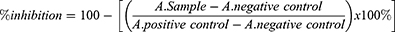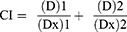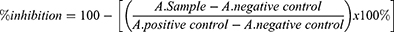Back to Journals » Journal of Experimental Pharmacology » Volume 14
Caffeic Acid Phenethyl Ester as a DHODH Inhibitor and Its Synergistic Anticancer Properties in Combination with 5-Fluorouracil in a Breast Cancer Cell Line
Authors Amalia E, Diantini A , Endang Prabandari E, Waluyo D, Subarnas A
Received 5 March 2022
Accepted for publication 11 July 2022
Published 23 July 2022 Volume 2022:14 Pages 243—253
DOI https://doi.org/10.2147/JEP.S365159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Paola Rogliani
Eri Amalia,1– 3 Ajeng Diantini,1 Erwahyuni Endang Prabandari,4 Danang Waluyo,4 Anas Subarnas1
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, Indonesia; 2Department of Pharmacology, Faculty of Science and Technology, Department of Pharmacy, Muhammadiyah University, Bandung, Indonesia; 3Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, Indonesia; 4Research Center for Vaccine and Drug, National Research and Innovation Agency, Bogor, Indonesia
Correspondence: Eri Amalia, Department of Pharmaceutics and Pharmaceutical Technology, Laboratory Building 1, Faculty of Pharmacy, Universitas Padjadjaran. Jl. Raya Bandung-Sumedang km.21. Jatinangor, West Java, 45363, Indonesia, Email [email protected]; [email protected]
Introduction: A combination of chemotherapy agents is the best choice in breast cancer treatment to increase the patient survival rate. 5-fluorouracil (5-FU) is one of the drugs applied in combination with other drugs to control and delay development of cancer cells. Nevertheless, the occurrence of multidrug resistance and dose-limiting cytotoxicity have limited the efficacy of 5-FU treatment. Therefore, the discovery of new anti-breast cancer drugs should be pursued.
Objective: To study potency of a promising naturally derived compound, caffeic acid phenethyl ester (CAPE), for breast cancer treatment in single and combination with 5-FU.
Methods: Cytotoxicity of CAPE, 5-FU, and 5-FU+CAPE was studied by in vitro MTT experiment in MCF-7 cell line, and RT-PCR analysis was used to evaluate the change in gene expression due to the treatment. Moreover, an enzymatic assay and molecular docking analysis were applied to evaluate the possible mechanism of substance-induced apoptosis.
Results: The study revealed that a single treatment of CAPE showed cytotoxicity with IC50 6.6 ± 1.0 μM and 6.5 ± 2.9 μM at 24 h and 48 h, respectively. Meanwhile, 5-FU showed cytostatic activity. The 5-FU + CAPE has a synergistic effect at 24 h treatment with a CI = 0.5 and an additive effect at 48 h treatment with CI = 1.0. CAPE was also found to enhances the mRNA expression of caspase-8 and BAX within 6 hours in combination with 5-FU compared to 5-FU treatment alone. Our study reveals a new mechanism of CAPE which is related to the inhibition of human dihydroorotate dehydrogenase (HsDHODH) with an IC50 of 120.7 ± 6.8 μM, by bound to the ubiquinone-binding site of the enzyme and could be responsible for inducing extrinsic and intrinsic apoptosis.
Conclusion: This study demonstrated the cytotoxicity of CAPE potential to induce apoptosis of breast cancer MCF-7 cell line single and cytotoxic–cytostatic combination with 5-FU. Therefore, further studies to develop CAPE and its derivatives will be required to discover new candidates for breast cancer agents.
