Back to Journals » Journal of Pain Research » Volume 12
ATHENA: A Phase 3, Open-Label Study Of The Safety And Effectiveness Of Oliceridine (TRV130), A G-Protein Selective Agonist At The μ-Opioid Receptor, In Patients With Moderate To Severe Acute Pain Requiring Parenteral Opioid Therapy
Authors Bergese SD , Brzezinski M , Hammer GB , Beard TL, Pan PH, Mace SE, Berkowitz RD, Cochrane K, Wase L , Minkowitz HS , Habib AS
Received 30 May 2019
Accepted for publication 19 October 2019
Published 14 November 2019 Volume 2019:12 Pages 3113—3126
DOI https://doi.org/10.2147/JPR.S217563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Katherine Hanlon
Sergio D Bergese,1 Marek Brzezinski,2 Gregory B Hammer,3 Timothy L Beard,4 Peter H Pan,5 Sharon E Mace,6 Richard D Berkowitz,7 Kristina Cochrane,8 Linda Wase,8 Harold S Minkowitz,9 Ashraf S Habib10
1School of Medicine, Stony Brook University, Stony Brook, NY, USA; 2School of Medicine, University of California San Francisco, VA Medical Center, San Francisco, CA, USA; 3Stanford University School of Medicine, Stanford, CA, USA; 4Clinical Research, Summit Medical Group/Bend Memorial Clinic, Bend, OR, USA; 5Wake Forest School of Medicine, Winston-Salem, NC, USA; 6Cleveland Clinic Lerner College of Medicine at Case Western Reserve University, Cleveland, OH, USA; 7Phoenix Clinical Research, Tamarac, FL, USA; 8Trevena, Inc., Chesterbrook, PA, USA; 9HD Research Corporation, Houston, TX, USA; 10Duke University Medical Center, Durham, NC, USA
Correspondence: Sergio D Bergese
School of Medicine, Stony Brook University, Health Sciences Center, Level 4, Room 060, Stony Brook, NY 11794, USA
Tel +1 631-444-2979
Fax +1-631-444-2907
Email [email protected]
Background: Pain management with conventional opioids can be challenging due to dose-limiting adverse events (AEs), some of which may be related to the simultaneous activation of β-arrestin (a signaling pathway associated with opioid-related AEs) and G-protein pathways. The investigational analgesic oliceridine is a G-protein-selective agonist at the μ-opioid receptor with less recruitment of β-arrestin. The objective of this phase 3, open-label, multi-center study was to evaluate the safety and tolerability, of IV oliceridine for moderate to severe acute pain in a broad, real-world patient population, including postoperative surgical patients and non-surgical patients with painful medical conditions.
Methods: Adult patients with a score ≥4 on 11-point NRS for pain intensity received IV oliceridine either by bolus or PCA; multimodal analgesia was permitted. Safety was assessed using AE reports, study discontinuations, clinical laboratory and vital sign measures.
Results: A total of 768 patients received oliceridine. The mean age (SD) was 54.1 (16.1) years, with 32% ≥65 years of age. Most patients were female (65%) and Caucasian (78%). Surgical patients comprised the majority of the study population (94%), most common being orthopedic (30%), colorectal (15%) or gynecologic (15%) procedures. Multimodal analgesia was administered to 84% of patients. Oliceridine provided a rapid reduction in NRS pain score by 2.2 ± 2.3 at 30 mins from a score of 6.3 ± 2.1 (at baseline) which was maintained to the end of treatment. No deaths or significant cardiorespiratory events were reported. The incidence of AEs leading to early discontinuation and serious AEs were 2% and 3%, respectively. Nausea (31%), constipation (11%), and vomiting (10%) were the most common AEs. AEs were mostly of mild (37%) or moderate (25%) severity and considered possibly or probably related to oliceridine in 33% of patients.
Conclusion: Oliceridine IV for the management of moderate to severe acute pain was generally safe and well tolerated in the patients studied.
ClinicalTrials.gov identifier: NCT02656875.
