Back to Journals » Cancer Management and Research » Volume 14
Clinical Characteristics, Prognostic Factors and Treatment Outcomes of Patients with Bone-Only Metastatic Breast Cancer
Authors Marie L, Braik D , Abdel-Razeq N, Abu-Fares H , Al-Thunaibat A, Abdel-Razeq H
Received 12 April 2022
Accepted for publication 20 July 2022
Published 23 August 2022 Volume 2022:14 Pages 2519—2531
DOI https://doi.org/10.2147/CMAR.S369910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Lina Marie,1 Dina Braik,1 Nayef Abdel-Razeq,2 Hala Abu-Fares,1 Ahmad Al-Thunaibat,1 Hikmat Abdel-Razeq1,3
1Department of Internal Medicine, King Hussein Cancer Center, Amman, Jordan; 2Department of Internal Medicine, Henry Ford Health System, Detroit, MI, USA; 3School of Medicine, the University of Jordan, Amman, Jordan
Correspondence: Hikmat Abdel-Razeq, Department of Internal Medicine, King Hussein Cancer Center, Queen Rania Al Abdullah Street, P.O. Box: 1269, Amman, 11941, Jordan, Tel +962-6 5300460, Ext: 1000, Email [email protected]
Introduction: Bone is the most frequent site of breast cancer metastasis. Differences between those who present with de novo bone-only metastasis (BOM) and those who progress to bone-only disease following a diagnosis of early-stage breast cancer are not clear. Such differences in clinical course might have an impact on the aggressiveness of treatment. This study presents the clinical and pathological features, along with treatment outcomes, of breast cancer patients with BOM in relation to the timing and type of bone metastasis.
Patients and Methods: Patients with breast cancer and BOM were retrospectively reviewed. De novo BOM was defined as bone metastasis diagnosed at presentation or within the first 4 months of follow-up. Treatment outcomes of patients with de novo, compared to those with subsequent BOM, are presented.
Results: 242 patients, median age (range) at diagnosis was 52 (27– 80) years were enrolled. The majority of the patients (77.3%) had de novo BOM with multiple sites of bone involvement (82.6%). At a median follow-up of 37.7 months, the median overall survival (OS) for patients with de novo BOM disease was significantly shorter than those who developed so subsequently; 40.8 months (95% CI, 51.1– 184.1) compared to 80.9 months (95% CI, 36.4– 47.9), p < 0.001. Tumor grade, hormone receptor status and type of bone lesions (lytic versus sclerotic) had a significant impact on survival outcomes.
Conclusion: Breast cancer with de novo BOM is a distinct clinical entity with unfavorable prognosis and is associated with shorter survival. Several risk factors for poor outcomes were identified and might inform treatment plans.
Keywords: breast cancer, bone metastasis, bone-only metastasis, de novo metastasis
Introduction
With an estimated 2.3 million new cases diagnosed in 2020, breast cancer continues to be the most commonly diagnosed cancer worldwide.1,2 Over the past decade, breakthroughs in the treatment of breast cancer with the implementation of novel and more effective therapeutic agents with better toxicity profile have improved the outlook of women diagnosed with the disease.
Breast cancer is not a single disease, and its heterogeneity can be a challenge. Understanding the interplay between the molecular and histopathological characteristics of the tumor, and their relationship with genetic and non-genetic risk factors, has had profound implications on treatment advances, which correlate with improved outcomes.3 Despite these advances, metastatic breast cancer remains a major leading cause of death. Though 5% or less of patients present with advanced-stage disease at diagnosis, such percentage may reach 20–30% in developing countries,4,5 and another third of patients with early-stage disease will develop distant metastasis during the course of their illness.6
Bone is a very common site of metastasis in patients with breast cancer, noted in 60%–80% of the patients and is the first site of metastasis in 25%–40%.7,8 Bone metastasis from solid tumors, including breast cancer, may lead to several skeletal-related events that may include bone pain, hypercalcemia, vertebral and non-vertebral pathological fractures, in addition to spinal cord compression that may mandate surgical intervention or radiation therapy.9,10
Compared to breast cancer with visceral metastasis, patients with bone-only metastasis (BOM) have better overall survival (OS).11,12 Bone pain commonly complicates bone metastasis, may limit patients’ mobility and impair their quality of life (QOL) leading to an increased demand for pain medications, including non-steroidal anti-inflammatory drugs (NSAIDs) and narcotics with their usual complications.13,14 Additionally, patients with BOM are more exposed to radiation therapy and surgical interventions,15 like internal fixations of pathological fractures, hip replacement and vertebroplasty.16–18 Bone-modifying agents, including bisphosphonates and denosumab, commonly utilized in patients with metastatic breast cancer to the bone, are associated with various short- and long-term adverse events.19
A limited number of studies identified the prognostic factors that may predict survival of breast cancer patients with BOM and their impact on treatment strategies and outcomes. In this study, we describe the clinical and tumor characteristics of patients with BOM whether diagnosed at presentation (de novo BOM) or subsequently following treatment for early-stage disease (subsequent BOM) and explore factors that may predict treatment outcomes.