Keywords: caffeic acid phenethyl ester, 5-fluorouracil, dihydroorotate dehydrogenase, DHODH, MCF-7, breast cancer
Introduction
Breast cancer causes high mortality in women, with an incidence of 2.3 million patients and mortality of 685,000 patients in 2020.1 The disease can be cured by immediate appropriate treatment in the initial stage; however, most patients are usually diagnosed at a later developmental stage. The complexity of breast cancer treatment leads to the use of combination drugs as the best approach to improve the therapeutic effectiveness of treatment, especially in patients with metastasis-stage breast cancer whereby several organ tissue functions are affected.2
5-Fluorouracil (5-FU) is an anti-metabolite of a pyrimidine analogue that inhibits thymidylate synthetase activity. This compound enters the cell via native uracil transport and is converted into three active metabolites: fluorouridine triphosphate (FUTP), fluorodeoxyuridine monophosphate (FdUMP), and fluorodeoxyuridine triphosphate (FdUTP); thus, it has the potential to arrest the cell cycle in the S phase and induce apoptosis.3,4 5-FU is used as first- and second-line breast cancer therapy in combination with other drugs, such as a combination of 5-FU, doxorubicin, and cyclophosphamide (FAC) or cyclophosphamide, methotrexate, and 5-FU (CMF).5 Nevertheless, 5-FU treatment is limited due to breast cancer resistance protein (BCRP)-mediated drug resistance and its toxic side effects.6 Therefore, the identification of new selective breast cancer drugs to be used as a single dose or in combination with other cytotoxic drugs is urgently required. An example of the successful finding of drug combination in cancer treatment was Teysuno™ (S-1), an oral fixed-dose combination containing tegafur, gimeracil, and oteracil at dose 1:0.4:1 molar ratio, respectively. Tegafur is a prodrug of 5-fluorouracil (5-FU); meanwhile, Gimeracil and Oteracil are modulators of 5-FU metabolism. The combination is designed to improve the 5-FU efficacy and safety ratio for the treatment of advanced gastric (stomach) cancer and several solid cancers including breast cancer.7,8
Our experiment focuses on elaborating on the potency of caffeic acid phenethyl ester (CAPE) to be used as a single treatment or combination with 5-FU for breast cancer treatment and predicting its mechanism of action. Caffeic acid phenethyl ester (CAPE) is a naturally derived bioactive substance of propolis mostly found in Brazil, India, America, and Romania. It was reported to have cytotoxic effects on several cancer cells, including in MCF-7 breast cancer cells.9–11 Several mechanisms of CAPE action to induce apoptosis have been reported, including inhibition of NFκB, disrupting mortalin-p53 complexes, thereby increasing p53 expression, and it has also been shown to inhibit MCF-7 cell migration in the laboratory.9–12 Research in the human promyelocytic leukaemia (HL-60) and human pancreatic cancer cell line (PANC-1 dan BxPC-3) revealed that CAPE could penetrate cells causing mitochondrial dysfunction and activating caspase-3 to induce apoptosis.12 Mitochondrial metabolism has been an important target in cancer drug discovery, due to its function as a central bioenergetic of cells including the cancer cells.13 The particular cancer drug targeting mitochondrial may cause mitochondrial dysfunction such as disruption of electron transport and ATP-synthesis, dysfunction of mitochondria to provide biomolecule, and lack of the number of mitochondria, which may lead to cancer cell death14,15
This study investigated the possible mechanism of CAPE to induce mitochondrial dysfunction, through its potency to inhibit enzyme dihydroorotate dehydrogenase (DHODH). DHODH is known as a potential target in cancer therapy. This enzyme is located in mitochondria and involves cellular bioenergetics and proliferation.3,16 Enzymatic analysis using purified human dihydroorotate dehydrogenase (HsDHODH) and molecular docking analysis was conducted to determine the interaction between HsDHODH and CAPE at the molecular level. HsDHODH is a flavoenzyme located in the inner membrane of mitochondria, which is involved in the pyrimidine biosynthesis pathway providing biomolecules required for several cellular activities including deoxyribonucleotide (DNA) and ribonucleotide (RNA) synthesis. This enzyme is also involved in the respiratory electron transport chain in mitochondria,3,16 thus, inhibition of this enzyme might potentially affect the viability of the cancer cells.
There are several pathways for apoptosis after the attachment of a compound to its binding site. Our study will evaluate the possible mechanism by evaluating the change in mRNA expression of several proteins involved in extrinsic apoptosis (caspase-8) and BAX-mediated intrinsic apoptosis using real-time PCR after 6 h of treatment.
Materials and Methods
Materials
CAPE with ≥98% purity was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). 5-Fluorouracil ≥99% (HPLC) powder was purchased from Sigma-Aldrich (Saint Louis, USA). Purified HsDHODH was provided by the Research Center for Vaccine and Drug, National Research and Innovation Agency (Bogor, Indonesia).