Keywords: acute pain, analgesia, patient-controlled, clinical trial
Introduction
Conventional parenteral opioids represent an essential resource in the management of moderate to severe pain in acute care and in the post-surgical setting.1,2 They have, however, a narrow therapeutic window and are associated with dose-limiting opioid-related adverse events (ORAEs) such as nausea, vomiting and opioid-induced respiratory depression (OIRD).1–3 The elderly and patients with comorbidities (e.g. obesity, sleep-apnea) may be at an increased risk of ORAEs.1 In recent years, the use of multimodal analgesia is recommended to minimize the dose and duration of opioids.4,5
The efficacy and even more importantly, the safety of conventional opioids is defined by their cellular mechanisms.6 Agents that bind to the μ-opioid receptor cause downstream signaling through two distinct pathways: G-protein and β-arrestin.6,7 Preclinical models suggest that activation of the β-arrestin pathway is associated with ORAEs, while activation of the G-protein pathway results in analgesia.8–10 Conventional opioids have a non-selective mechanism, activating both pathways, thus achieving potent analgesia often at the expense of safety and/or tolerability.9,10 Selective activation of G-protein signaling may represent a mechanism that offers effective analgesia with less risk of adverse events (AEs).6,7
Selective or biased opioid-receptor agonists are a new class of medications with the potential to widen the therapeutic window. Oliceridine (TRV130; Trevena Inc., Chesterbrook, Pennsylvania), an investigational IV opioid, is a novel, centrally acting μ-opioid receptor agonist that is selective (or “biased”) toward signaling through the G-protein pathway. Oliceridine exhibits less β-arrestin recruitment to the μ-receptor than full agonist opioids such as morphine or fentanyl.6 In nonclinical studies, oliceridine was associated with potent analgesic effects while causing less gastrointestinal dysfunction and respiratory depression compared to morphine.6 Similarly, phase 3 randomized, controlled clinical trials suggest that oliceridine offers the potential for effective analgesia with improved safety and tolerability compared to conventional opioids.11,12
The aim of the phase 3 ATHENA study was to evaluate the safety and effectiveness of oliceridine in patients with moderate to severe acute pain undergoing a wide range of surgical procedures, or with non-surgical medical conditions, warranting parenteral opioids in either setting.
Materials And Methods
Study Overview
ATHENA was a phase 3, multicenter, open-label clinical study (Clinical Trial Identifier: NCT02656875), conducted from December 2015 to May 2017 at 41 sites in the United States, including ambulatory surgical centers, hospital-based outpatient and inpatient settings, and emergency departments. This study evaluated the safety and effectiveness of oliceridine in patients with moderate to severe acute pain, warranting the use of a parenteral opioid. In order to accurately represent IV opioid use in a broad, “real world” setting, the study was designed to be less restrictive for patient eligibility criteria, treatment protocol requirements, patient population, and mode of administration.
The study was approved by the Institutional Review Board or Independent Ethics Committee at each investigational site and was conducted in compliance with the Declaration of Helsinki and all International Conference on Harmonization Good Clinical Practice Guidelines. All patients provided written informed consent before participating in the study. The list of participating trial sites is provided in the supplementary table 1 (Supplementary Table1).
Patients
Eligible patients were 18 years of age or older experiencing moderate to severe acute pain following surgery or with a painful non-surgical medical condition, defined as ≥4 on an 11-point numeric rating scale (NRS), ranging from 0 = no pain to 10 = worst pain. Baseline NRS ratings were obtained within 30 mins prior to receiving the first dose of oliceridine.