Methods
Patient Selection
Retrospectively, we reviewed all consecutive adult breast cancer patients diagnosed and treated at our institution between July 2006 and December 2018. All patients with BOM breast cancer were included and classified according to the time of diagnosis with bone metastasis. Patients were labeled as having de novo BOM disease if they had bone metastasis at or within 4 months, of their initial diagnosis of breast cancer. Patients who progressed with BOM following the curative management of their primary breast tumor or after a minimum of 4 months of treatment with neoadjuvant and/or adjuvant systemic therapy are labelled as “subsequent BOM”.20 Bone metastasis was diagnosed on the basis of imaging studies using bone scan, computed tomography (CT) scan, positron emission tomography (PET) scan, or magnetic resonance imaging (MRI).
Furthermore, clinical characteristics including age at diagnosis, tumor grade, subtype, hormone receptor (HR), human epidermal growth factor receptor-2 (HER2) status, sites of BOM, number of sites of bone metastasis; single versus multiple, were analyzed. Treatment modalities including endocrine therapy and chemotherapy were obtained from medical records. The need for bone-modifying agents like bisphosphonates, radiotherapy, and/or vertebroplasty was also described.
Estrogen (ER) and progesterone (PR) receptors were defined as per the College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO). Both were considered positive if staining of tumor cell nuclei was 1% or more. HER2 was tested using immune histochemical staining (IHC) and scored zero to +3; tumors were considered negative if IHC scores were zero or +1 and positive if +3. Fluorescence in situ hybridization (FISH) was performed for tumors with +2 scores (considered equivocal).
The study was conducted in accordance with the international and local regulations and guidelines on human research including the 1964 Helsinki declaration and its later amendments. The study (protocol number:19-KHCC-72) was approved by the Institutional Review Board (IRB) at King Hussein Cancer Center. Given the retrospective nature of data collection and lack of patients’ identifier, informed consent was waived by the IRB.
Statistical Analysis
A descriptive analysis of patients’ information was done. Categorical data, such as age group, grade, and other factors, were presented as counts and percentages. In general, continuous variables were analyzed using t-test or a non-parametric test depending on the assumptions required for each test. The Kaplan–Meier method was used to estimate OS curves and progression-free survival. A Log rank test was used to compare patients’ survival times between different factor groups. The OS time was calculated from the date of breast cancer diagnosis to death from any cause; patients who were alive at the last follow-up were censored at that time. The progression-free survival (PFS) time was calculated from the date of diagnosis till the first evidence of disease progression in bone, metastasis to any other site or death due to any cause. Survival was expressed as a median with 5-year survival rate. A significance criterion of p <0.05 was used in the analysis. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Patients Characteristics
A total of 242 patients, 2 males, were included in the final analysis. The median age (range) at the time of breast cancer diagnosis was 52 (27–80) years. The majority (n = 187, 77.3%) of identified patients presented with de novo bone-only metastatic breast cancer (mBC), while 55 (22.7%) patients were initially diagnosed with early-stage disease, then progressed with bone-only metastasis. Table 1 summarizes patients’ characteristics.
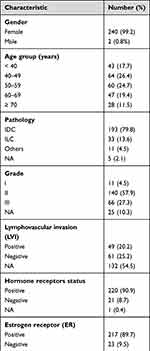 |
Table 1 Clinical Characteristics (n = 242) |
Tumor Characteristics
Invasive ductal carcinoma (IDC) was the most common pathology (n = 193, 79.8%). High-grade (grade III) tumors were identified in 66 (27.3%) patients and 44 (18.2%) had HER2-positive disease. Majority (n = 220, 90.9%) of patients had hormone receptor (HR)-positive disease, while 13 (5.4%) were triple-negative (hormone-receptor negative and HER2-negative), Table 1.