MCF-7 Cell Cultures
MCF-7 cells (HTB-22) were purchased from ATCC. MCF-7 cells were cultured in RPMI and supplemented with 10% FBS and 1% penicillin–streptomycin solution (all Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured in T25 tissue culture flasks at 37°C in a 5% CO2 incubator and were subcultured every 2–3 days before they reached confluency to keep the cultures healthy and actively growing. The experiments were performed with cells at 70–80% confluence.
In vitro Cytotoxic Activity of 5-FU, CAPE and the Combination of 5-FU+CAPE on the MCF-7 Cell Line
The cytotoxic effects of CAPE and 5-FU were evaluated using the MTT ((4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. MCF-7 cells at a density of 5 × 103 cells/well were seeded in treated 96-well plates and maintained for 24 h at 37℃ in 5% CO2 to allow the cells to adhere to the bottom of the plates. The cultures were then treated with series of 5-FU (7.8, 15.6, 31.3, 62.5, 125, 250, 500, and 1000 µM), and CAPE (0.8, 1.6, 3.1, 6.3, 12.5, 25, 50, and 100 µM) concentrations. A combination of the 5-FU and CAPE (5-FU+CAPE) was prepared in a ratio of 10:1 (7.8 µM+0.8 µM, 15.6 µM+1.6 µM, 31.3 µM+3.1 µM, 62.5 µM+6.3 µM, 125 µM+12,5 µM, 250 µM+25 µM, 500 µM+50 µM, 1000 µM+100 µM). The cells were treated for 24 h and 48 h at 37℃ in 5% CO2 and each concentration was tested in triplicate. Untreated cells were used as a positive control and wells without cells were the negative control. After 24 h and 48 h incubation, the medium was aspirated and replaced with 100 µL of freshly prepared medium containing 0.5 mg/mL of MTT and incubated for 3–4 h. The MTT solution was removed from the well, and 100 µL of DMSO was added to solubilise the formazan crystals. Finally, the absorbance was measured at a wavelength of 450 nm using a Multiscan® Ex Thermo labystem multiplate reader (Vantaa, Finland).
The IC50 value was analyzed in logarithmic equation, and % inhibition of cell proliferation was calculated by the equation:
Statistical analysis was analyzed by two-way ANOVA in SPSS v23. (IBM Corp) software.
The combination index was calculated by the formula:
Where:
(D)1 and (D)2 were concentrations of drugs in combination treatment to exert 50% effect; (Dx)1 and (Dx)2 were concentrations of single drug treatment to exert 50% effect; CI < 1 indicates synergy; CI = 1 indicates additivity; CI > 1 indicates antagonism.
Analysis of mRNA Expression by Real-Time PCR
MCF-7 Cell Culture
The MCF-7 cell lines were seeded in a 6-well plate with a density of 3.5 × 105 cells/well and were maintained for 24 h at 37℃ with 5% CO2. The cells were treated with CAPE (6.5 µM), 5-FU (3.1 µM), 5-FU+CAPE (2.6 µM+1.1 µM) to get the IC50 at 48 h treatment and incubated for 6 h at 37°C with 5% CO2. After 6 h, the morphological change of cells was observed by a phase-contrast inverted microscope. The experiment was continued by aspirating the media from each well, and the cells were with 500 µL PBS and adding 500 µL of 0.05% trypsin incubated for 5 minutes at 37°C. The cells were collected in a centrifuge tube and added with 500 µL of RPMI 1640, supplemented with 10% FBS and 1% Penicillin-streptomycin, and centrifuged for 3 minutes at 1600 rpm at room temperature. The media were discarded, added with 500 µL PBS, and stored at −80℃ until used.
RNA Extraction& RT-qPCR Analysis
RNA was extracted from treated cells using the Quick-RNA Miniprep Plus Kit (Zymo Research, CA, USA), and real-time PCR analysis was conducted using Rotor-Gene Q (Qiagen, Hilden, Germany) and a SensiFASTTM SYBR R Hi-ROX kit (Bio, London, UK). The PCR cycling conditions were an initial denaturation at 45°C for 5 minutes and 95°C for 2 minutes, followed by 40 cycles at 95°C for 1 second and 60°C for 40 seconds. The data were normalised to GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) as a reference gene and relative mRNA expression was calculated using the 2−(ΔΔCt). The difference in gene expression between single and combination treatment was analysed by one-way ANOVA with the Tukey post hoc in SPSS v23.0 (IBM Corp.) software.