Exclusion criteria included (a) medical conditions that could have confounded the effectiveness of oliceridine (such as acute pain without a specific etiology, undifferentiated acute abdominal pain, acute breakthrough pain in palliative “end of life” care, and pain associated with advanced cancer [somatic, visceral, or neuropathic] or with concurrent use of chemotherapeutic or biologic agents for the treatment of cancer), (b) participation in another study of oliceridine, (c) American Society of Anesthesiologists (ASA) Physical Status classification of IV or higher, (d) Emergency Severity Index of 1,13 (e) hypersensitivity to opioids, (f) signs of hemodynamic instability or respiratory insufficiency, (g) any clinically significant medical, surgical or postsurgical, psychiatric, and/or substance abuse condition that could confound interpretation of study outcomes, (h) clinically significant abnormal ECG including a QT interval using Fridericia’s correction for rate (QTcF) interval of >450 msec in males or >470 msec in females; as well as clinically significant abnormal laboratory values (known or obtained at screening) indicative of hepatic impairment (defined as total bilirubin >2×upper limit of normal [ULN], aspartate aminotransferase [AST] ≥1.5×ULN AND alanine aminotransferase [ALT] ≥1.5×ULN) or renal impairment (defined as estimated glomerular filtration rate ≤29 mL/min/1.73 m2).
Treatment
After screening, enrolled patients were treated with intravenous (IV) oliceridine via clinician-administered bolus dosing and/or patient-controlled analgesia (PCA). For IV bolus dosing, a loading dose of 1 to 2 mg was administered and a supplemental dose of 1 mg was given within 15 mins if needed. Subsequent doses of 1 to 3 mg were administered every 1 to 3 hrs on as-needed basis (PRN).
In settings where rapid analgesia was required (eg, emergency departments, post-anesthesia care units), loading doses of 1 to 3 mg were administered and supplemental doses of 1 to 3 mg every 5 mins PRN were allowed. Subsequent doses of 1 mg to 3 mg every 1 to 3 hrs were used if clinically indicated.
For PCA, a loading dose of 1.5 mg and a demand dose of 0.5 mg were administered using a 6-mins lockout interval. If clinically indicated, 1 mg supplemental doses were allowed PRN as early as 15 mins after the initial dose, taking into consideration, the patient’s utilization of PCA demand doses, individual patient need, and previous response to oliceridine treatment.
The dosing limit for oliceridine was 60 mg in the first 12 hrs. In patients reaching this limit, or in patients with pain not adequately controlled with oliceridine as determined by the treating physician, oliceridine treatment was discontinued and conventional treatment initiated.
Treatment duration for each patient was determined by the clinical need for parenteral opioid therapy. The maximal duration of oliceridine treatment was limited to 14 days. “End of treatment” was considered as the period within 24 hrs after the last dose of oliceridine. The duration of the post-treatment follow-up period was limited to 3 days post treatment; except when serious AEs occurred, when the patient was followed until the AE was resolved (Supplementary Figure 1).
Prior And Concomitant Medications
Prior medications were defined as those taken within 14 days before the first dose of oliceridine and concomitant medications were those taken after the first dose of oliceridine. There was no restriction on prior use of opioids and/or non-opioid analgesics; perioperative use of local anesthetics, epidural and intrathecal opioids were also allowed. Likewise, there was no restriction on prior and concomitant use of anxiolytics, sedatives, and hypnotics. Concomitant non-opioid analgesics used as part of a multimodal analgesic approach were permitted based on study site clinical guidelines. Use of other parenteral and/or oral opioids during treatment with oliceridine was not allowed; in the event, opioids were used, oliceridine administration was discontinued and patients managed with conventional opioids. There were no restrictions on medications prescribed to manage opioid-related AEs such as antiemetics and laxatives.
Medication Exposure And Safety And Tolerability Evaluation
Patient exposure to oliceridine was reported as cumulative dose administered (i.e., total number of milligrams of oliceridine received during the acute treatment period) and as cumulative duration of treatment (i.e., total hours from the first to last dose of oliceridine). For patients receiving only a single dose, the duration of treatment was set to “0” hours.