Bone Involvement
Solitary bone metastasis was reported in 42 (17.4%) patients, while 200 (82.6%) patients had multiple sites of bone involvement. Vertebral column metastases was reported in 130 (53.7%) patients. Bone metastasis was lytic in 90 (37.2%) patients, 49 (20.2%) had sclerotic disease and 20 (8.3%) patients had mixed (lytic and sclerotic) bone metastasis, Table 2.
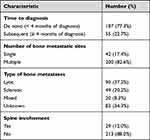 |
Table 2 Features of Bone Metastasis |
Treatment
Endocrine therapy (ET) was utilized in the first-line setting in 211 (87.2%) patients. Among those, 128 (60.7%) received aromatase inhibitors (AI), while 83 (39.3%) patients received tamoxifen, with or without gonadotropin-releasing hormone (GnRH) agonists. Only 57 (23.6%) patients received chemotherapy through their course of management, mostly because of disease progression or negative hormone-receptor status. Bisphosphonate, mainly intravenous zoledronic acid, was utilized in 209 (86.4%) of patients. Palliative radiation therapy to at least one site of bone metastasis was given to 101 (41.0%), and 29 (12.0%) underwent vertebroplasty.
Treatment Outcomes
At a median follow-up of 37.7 months, the 5-year OS for the whole group was 41.8%. Survival of patients with subsequent BOM was significantly better than those with de novo BOM (60.5% vs 35.5%, respectively, log-rank p < 0.0001), Figure 1A. Similarly, the 5-year PFS for the whole group was 22.8% (13.4% for de novo and 49.0% for the subsequent BOM group), with a median time to progression of 19.4 (95% CI, 16.07–20.62) months for those diagnosed with de novo BOM at presentation and 50.7 (95% CI, 32.98–126.7) months for patients with subsequent BOM (Log-rank p = 0.0001), Figure 1B. At time of last follow-up, 12 (4.9%) of 242 patients had died and majority (n = 185, 76.4%) had disease progression in the bone (n = 48, 19.8%) or visceral organs (n = 137, 56.6%), while another 45 (18.6%) continued to have a stable disease.
 |
Figure 1 Overall survival (A) and progression-free survival (B) for both groups; de novo bone-only metastasis (BOM) and subsequent metastasis. |
Table 3 summarizes the prognostic factors that affected survival for BOM. Histologic grade impacted survival significantly; women with poorly differentiated tumors (grade-III) had 5-year OS of 26.6%, compared to 64.9% and 43.6% for grades I and II, respectively (log-rank p = 0.01), Figure 2. Additionally, 5-year PFS was 9.6% for patients with grade-III disease, compared to 27.3% and 23.4% for those with grade-I and II, respectively (log-rank p = 0.005), Figure 2B.
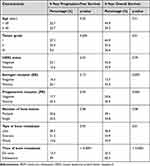 |
Table 3 Prognostic Factors Affecting Survival of Patients with BOM |
 |
Figure 2 Overall survival (A) and progression-free survival (B) according to tumor grade. |
The nature of bone metastasis also affected treatment outcomes, with a 5-year overall survival of 46.0%, 54.9%, and 43.0% for patients with lytic, sclerotic, or mixed bone metastasis, respectively (log-rank p = 0.01), Figure 3A. However, no difference was observed in PFS (log-rank p = 0.934), Figure 3B. We also studied the impact of tumor subtype, based on hormonal receptor (HR) and HER2 status, on treatment outcomes. Longer overall survival was observed in patients with HR-positive/HER2-negative subtype (5-year OS of 45.9%) compared to 34.2% for those with HR-positive/HER2-positive subtype and 11.5% for those with triple-negative disease (log-rank p = 0.052), Figure 4A. However, no significant differences were observed in PFS among the different groups, Figure 4B. In multivariate analysis, de novo bone metastasis versus subsequent metastasis (hazard ratio = 2.81, 95% CI, 1.65–4.79, p = 0.0001), and to a lesser extent lytic versus sclerotic bone lesions (hazard ratio = 1.18, 95% CI, 0.46–1.55) were associated with poor overall survival, while de novo bone metastasis, HER2 positivity and high tumor grade were all associated with poor PFS (Table 4).