The PCR Primers sequence of BAXand GAPDH were referred to Yu et al, 2013; meanwhile, for caspase-8 was referred to Farghadani et al, 2019.17,18 During the experiment, the GAPDH mRNA expression was determined as a reference gene. The PCR Primers sequence was purchased from Macrogen, Singapore, as follows:
BAX: Forward Primer GATGCGTCCACCAAGAAGC
Reverse Primer AAGTCCAATGTCCAGCCCAT
Caspase-8: Forward Primer GTTGTGTGGGGTAATGACAATCT
Reverse Primer TCAAAGGTCGTGGTCAAAGCC
GAPDH: Forward Primer TGAACGGGAAGCTCACTGG
Reverse Primer GCTTCACCACCTTCTTGAT GTC
HsDHODH Inhibition by CAPE and 5-FU
The inhibitory ability of CAPE and 5-FU on HsDHODH was assessed by an enzymatic biochemical method in vitro. A dilution series of CAPE in water (50 mM, 10 mM, 1 mM, 100 µM, 10 µM, 1 µM, 100 nM, 10 nM, 1 nM, and 0.1 nM) and 2 µL was added to the plate well to give a final concentration of 500 µM, 100 µM, 10 µM, 1 µM, 100 nM, 10 nM, 1 nM, 0.1 nM, 0.01, and 0.001 nM CAPE. A dilution series of 5-FU in water was prepared (0.4 M, 0.04 M, 3.8 mM, 0.4 mM, 0.04 mm, and 3.8 µM) and 2 µL was added to the plate well to give a final concentration of 5-FU in the system of 0.04 M, 3.8 mM, 0.4 mM, 0.04 mM, 3.8 µM and 0.004 µM. Two µL of water was used as a positive and negative control. Then, 190 µL of assay mixture [100 mM HEPES pH 8, 10% (v/v) glycerol, 50 mM NaCl, 0.05% (w/v) Triton X-100, 120 μM DCIP, and 18 μM decylubiquinone] was added to each well and homogenised by shaking at 500–700 rpm for 30 second. The absorbance was measured in the kinetic mode on a Multiskan Go multiplate reader (Thermo) at 600 nm, 25℃ for 1 minute to record the background. Then, 8 µL of 5 mM L-DHO was added to each well except for the positive control and homogenised by shaking at 500–700 rpm for 30 seconds. The absorbance was read at 600 nm and the inhibition activity was calculated as follows:
where A. sample is the decrease in the absorbance of the sample, A. positive control is the average decrease in absorbance of the positive control well and A. negative control is the average decrease in absorbance of the negative control well. The IC50 values were analysed in GraphPad Prism 7.05 software.
Molecular Docking Complex of CAPE to HsDHODH
The mechanism of CAPE to inhibit HsDHODH activity was evaluated by molecular docking using AutoDock 4.2 software. The crystal structure of human DHODH [PDB ID: 5ZF8] refers to PDB (https://www.rcsb.org/structure/5ZF8). This structure has 366 amino acid residues and a molecular weight of 44.17 kDa. The macromolecule 5ZF8 was prepared by the addition of hydrogen atoms. The three-dimensional structure of CAPE was obtained from the PubChem database (CID: 5281787). The ligand has eight active torsions and the centre of the coordinate grid map was arranged at x = −6.598, y = 38.996 and z=−5.639 with a 100x100x100 point grid map. The Lamarckian genetic algorithm was selected and 25 docking runs were performed, with 150 population size and medium 2.5 × 106 evaluation energy. Molecular docking was continued using the lowest conformation binding energy.
Statistical Analysis
The cytotoxicity experiments were performed in triplicate, with three independent experiments. SPSS software version 23.0 was used for statistical analysis and a P-value <0.05 was considered statistically significant. A two-way ANOVA was performed for the in vitro cytotoxic assay of the MCF-7 cell line to analyse the effect of all doses for 24 h and 48 h, and one-way ANOVA was used to compare the significant difference in IC50 values between the different treatment durations. One-way ANOVA with Tukey post hoc was performed for mRNA expression analysis.