AEs reported are those occurring during treatment with oliceridine and post-treatment follow-up; or any pre-existing AEs that have worsened in severity during the treatment phase and post-treatment follow-up. The safety and tolerability of oliceridine were assessed by (1) incidence of observed or self-reported AEs, coded based on verbatim reported terms, using the Medical Dictionary for Regulatory Activities (MedDRA, Version 19.0), as well as, (2) other safety and tolerability evaluations including, somnolence or sedation, opioid withdrawal symptoms, vital signs, oxygen saturation by pulse oximetry, physical examination, clinical laboratory tests, and electrocardiograms (ECG). Vital signs, physical examinations, blood chemistry and hematology testing were conducted at baseline (i.e., within 24 hrs from the first oliceridine dose) and after the last oliceridine dose was administered. The intensity of an AE was classified as mild, moderate or severe. An AE was considered “serious”, if in the view of the investigator it resulted in death, a life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions.
Somnolence and sedation were assessed using the Moline-Roberts Pharmacologic Sedation Scale (MRPSS) within 30 mins of the first oliceridine dose and within 1 hr from the last oliceridine dose. The MRPSS-scale includes 6 levels of sedation ranging from “none/minimal” to “general anesthesia.” Additional reports of somnolence and sedation were recorded as AEs based on occurrence.14
Opioid withdrawal symptoms were assessed, by patients, the day after the last dose of oliceridine using the Subjective Opiate Withdrawal Scale (SOWS) questionnaire.15 Patients rated the intensity of 16 symptoms on a scale of 0 (not at all) to 4 (extremely). The total SOWS score was derived by summing the scores for each of the 16 individual symptoms (range, 0 to 64). Total SOWS scores were also classified by severity.16
Oxygen saturation was monitored continuously by pulse oximetry starting within 30 mins of the first oliceridine dose through the treatment period and recorded based on institutional requirements. ECGs were performed at baseline, within 30 mins prior to the first oliceridine dose (in surgical patients only), 60 mins after the first dose of oliceridine, and every 24 hrs during the treatment period. Changes from baseline were recorded for heart rate, PR interval, RR interval, QRS interval, QT interval, and QT interval corrected for heart rate (QTcF; Fridericia).
Effectiveness Evaluation
The analgesic effectiveness of oliceridine was assessed using the change from baseline in the 11-point NRS for pain intensity. NRS pain scores were evaluated within 30 mins before administration of the first oliceridine dose, and 30 mins (± 10 mins) after administration of the first dose. After these measurements, pain scores were not evaluated at specific time points, but were collected at any time thereafter during the treatment period based on each institution’s standard of care as determined by the patient’s treating clinician.
Statistical Methods
As an observational safety study, no formal sample size calculations were conducted. A sample size of approximately 1,000 patients was planned based on the desired number of patient exposures. The safety analysis population included all enrolled patients who received at least one dose of oliceridine, while the efficacy population included all enrolled patients who received at least one dose of oliceridine and had at least one post-dose NRS pain score. To estimate dose-related effects on safety and effectiveness parameters, patients were stratified by cumulative dose subgroup: ≤4 mg, >4 to 8 mg, >8 to 16 mg, >16 to 36 mg, and >36 mg. These dose groups were chosen based on utilization data from a previously completed phase 2 randomized, double-blind, placebo-controlled study with an active comparator to evaluate the analgesic efficacy of oliceridine compared to placebo and morphine following abdominoplasty17 with extrapolation to the 2 to 3 days of exposure expected in this study.
Patients who only received oliceridine via bolus dosing were included in the “bolus group”. Those who received at least one dose via a PCA device were included in the “PCA group”, even if they received additional supplemental bolus doses of oliceridine during their course of therapy. Safety data were summarized using descriptive statistics for continuous (means and standard deviation [SD]) and categorical (number and percentage of patients) variables. For SOWS (based on scoring on a 16-symptom scale), if data from ≥8 symptoms were available, the analysis was based on the mean scores of the non-missing symptoms to calculate the total score. If data for >8 symptoms were not reported, the total score was recorded as “missing”. NRS scores at baseline and 30 mins after the first dose of oliceridine and change from baseline to 30 mins were tabulated by the cumulative dose group. No formal statistical testing was performed on the effectiveness results.