 |
Table 4 Multivariate Analysis and Impact of Prognostic Factors on PFS |
 |
Figure 3 Survival according to type of bone metastasis: (A) overall survival, (B) progression-free survival. |
 |
Figure 4 Survival according to hormonal receptor (HR) and HER2 status: (A) overall survival, (B) progression-free survival. |
Discussion
Bone is the most common site of metastasis in breast cancer; some postmortem studies had shown that up to 75% of patients may have bone metastasis during the course of their disease.21–23 Several studies had used preclinical models for a better understanding of tumor biology and to explain its natural course.24–26 Bone metastasis, alone or when associated with visceral involvement, may result in significant bone pain and may impair patients’ quality of life. Bone-only metastasis is usually treated less aggressively, especially so in patients with HR-positive and HER2-negative subtypes. Endocrine treatment including tamoxifen, aromatase inhibitors (AI) or fulvestrant, mostly with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, are the first choice to treat patients with HR-positive and HER2-negative disease.27,28 Options for subsequent therapies include another endocrine therapy (with or without CDK4/6 inhibitors, if not used in the first-line setting), or exemestane with everolimus.29,30 Patients with PIK3CA (phosphoinositide 3-kinase) mutation can be offered alpelisib, a PIK3CA-inhibitor, along with fulvestrant (if not previously used).31,32 Chemotherapeutic agents are options for HR-negative or resistant tumors, as well as for patients in whom a rapid response is desired.33 Patients with HER2-positive BOM disease are less-commonly encountered. Agents targeting and blocking HER2 receptor are important in the first- and subsequent-line settings, mostly used in combination with chemotherapy or endocrine therapy (in hormone receptor-positive disease).34–38
Bone-only metastasis is a unique entity that describes patients who present with bone involvement but with no other organ metastasis. BOM is not extensively evaluated by large prospective trials as a distinct entity due to the fact that bone metastasis, until the most recent modification, is considered a non-measurable disease, based on RECIST criteria.39
Compared to other breast cancer patients with visceral metastasis, patients with BOM usually have better prognosis.40–43 A recent French study identified a total of 20,095 women with metastatic breast cancer; 22.4% (n = 5041) of them had BOM. Regardless of the HR or HER2 status, patients with BOM had a better adjusted OS compared with non-BOM metastatic breast cancer; 52.1 months (95% CI, 50.3–54.1) versus 34.7 months (95% CI, 34.0–35.6), respectively. Progression-free survival (PFS) was also better; 13.1 months (95% CI, 12.6–13.8) versus 8.5 months (95% CI, 8.3–8.7), respectively.44
Multiple studies have shown that the survival of patients with de novo metastatic breast cancer is significantly better compared to that of patients who relapse following treatment for early-stage disease.45–48 Very few, however, had addressed such difference in those with BOM. Our study clearly illustrates that patients who present with de novo metastatic BOM have significantly worse outcome compared to those who develop so following a presentation with early-stage disease (5-year OS 36% versus 61%, p < 0.0001). Similar trends were also observed for PFS (14% versus 49%, p < 0.0001). Such data may carry a significant impact on the aggressiveness of anti-cancer therapy and may help physicians address patients with their anticipated disease course. Similar conclusions were reached by a study from MD Anderson Cancer Center (MDACC) in which a total of 1048 evaluable patients with BOM were identified, the majority of them were HR-positive/HER2-negative (78%). Median OS from the time of breast cancer diagnosis was 8.7 years (95% CI, 8.0–9.7) and varied significantly by tumor subtype. Survival was significantly shorter (p < 0.0001) for the 442 patients with de novo BOM disease (defined similar to the one used in our study).49 However, a smaller study from Korea reached different conclusions. In this study, medical records of 110 breast cancer patients were retrospectively reviewed to identify patients with BOM. Contrary to our study cohort, only a minority of the patients presented with de novo BOM (n = 19, 17.3%), while the majority (n = 91, 82.7%) had BOM following a diagnosis and treatment of early-stage disease (subsequent BOM group). At a median follow-up of 55 months, the estimated 10-year OS was 35% for the whole group. Similarly, contrary to our findings, the 5-year OS for patients with de novo BOM was better than that for patients with subsequent bone metastasis (60.2% vs 44.1%); nonetheless, the difference was not statistically significant (p = 0.136).42
Generally, regardless of the metastatic sites, positive hormonal receptor status in metastatic breast cancer confers a better survival compared to those with HR-negative, HER2-positive or those with triple-negative disease.50–52 This also holds true for those with BOM as illustrated in our study (Figure 4) and in another MDACC study, as well.11 In the Korean study discussed above, a multivariate analysis showed that solitary bone metastasis (hazard ratio = 0.32; 95% CI, 0.14–0.72), therapy with bisphosphonate [hazard ratio = 0.18; 95% CI, 0.07–0.43], and ER-positivity (hazard ratio = 0.51; 95% CI, 0.28–0.94), were significantly associated with improved overall survival.42
Our findings, in addition to previously published studies, can be used to develop a scoring system, based on clinical and pathological features, that may potentially help physicians tailor treatment plans based on the aggressiveness of the disease, as judged by such scoring system.