Results
In vitro Cytotoxic Activity of 5-FU, CAPE, and a Combination of 5-FU + CAPE on MCF-7 Cell Line
The in vitro MTT assay of the MCF-7 cell line treated with a single dose of 5-FU revealed that 5-FU exerted cytostatic activity, whereby the viability of MCF-7 cells was >90% at 1000 μM during 24 h treatment. However, the cytotoxicity significantly increased after 48 h treatment with an IC50 of 3.1 ± 0.9 µM, with a significant difference (p < 0.001) between 24 h and 48 h for all doses (Figure 1A). CAPE had an IC50 of 6.6 ± 1.0 µM and 6.5 ± 2.9 µM at 24 h and 48 h, respectively (p = 0.948). Cytotoxic activity was immediately observed after 24 h treatment, but there was no further increase after 48 h treatment (Figure 1B).
Regarding the combination of 5-FU+CAPE, the cytotoxic activity of 5-FU in the 24 h treatment (IC50 45.1 ± 4.8 µM) increased significantly compared to the single dose (>1000 µM). Similarly, the cytotoxicity of CAPE in combination (IC50 3.5 ± 0.4 µM) was significantly different (p = 0.007) from the single-dose (IC50 6.6 ± 1.0 µM). The combination of 5-FU+CAPE showed a synergistic effect with a combination index (CI) of 0.5 (Figure 2A). The combination treatment for 48 h slightly increased the cytotoxic effect of 5-FU from IC50 3.1 ± 0.9 µM for a single dose to IC50 2.6 ± 0.5 µM in combination (p = 0.423), whereas the IC50 value of CAPE significantly decreased from 6.5 ± 2.9 µM for a single dose to 1.1 ± 0.1 µM in combination (p = 0.033). The combination of 5-FU+CAPE for 48 h showed an additive effect with a CI = 1.0 (Figure 2B).
mRNA Expression Analysis
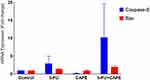
Six hours of 5-FU treatment increased the mRNA expression of the pro-apoptotic caspase-8 and BAX by 3.0 ± 2.9 fold and 1.4 ± 0.2 fold of the untreated control, respectively, indicatinsic and intrinsic apoptotic mechanisms occur. Whereas 6 h of CAPE treatment with CAPE did not increase the mRNA expression o caspase-8and BAX, indicated by 0.2 ± 0.1 and 0.9 ± 0.4 fold of the untreated control, respectively. Interestingly, the combination of 5-FU+CAPE increased the expression of caspase-8 and BAX by 10.3 ± 13.1and 2.01 ± 0.56 fold of the untreated control, respectively, this value was higher than the single treatment (Figure 3). This result suggests that CAPE may enhance the cytotoxicity of 5-FU via extrinsic apoptosis and intrinsic apoptosis. The mRNA expression was normalized to GAPDH and was analyzed by 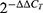 Method.19
Method.19
HsDHODH Inhibition by CAPE and 5-FU
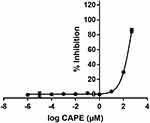
The biochemical enzymatic assay revealed that 5-FU did not inhibit the activity of HsDHODH at a concentration of 0.004 µM to 0.04 M, whereas CAPE inhibited the activity of HsDHODH with an IC50 of 120.74 ± 6.78 µM (Figure 4).
Molecular Docking Complex of CAPE to HsDHODH
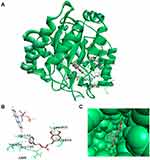
Docking analysis revealed that CAPE is bound to the ubiquinone-binding site of HsDHODH adjacent to the flavin mononucleotide (FMN) with orotate as the substrate (Figure 5A). CAPE has eight torsions and forms a hydrogen bond (green colour) between the O atom and Arg 136 (Figure 5B). The Pi-alkyl bond interaction between aromatic 1 with Val 143 and FMN and the interaction between the second aromatic with Leu 359 are shown in pink. The Pi-sigma interaction between aromatic 1 with Val 134 and the second aromatic with Ala 59 are shown in purple. As shown in Figure 5C, the structure of CAPE allows it to form a complex with HsDHODH with ΔG –11.13 kcal/mol and inhibition constant Ki of 6.95 nM, indicating that CAPE had a good affinity with the enzyme.