Results
Patient Disposition
Among 1,038 patients enrolled in the ATHENA study, 768 patients were treated with oliceridine and were included in the safety and efficacy analysis populations; 698 (91%) patients completed the study (Figure 1). More than half of all patients enrolled were females (65%) and Caucasian (78%) (Table 1). Patients’ mean age (SD [range]) was 54.1 (16.1 [18–89]) years: 68% were <65 years of age; 24% were ≥65 to <75; and 8% were ≥75 years of age. Almost half of all patients (46%) had a body mass index (BMI) ≥30 kg/m2.
 |
Table 1 Demographic And Baseline Characteristics Of Patients In The ATHENA Study |
 |
Figure 1 Patient disposition during the ATHENA study. |
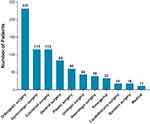
Of those enrolled, 94% were in surgical settings, with orthopedic (30%), colorectal (15%) or gynecologic (15%) procedures as the most common surgical procedures (Figure 2). All enrolled patients had at least one comorbid condition. The most common medical comorbidities are shown in Figure 3.
 |
Figure 3 Medical Comorbidities. All patients had at least one underlying medical condition. The most common (≥10% of all treated patients) medical conditions are presented. |
Prior to the first dose of oliceridine, 76% of patients received local anesthetics (eg, bupivacaine, lidocaine, ropivacaine for intraoperative pain management as nerve blocks, for local infiltration, anesthesia adjunct), 69% of patients received non-steroidal anti-inflammatory drugs (NSAIDs) and 48% of patients received oral opioids (Table 2).
 |
Table 2 Prior And Concomitant Medications |
Overall, 84% of the patients received multimodal analgesic therapy concomitantly with oliceridine (Table 2). Many analgesics used perioperatively as part of a multimodal regimen were continued during the treatment phase and administered concomitantly with oliceridine. Opioids alone or in combination with acetaminophen were used after the last dose of oliceridine in 78% of the patients.
Exposure To Oliceridine
Oliceridine was administered by bolus dosing (exclusively) and by PCA device (at least once) in 55% and 45% of patients, respectively (Table 3). The median cumulative oliceridine dose was 19.3 mg, ranging from 0.9 to 223.5 mg. The median cumulative duration of oliceridine exposure was 20.3 hrs, ranging from 0 (for patients who received a single oliceridine dose) to 142.7 hrs. The duration of oliceridine exposure was 0 to 26.8 hrs in the cumulative dose group of ≤4 mg that also included patients receiving a single dose. The duration increased proportionately with each increasing cumulative dose group, with the largest exposure in the cumulative dose group of >36 mg across a broader range of duration from 5.8 to 142.7 hrs (6 days). Cumulative oliceridine dose and duration of exposure increased with patient age. Patients ≥65 to <75 years and those ≥75 years old had greater duration of oliceridine exposure (36.9 hrs and 38.3 hrs, respectively) than patients <65 years old (27.6 hrs). There were no meaningful differences in the exposure to oliceridine between the obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2) patients.
 |
Table 3 Exposure To Oliceridine |
Safety And Tolerability
In the overall population, 64% of patients reported at least one AE during the study (Table 4). Most AEs were of mild (37%) or moderate (25%) intensity. Severe AEs were reported in 2% of patients. The proportion of AEs categorized by the intensity of mild, moderate or severe were similar across all the cumulative dose groups (Table 4). Overall, 2% of patients had AEs leading to early discontinuation. The most frequent AEs reported were nausea (31%), constipation (11%), and vomiting (10%). The incidence of events increased with a higher cumulative dose of oliceridine; the >16–36 mg and >36 mg dose groups also had the longest duration of exposure that could have confounded the dose–response relationship. AEs “probably” or “possibly” related to oliceridine were reported in 33% of patients; with a low incidence of nausea (18%), vomiting (7%), and constipation (6%). The use of concomitant medications for nausea, vomiting, and constipation increased in patients with higher cumulative doses of oliceridine (Supplementary Table 2). There were no differences observed in the overall incidence of AEs with administration via bolus (63%) or PCA (65%). Likewise, the incidence of most common AEs of nausea, vomiting and constipation did not differ for the two routes of administration.