Our study is not without limitations. The retrospective nature of the study and the relatively small population of patients included from a single center may limit the generalization of our conclusions. However, we believe our study presents a real-world data for a cohort of patients treated on a unified clinical practice guidelines at a tertiary care cancer center.
Conclusions
Bone-only metastatic breast cancer is a distinct clinical entity and is usually associated with favorable prognosis. On the other hand, de novo BOM compared to subsequent metastasis following a diagnosis of early-stage disease, is less favorable and is usually associated with shorter survival. Triple-negative, HER2-positive, high-grade tumors and lytic or multiple bone lesions may reflect negatively on treatment outcome of bone-only metastatic breast cancer.
Data Sharing Statement
All data related to this research can be shared upon request to the corresponding author.
Acknowledgment
The abstract of this paper was presented at the 43rd Annual San Antonio Breast Cancer Symposium (SABCS) as a poster presentation with interim findings. The abstract was published as “Poster Abstracts” in: Cancer Res (2021) 81 (4_Supplement): PS14-22, available at: https://aacrjournals.org/cancerres/article/81/4_Supplement/PS14-22/648425/Abstract-PS14-22-Clinical-characteristics.
The authors would like to thank Ms. Ryan Bater and Mrs. Alice Hadaddin for their help in preparing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, took part in drafting, revising and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work
References
1. Lei S, Zheng R, Zhang S, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021;41(11):1183–1194.
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249.
3. Feng Y, Spezia M, Huang S, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106.
4. Abdel-Razeq H, Mansour A, Jaddan D. Breast Cancer Care in Jordan. JCO Glob Oncol. 2020;6:260–268.
5. Abdel-Razeq H, Tamimi F, Abdel-Razeq N, et al. Late presentation and suboptimal treatment of breast cancer among Syrian refugees: a retrospective study. J Int Med Res. 2021;49(5):3000605211018448.
6. Liede A, Jerzak KJ, Hernandez RK, Wade SW, Sun P, Narod SA. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res Treat. 2016;156(3):587–595.
7. Manders K, van de Poll-Franse LV, Creemers GJ, et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6(1):1–8.
8. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–66.
9. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl 8):1588–1594.
10. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–6249s.
11. Parkes A, Warneke CL, Clifton K, et al. Prognostic factors in patients with metastatic breast cancer with bone-only metastases. Oncologist. 2018;23(11):1282–1288.
12. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
13. Cramarossa G, Chow E, Zhang L, et al. Predictive factors for overall quality of life in patients with advanced cancer. Support Care Cancer. 2013;21:1709–1716.
14. Caissie A, Culleton S, Nguyen J, et al. EORTC QLQ-C15-PAL quality of life scores in patients with advanced cancer referred for palliative radiotherapy. Support Care Cancer. 2012;20(4):841–848.
15. Rossi L, Longhitano C, Kola F, Del Grande M. State of art and advances on the treatment of bone metastases from breast cancer: a concise review. Chin Clin Oncol. 2020;9(2):18.
16. Schurman DJ, Amstutz HC. Orthopedic management of patients with metastatic carcinoma of the breast. Surg Gynecol Obstet. 1973;137(5):831–836.
17. Pretell J, Rodriguez J, Blanco D, Zafra A, Resines C. Treatment of pathological humeral shaft fractures with intramedullary nailing. A retrospective study. Int Orthop. 2010;34(4):559–563.
18. Kim WS, Kim KH. Percutaneous osteoplasty for painful bony lesions: a technical survey. Korean J Pain. 2021;34(4):375–393.
