
6. Zhao S, Wu W, Kang R, Wang X. Significant increase in depression in women with primary dysmenorrhea: a systematic review and cumulative analysis. Front Psychiatry. 2021;12:686514. doi:10.3389/fpsyt.2021.686514
7. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;2015(7):CD001751. doi:10.1002/14651858.CD001751.pub3
8. Chen CX, Carpenter JS, LaPradd M, Ofner S, Fortenberry JD. Perceived ineffectiveness of pharmacological treatments for dysmenorrhea. J Womens Health. 2021;30(9):1334–1343. doi:10.1089/jwh.2020.8581
9. Doğan H, Eroğlu S, Akbayrak T. The effect of kinesio taping and lifestyle changes on pain, body awareness and quality of life in primary dysmenorrhea. Complement Ther Clin Pract. 2020;39:101120. doi:10.1016/j.ctcp.2020.101120
10. Doğan H, Demir Çaltekin M, Onat T, Aydoğan Kırmızı D, Başer E, Yalvaç ES. Approaches of dealing with primary dysmenorrhea and relationship between kinesiophobia and pain severity. Konuralp Tip Dergisi. 2020;12(3):551–556. doi:10.18521/ktd.727929
11. Borlack RE, Shan S, Zong AM, Khlevner J, Garbers S, Gold MA. Electrodermal activity of auricular acupoints in pediatric patients with functional abdominal pain disorders. J Pediatr Gastroenterol Nutr. 2021;73(2):184–191. doi:10.1097/MPG.0000000000003137
12. He BJ, Tong PJ, Li J, Jing HT, Yao XM. Auricular acupressure for analgesia in perioperative period of total knee arthroplasty. Pain Med. 2013;14(10):1608–1613. doi:10.1111/pme.12197
13. Kong X, Fang H, Li X, Zhang Y, Guo Y. Effects of auricular acupressure on dysmenorrhea: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol. 2023;13:1016222. doi:10.3389/fendo.2022.1016222
14. Burnett M, Lemyre M. No 345-primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2017;39(7):585–595. doi:10.1016/j.jogc.2016.12.023
15. Societies(WFAS) WFoA-M. Auricular point. World J Acupunct Moxibustion. 2013;23(03):12–21.
16. Wang YJ, Hsu CC, Yeh ML, Lin JG. Auricular acupressure to improve menstrual pain and menstrual distress and heart rate variability for primary dysmenorrhea in youth with stress. Evid Based Complement Alternat Med. 2013;2013:138537. doi:10.1155/2013/138537
17. Chang LH, Hsu CH, Jong GP, Ho S, Tsay SL, Lin KC. Auricular acupressure for managing postoperative pain and knee motion in patients with total knee replacement: a randomized sham control study. Evid Based Complement Alternat Med. 2012;2012:528452. doi:10.1155/2012/528452
18. Hsieh CH. The effects of auricular acupressure on weight loss and serum lipid levels in overweight adolescents. Am J Chin Med. 2010;38(4):675–682. doi:10.1142/S0192415X10008147
19. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi:10.1111/j.1365-2702.2005.01121.x
20. Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30(6):853–867. doi:10.1097/00006842-196811000-00006
21. Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–57. doi:10.3760/cma.j.issn.0253-2727.2019.01.010
22. Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2018;218(4):390–400. doi:10.1016/j.ajog.2017.08.108
23. Chen L, Tang L, Guo S, Kaminga AC, Xu H. Primary dysmenorrhea and self-care strategies among Chinese college girls: a cross-sectional study. BMJ Open. 2019;9(9):e026813. doi:10.1136/bmjopen-2018-026813
24. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi:10.15585/mmwr.rr6501e1
25. Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. N Engl J Med. 2016;374(15):1480–1485. doi:10.1056/NEJMsr1601307
26. Oleson TD, Kroening RJ, Bresler DE. An experimental evaluation of auricular diagnosis: the somatotopic mapping or musculoskeletal pain at ear acupuncture points. Pain. 1980;8(2):217–229. doi:10.1016/0304-3959(88)90009-7
27. Yeh CH, Chiang YC, Hoffman SL, et al. Efficacy of auricular therapy for pain management: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2014;2014:934670. doi:10.1155/2014/934670
28. Artioli DP, Bertolini GR. Auriculotherapy: neurophysiology, points to choose, indications and results on musculoskeletal pain conditions: a systematic review of reviews. Br J Pain. 2019;2(4):356–361.
29. Cha NH, Sok SR. Effects of auricular acupressure therapy on primary dysmenorrhea for female high school students in South Korea. J Nurs Scholarsh. 2016;48(5):508–516. doi:10.1111/jnu.12238
30. Xiang D, Situ Y, Liang X, Cheng L, Zhang G. Ear acupuncture therapy for 37 cases of dysmenorrhea due to endometriosis. J Tradit Chin Med. 2002;22(4):282–285.
31. Yeh ML, Hung YL, Chen HH, Wang YJ. Auricular acupressure for pain relief in adolescents with dysmenorrhea: a placebo-controlled study. J Altern Complement Med. 2013;19(4):313–318. doi:10.1089/acm.2011.0665
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