 |
Table 4 Overall Summary Of AEs (Safety Analysis population) |
Serious adverse events (SAEs) were observed in 26 patients (3%) (Table 4). Most SAEs were due to complications of surgery (eg, bleeding and infection, postoperative ileus), secondary to an underlying medical condition (eg, endometrial cancer) or secondary to opioid therapy (eg, nausea, bowel dysfunction, respiratory depression, hypoxia, and syncope). All SAEs resolved or were resolving at the time of study completion. Only 3 patients experienced SAEs considered by the investigator as “possibly” related to oliceridine: post-operative ileus (1 patient), respiratory depression with respiratory rate <8 breaths/minute within 5 hrs of receiving oliceridine (1 patient), hepatic and renal failure confounded by surgical-related complications (1 patient).
The incidence of AEs in obese patients (defined as BMI≥ 30 kg/m2) was 61% (vs 64% in the overall population). The incidence of AEs in the elderly patients (>65 to <75 and ≥75 years old) was 61% and 66%, respectively, in the two elderly groups vs 64% in the overall population. Also, the incidence of AEs considered as “probably” or “possibly” related to oliceridine was similar in the elderly compared to the overall population (30% in the age group >65 to <75 years; 34% in those ≥75 years; vs 33% in the overall population).
There were 46% of patients at baseline, 66% at 30 mins after oliceridine dosing, and 93% at the end of the study that reported “none to minimal sedation” in the MRPSS (Supplementary Table 3). Likewise, the AE of somnolence (and sedation) reported by patients in all oliceridine dose groups was minimal (Table 4).
The mean opioid withdrawal symptom score was low (3.9) and comparable across groups (ranging from 3.3 to 4.1) (Supplementary Table 4). A total of 97% of patients reported “none to mild” withdrawal symptoms (i.e., SOWS score <17), 3% reported moderate symptoms (score ≥17 to ≤32), and less than 1% of patients reported severe symptoms (score >32) (Supplementary Table 4).
Twenty patients had at least one hepatic laboratory result, meeting “potentially clinically significant abnormal liver function test” (PCSA LFT) criteria. One of these patients experienced an SAE considered “possibly” related to oliceridine by the investigator and previously described. No patient experienced jaundice. Causality assessments conducted by an independent panel of experts reported that none of the LFT abnormalities were likely the result of oliceridine treatment.
The incidence of respiratory AEs associated with vital sign assessments is shown in Table 5. The percentage of patients with an oxygen saturation <90% increased from the lower to higher oliceridine cumulative dose groups (2–8%); no clinically relevant sequelae were noted. For the respiratory AEs associated with vital sign assessments and typically considered opioid-related, there was no apparent dose-effect noted across increasing oliceridine cumulative dose groups. Only one of these (the SAE) was considered by the investigator to be related to oliceridine (previously discussed) and no patients required the use of naloxone during oliceridine treatment. No other clinically relevant abnormalities were observed for vital signs. Likewise, there were no other clinically meaningful laboratory or serum chemistry changes associated with oliceridine.
 |
Table 5 Incidence Of Respiratory AEs And Associated Vital Signs |
Changes from baseline in ECG parameters were minimal and similar across cumulative oliceridine dose groups over time. Twenty-two patients distributed across all cumulative oliceridine dose groups met one or both predefined QTcF criteria. Half of these patients had ≥1 potential confounding factor that may have prolonged the QT interval (eg, electrolyte abnormalities, use of concomitant vasopressin, levofloxacin, amitriptyline, amiodarone, azelastine, or medical condition as in right bundle branch block or left bundle branch block); none of these patients had any AE or ECG assessment abnormalities suggestive of ventricular extrasystoles, premature ventricular contractions, or ventricular tachycardia.