Discussion
CAPE is a bioactive substance of propolis found in Brazil, Romania, and India, with several activities, such as anti-inflammation, antimicrobial, as well as anticancer properties.9,10,20 Nevertheless, not all propolis contains CAPE, as we found that stingless bee propolis from South Sulawesi-Indonesia did not contain CAPE as a major bioactive substance.21 This study investigated the potential of CAPE as an anticancer agent, by its activity in MCF-7 cell line due to its character is known to be similar to Luminal A type of breast cancer, which occurs in 80% of the breast cancer patient. The experiment showed that CAPE exerted cytotoxic activity against the MCF-7 breast cancer cell line. Moreover, CAPE had a synergistic effect with 5-FU with a CI of 0.5 in 24 h treatment. CAPE is known as a potent cytotoxic agent against the proliferation of MCF-7 cell lines with an IC50 6.6 µM at 24 h. However, the activity did not increase after 48 h treatment, which was predicted due to the instability of CAPE in the culture medium. A previous study by Wadhwa et al reported that the hydrolysis of CAPE reduces the CAPE concentration, simultaneously increasing the formation of caffeic acid as well as other unknown substances.11 In the present study, the hydrolysis of CAPE likely occurred during the prolonged incubation for 48 h in the culture media, thereby reducing the cytotoxic activity of CAPE against the MCF-7 cell line. However, this phenomenon will probably not occur in human plasma, since the stability of CAPE is affected by the incubation medium and incubation in human plasma for 6 h does not affect the stability of CAPE.22 Further studies to optimise the CAPE structure are necessary to maintain stability and increase the selectivity of CAPE derivatives on breast cancer cell lines.
The combination of 5-FU+CAPE for 24 h treatment had a synergistic effect with a CI = 0.5, whereas the 48 h treatment had an additive effect with a CI = 1.0. There was no significant difference between the single-dose 5-FU treatment and the combination at 48 h incubation, resulting in a higher CI value which indicated the additive effect. Interestingly, 5-FU showed cytostatic activity at a concentration <1000 µM and CAPE showed cytotoxic activity at concentration <100 µM. Apoptosis of the MCF-7 cells after 24 h and 48 h treatment was due to the activity of CAPE and 5-FU, respectively. This study suggests that the combination of 5-FU+CAPE as cytostatic and cytotoxic compounds will be efficient to reduce the concentration of 5-FU for breast cancer treatment, thus diminishing undesirable side effects. Further in vitro and in vivo experiments with the use of additional cell lines are required to establish clinically achievable concentration of CAPE in a single dose and combination with 5-FU for the treatment of breast cancer in the future.
We observed that some of the MCF-7 cells begin to shrink at 6 h after CAPE treatment, which indicates the beginning of apoptosis (data not reported). Nevertheless, it does not describe the significant change in caspase-8 and BAX mRNA expression. A study conducted by Watabe et al reported that Bax activation occurred after 12 h treatment analyzed by Western blot analysis.10 Therefore, a study in prolonging the treatment period and evaluation of cleaved protein by Western blot analysis should be performed to evaluate the mechanism of action of CAPE. Interestingly, the combination 5-FU+CAPE increased the expression of caspase-8 and BAX compared to the single 5-FU treatment, indicating that CAPE enhances the cytotoxic activity of 5-FU via the activation of extrinsic and intrinsic apoptotic pathway.