19. Brooks GA, Landrum MB, Kapadia NS, et al. Impact of the Oncology Care Model on Use of Supportive Care Medications During Cancer Treatment. J Clin Oncol. 2022;1:JCO2102342.
20. Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36(4):476–482.
21. Tahara RK, Brewer TM, Theriault RL, Ueno NT. Bone Metastasis of Breast Cancer. Adv Exp Med Biol. 2019;1152:105–129.
22. Cummings MC, Simpson PT, Reid LE, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23–31.
23. Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–180.
24. Mercatali L, Spadazzi C, Miserocchi G, et al. The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts. Int J Mol Sci. 2016;17(11):1827.
25. Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549.
26. Mercatali L, La Manna F, Miserocchi G, et al. Tumor-Stroma Crosstalk in Bone Tissue: the Osteoclastogenic Potential of a Breast Cancer Cell Line in a Co-Culture System and the Role of EGFR Inhibition. Int J Mol Sci. 2017;18(8):1655.
27. Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369.
28. Campos SM, Guastalla JP, Subar M, Abreu P, Winer EP, Cameron DA. A comparative study of exemestane versus anastrozole in patients with postmenopausal breast cancer with visceral metastases. Clin Breast Cancer. 2009;9(1):39–44.
29. Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, Phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–998.
30. André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32(2):208–217.
31. Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a Phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498.
32. Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;2014(6):847.
33. Sledge GW, Hu P, Falkson G, Tormey D, Abeloff M. Comparison of chemotherapy with chemohormonal therapy as first-line therapy for metastatic, hormone-sensitive breast cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2000;18(2):262–266.
34. Perez EA, Barrios C, Eiermann W, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: primary Results From the Phase III MARIANNE Study. J Clin Oncol. 2017;35(2):141–148.
35. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742.
36. Murthy RK, Loi S, Okines A, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382(7):597–609.
37. Rugo HS, Im SA, Cardoso F, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: a Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7(4):573–584.
38. Lee SJ, Park S, Ahn HK, et al. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat. 2011;43(2):89–95.
39. Litière S, Isaac G, De Vries EGE, et al. RECIST 1.1 for Response Evaluation Apply Not Only to Chemotherapy-Treated Patients But Also to Targeted Cancer Agents: a Pooled Database Analysis. J Clin Oncol. 2019;37(13):1102–1110.
40. Zhang L, Zhang J, Li Z, Wu Y, Tong Z. Comparison of the clinicopathological characteristics and prognosis between Chinese patients with breast cancer with bone-only and non-bone-only metastasis. Oncol Lett. 2020;20(4):92.
41. Curtit E, Bazan F, Chaigneau L, et al. Prolonged overall survival for patients with bone-only metastases at presentation of metastatic breast cancer. Ann Oncol. 2018;29(suppl):
42. Ahn SG, Lee HM, Cho SH, et al. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med J. 2013;54(5):1168–1177.
43. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77(2):336–340.
44. Bertho M, Fraisse J, Patsouris A, et al. Real-life prognosis of 5041 bone-only metastatic breast cancer patients in the multicenter national observational ESME program. Ther Adv Med Oncol. 2021;21:13.
45. McKenzie HS, Maishman T, Simmonds P, et al. Survival and disease characteristics of de novo versus recurrent metastatic breast cancer in a cohort of young patients. Br J Cancer. 2020;122(11):1618–1629.
46. Tripathy D, Brufsky A, Cobleigh M, et al. De Novo Versus Recurrent HER2-Positive Metastatic Breast Cancer: patient Characteristics, Treatment, and Survival from the SystHERs Registry. Oncologist. 2020;25(2):e214–e222.
47. Yamamura J, Kamigaki S, Fujita J, Osato H, Komoike Y. the difference in prognostic outcomes between de novo stage IV and recurrent metastatic patients with hormone receptor-positive, HER2-negative breast cancer. Vivo. 2018;32(2):353–358.
48. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139.
49. Parkes A, Clifton K, Al-Awadhi A, et al. Characterization of bone only metastasis patients with respect to tumor subtypes. NPJ Breast Cancer. 2018;4(2):1–7.
50. Emi Y, Kitamura K, Shikada Y, Kakeji Y, Takahashi I, Tsutsui S. Metastatic breast cancer with HER2/neu-positive cells tends to have a morbid prognosis. Surgery. 2002;131(Suppl 1):S217–S221.
51. Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control. 2010;17(3):173–176.
52. Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