Effectiveness
The mean NRS pain score at baseline was 6.3 ± 2.1. The mean change from baseline in the NRS pain score was −2.2 ± 2.3 at 30 min after the first dose, indicating a rapid reduction in pain intensity (Figure 4). The reductions were comparable across cumulative oliceridine dose groups, ranging from −2.8 to −1.9.
In patients with follow-up assessment available at their end of treatment (n=225), the mean change from baseline in NRS pain scores was −3.1 ± 3.1 across dose groups (ranging from −3.5 to −2.8), indicating maintenance of pain reduction.
Discussion
The salient finding of this phase 3, multicenter, open-label study was that oliceridine administered alone or as a component of multimodal analgesia in adult patients having moderate to severe pain with surgical procedures or medical conditions was generally safe and well tolerated in a broad-based population. The inclusion and exclusion criteria used in the ATHENA trial were intentionally designed to enroll a more clinically diverse population with fewer protocol restrictions than typically included in the setting of a randomized controlled clinical trial, reflecting a study population and a setting, more directly generalizable to real-world practice settings; particularly in the post-surgical setting, as 94% of the study participants were enrolled from this setting. The pattern, type, incidence and severity of AEs did not differ from those observed in prior randomized clinical trials.
Effectiveness
Oliceridine was associated with a potent analgesic effect and a rapid onset of action (2.2-point reduction of pain score within 30 mins) consistent with previous reports.11,12 For purposes of context, it is believed that the morphine milligram equivalent (MME) of oliceridine when initiating treatment is approximately 5:1 for the first dose (Data on file). The rapid reduction in NRS pain score along with sustained decreased pain intensity until the end of treatment demonstrated the analgesic effectiveness of oliceridine. Lack of efficacy leading to discontinuation was reported in less than 5% of patients.
It is noteworthy that despite patients receiving local anesthetics (76%), NSAIDs (69%); and oral opioids (48%) prior to the first dose of oliceridine, the average pain score prior to oliceridine administration was 6.3, further supporting the need for potent analgesics including parenteral opioids. The 84% rate of multimodal analgesia use in ATHENA, particularly in the post-surgical setting, is similar to the findings reported from a recent study that evaluated the utilization patterns of multimodal pain management in joint arthroplasty recipients in the United States, wherein 86% of patients received multimodal analgesia.18
Safety
Oliceridine demonstrated a favorable safety and tolerability profile, notably in patients at risk for opioid-related complications. AEs were experienced by 64% of patients. Nausea, constipation, and vomiting were documented in 31%, 11% and 10% of the patients, respectively. For those considered “probably” or “possibly” related to oliceridine, the reported incidence was lower for nausea (18%), vomiting (7%), and constipation (6%). The rates of serious SAEs and AEs leading to discontinuation were low, and no deaths were reported in the study. There was one SAE of respiratory depression which resolved, and most of the patients experienced “none to minimal” sedation/somnolence. The protocol allowed the use of antiemetics as prophylaxis. Thus, the incidence of nausea and vomiting related specifically to oliceridine alone is difficult to interpret.
A recently conducted retrospective analysis from a large healthcare system including 21 acute care hospitals and 135,379 patients undergoing surgical and endoscopic procedures and receiving conventional opioids reported at least one AE in 73% of patients.19 This retrospective analysis found that opioid-related AEs were associated with increased inpatient mortality (odds ratio [OR], 28.8; 95% CI, 24.0–34.5), prolonged length of stay (OR, 3.1; 95% CI, 2.8–3.4), high cost of hospitalization (OR, 2.7; 95% CI, 2.4–3.0), and higher rate of 30-day readmission (OR, 1.3; 95% CI, 1.2–1.4).19 In the ATHENA trial, AEs were reported in 64% of patients and no deaths were reported. ATHENA did not investigate the length of stay, 30-day readmission or cost of hospitalization.