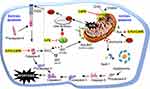
To date, several mechanisms of the action of CAPE have been reported related to mitochondrial dysfunction. Nevertheless, to our knowledge, the potential target of CAPE remains unknown. The present study showed that CAPE but not 5-FU could inhibit HsDHODH, suggesting that apoptosis is induced by CAPE entering the cells towards the mitochondrial membrane and binding to the ubiquinone-binding site of the HsDHODH enzyme in the inner membrane mitochondrial. The CAPE-HsDHODH complex forms by the hydrogen bond between the O atom of CAPE with arginine A136 of the enzyme. In normal conditions, HsDHODH is involved in the oxidation of dihydroorotate to orotate, simultaneously reducing FMN to FMNH2. This reaction induces ubiquinone, which quickly diffuses into the inner membrane mitochondria, allowing oxidation of FMNH2 back to FMN and hence further rotation formation.16 Therefore, in the presence of CAPE at the ubiquinone-binding site, FMNH2 may remain in reduced form and block further reaction, thereby reducing the uridine monophosphate (UMP) supply. Orotate and UMP are required for DNA/RNA biosynthesis, therefore limited UMP will arrest the cell cycle in the S stage and induce intrinsic apoptosis. However, the experimental results indicate immediate cell death and expression of caspase-8, so the apoptosis of CAPE is more likely due to activation of the extrinsic apoptosis pathway. As shown in Figure 6, inhibition of HsDHODH by CAPE may disrupt the mitochondrial transport chain and perturb the mitochondrial membrane potential, leading to the accumulation of reactive oxygen species (ROS) including superoxidase (O2•−) due to continuous orotate formation and reverse electron transfer. The formation of mitochondrial superoxidase induces accumulation of p53, activation of which will activate FaDD (Fas-associated Death Domain) and activation of procaspase-8 to form active caspase-8, in turn, activation of caspase-3 inducing cell apoptosis.23–25 Caspase-8 could also activate intrinsic apoptosis by cleaving the BH3 interacting domain death agonist (BID) to form an active BID and a Bax-BID transition complex. The activation of Bax releases cytochrome c from the mitochondria, which recruits Apaf-1 to form the apoptosome complex and activates procaspase-9 into active caspase-9, which then induces caspase-3, an apoptosis executor of cell death in a programmable manner.26–28 This new mechanism could be exploited to develop CAPE derivatives targeting HsDHODH or other mitochondrial enzymes for breast cancer treatment.
Conclusion
CAPE has optimum cytotoxic activity in 24 h incubation with IC50 of 6.6 ± 1.0 µM. Nevertheless, the cytotoxicity is reduced for longer incubation times, which is predicted due to the instability of the CAPE structure in incubation culture media. Therefore, further study to investigate its stability in different media and human plasma will be required. The mechanism of action of CAPE as an inhibitor of NFκB and inhibit human DHOH is different from the mechanism of action of 5-FU as thymidylate synthetase activity. These mechanisms could be important for CAPE to be developed as an anticancer agent in single treatment or in combination with 5-FU.
Acknowledgment
This work was supported by the Ministry of Research, Technology & Higher Education, directorate general of research and development strengthening, Indonesia.
We would also like to thank Dr. Ahmad Faried, PhD, Sp. BS(K), Tenny Putri W., and Nurul Qomarilla and team member of Cell Culture and Cytogenetic Laboratory, Faculty of Medicine Universitas Padjadjaran for supporting us with the MCF-7 cell line.
Funding
The present study was supported by the Ministry of Research, Technology & Higher Education, directorate general of research and development strengthening (Indonesia; grant no. 1123s/UN6.O/LT/2019).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Breast cancer; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
2. Lee JH, Nan A, Atkinson GM, Moorthy RS. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv. 2012;2012:1–17. doi:10.1155/2012/527516
3. Amalia E. Diantini A, Subarnas A. Overview of current and future targets of breast cancer medicines. J Pharm Sci Res. 2019;11:2385–2397.
4. Sharder A, Rabbani G, Chowdhury HK. Molecular basis of drug interactions of methotrexate, cyclophosphamide and 5-fluorouracil as chemotherapeutic agents in cancer. Biomed Res Ther. 2015;2:196–206.
5. World Health Organization. Guidelines for management of breast cancer. EMRO Technical Publication Series 31. World Health Organization; 2006.
6. Yuan J-H, Cheng J-Q, Jiang L-Y, et al. Breast cancer resistance protein expression and 5-fluorouracil resistance. Biomed Environ Sci. 2008;21:290–295. doi:10.1016/S0895-3988(08)60044-6
7. Peters GJ, Noordhuis P, Van Kuilenburg ABP, et al. Pharmacokinetics of S-1, an oral formulation of ftorafur, oxonic acid and 5-chloro-2,4-dihydroxypyridine (molar ratio 1:0.4:1) in patients with solid tumors. Cancer Chemother Pharmacol. 2003;52(1):1–12. doi:10.1007/s00280-003-0617-9
8. Matt P, Van Zwieten-Boot B, Rojas GC, et al. The European medicines agency review of tegafur/gimeracil/oteracil (teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: summary of the scientific assessment of the committee for medicinal products for human use (CHMP). Oncologist. 2011;16:1451–1457. doi:10.1634/theoncologist.2011-0224
9. Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O’Connor OA. Propolis and its active component, Caffeic Acid Phenethyl Ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J Cancer Sci Ther. 2013;5:334–342.
10. Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 Cells. J Biol Chem. 2004;279:6017–6026. doi:10.1074/jbc.M306040200
11. Wadhwa R, Nigam N, Bhargava P, et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J Cancer. 2016;7:1755–1771. doi:10.7150/jca.15170
12. Chen M-J, Shih S-C, Wang H-Y, et al. CAPE induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction pancreatic cancer cells. J Evid Based Complementary Altern Med. 2013;2013:1–7.
13. Porporato PE, Filigheddu N, Bravo-San Pedro JM, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Nat Publ Gr. 2017;28(3):265–280.
14. Sanchez-Alvarez R, Martinez-Outschoorn UE, Lamb R, et al. Mitochondrial dysfunction in breast cancer cells prevents tumor growth Understanding chemoprevention with metformin. Cell Cycle. 2013;12(1):172–182. doi:10.4161/cc.23058
15. Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med. 2014;13(4):35–43.
16. Miyazaki Y, Inaoka DK, Shiba T, et al. Selective cytotoxicity of Dihydroorotate dehydrogenase inhibitors to human cancer cells under hypoxia and nutrient-deprived conditions. Front Pharmacol. 2018;9:1–13. doi:10.3389/fphar.2018.00997
17. Yu J-H, Zheng G-B, Liu C-Y, et al. Dracorhodin perchlorate induced human breast cancer MCF-7 apoptosis through mitochondrial pathways. Int J Med Sci. 2013;10:1149–1156. doi:10.7150/ijms.6275
18. Farghadani R, Seifaddinipour M, Rajarajeswaran J, et al. In vivo acute toxicity evaluation and in vitro molecular mechanism study of antiproliferative activity of a novel indole Schiff base β -diiminato manganese III complex in hormone-dependent and triple negative breast cancer cells. Peer J. 2019;7:e7686. doi:10.7717/peerj.7686
19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
20. Kitamura H. Effects of propolis extract and propolis-derived compounds on obesity and diabetes: knowledge from cellular and animal models. Molecules. 2019;24:1–53. doi:10.3390/molecules24234394
21. Amalia E, Diantini A, Subarnas A. Water‑soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF‑7 cell line. Oncol Lett. 2020;20:274. doi:10.3892/ol.2020.12137
22. Celli N, Dragani LK, Murzili S, Pagliani T, Poggi A. In vitro and in vivo stability of Caffeic acid phenethyl ester, a bioactive compound of propolis. J Agric Food Chem. 2007;55:3398–3407. doi:10.1021/jf063477o
23. Schuler M, Bossy-Wetzel B, Goldstein JC, Fitzgerald P, Green DR. Induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi:10.1074/jbc.275.10.7337
24. Ehrhardt H, Ha¨cker S, Wittmann S, et al. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene. 2008;27:783–793. doi:10.1038/sj.onc.1210666
25. Khutornenko AA, Dalina AA, Chernyak BV, Chumakov PM, Evstafieva AG. The role of Dihydroorotate dehydrogenase in apoptosis induction in response to inhibition of the mitochondrial respiratory chain complex III. Acta naturae. 2014;6:69–74. doi:10.32607/20758251-2014-6-1-69-75
26. Musumeci G, Castrogiovanni P, Trovato FM, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16:20560–20575. doi:10.3390/ijms160920560
27. Tummers B, Green DR. Caspase-8; regulating life and death. Immunol Rev. 2017;277:76–89. doi:10.1111/imr.12541
28. Loreto C, La Rocca G, Anzalone R, et al. The role of intrinsic pathway in apoptosis activation and progression in peyronie’s disease. Biomed Res Int. 2014;2014:1–10. doi:10.1155/2014/616149
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.