Opioid-induced nausea and vomiting is one of the main causes of PONV20 with a reported incidence of 30% vomiting, and nausea ranging from 50 to as high as 80% in high-risk patients.21 Although a non-life-threatening side effect, PONV can have a substantial impact on patient outcomes, including quality of life.22 Thus, the findings of the low rates of nausea and vomiting associated with oliceridine in this trial are important in the context of strategies for the management of postoperative nausea and vomiting to enable earlier mobilization and discharge.
Opioid-induced respiratory depression (OIRD), a potentially fatal complication associated with conventional opioids in the acute setting involves complex and inter-related factors.23 Depending on the type of surrogate measures used the rates varied, for eg, with naloxone utilization as a surrogate, the incidence of respiratory depression was 1.9–2.0%, while using oxygen saturation <90% or <85% as surrogate resulted in incidences as high as 22%.24 In one meta-analysis, 1.2% to 11.5% of patients receiving parenteral opioids via a PCA device after major surgery had respiratory depression.24 Using the Anesthesia Closed Claims Project database of 9,799 claims, OIRD was “probably”, “possibly”, or “definitely” reported in 92 claims, and more than half of these claims were associated with death.25 In addition to the clinical consequences, the occurrence of OIRD in patients undergoing major surgical procedures has been shown to significantly increase total inpatient costs, patient in-hospital length of stay, and readmission rates.1 To reduce the risk of respiratory events, clinicians may limit opioid dosing, potentially resulting in inadequate analgesia.26 In the ATHENA study, oxygen saturation <90% was reported in 6% and none of the patients required naloxone. These findings support the early phase 3 trials which compared oliceridine with morphine in treating acute pain after bunionectomy or abdominoplasty.11,12 In both these studies, oliceridine was associated with potentially improved respiratory safety profile.11,12 Together, the findings of the low incidence of respiratory depression are consistent with the selective nature of oliceridine on the G-protein effector pathway.
In marked contrasts to conventional opioids,27–29 the incidence of AEs/SAEs leading to early discontinuation in this study was similar in the elderly (aged >65 years of age) and obese subgroups (BMI ≥30 kg/m2) when compared with the overall population.
A few patients in the ATHENA study experienced QT prolongation, but there were no instances of ventricular arrhythmias. Previous studies of oliceridine in healthy volunteers in a thorough QT (tQT) study found no evidence of any clinically significant effect at the highest proposed clinical dose of 3 mg on cardiac repolarization (Data on file). However, at the supratherapeutic dose of 6 mg, a minor, transient effect, with the upper one-sided 95% confidence limit of the mean placebo-adjusted change from baseline in QTcF exceeded the 10 msec, at 2.5 mins, 1 hr, and 2 hrs post-dose (Data on file). Subsequently, in the randomized clinical trials of oliceridine, there was no evidence of QT prolongation.11,12 A recent study of about 500 non-cardiac surgery patients reported QT prolongation as a common occurrence, and perioperative medication, including anesthetics (isoflurane), analgesics (ketorolac and methadone), and antibiotics, was one of the causative factors.30 Unlike the phase 3 controlled clinical trials of oliceridine, the study design of ATHENA did not have any restrictions on the use of prior or concomitant medications, and indeed in half of the reported cases, patients received medications known to cause QT prolongation. Thus, the results could be confounded by the cumulative effect of several drugs likely contributing to the postoperative QTc interval prolongation. Further tQT evaluation is underway.
There are several limitations to the ATHENA study. First, the open-label design can introduce bias in the findings of the study. Secondly, most patients received local anesthetics, NSAIDs, and/or other opioids prior to the first dose of oliceridine. The non-opioid multimodal analgesics were also allowed as part of the standard of care. The influence of the prior medications, including opioids and concomitant non-opioid analgesics contributing to the adverse events cannot be ruled out. In addition, although the protocol defined that no concomitant opioids could be administered during oliceridine treatment, several patients received opioids after the last dose of oliceridine. This could have further contributed to the AEs observed in the study. Lastly, the lack of a control group limits the true estimation of the AEs.
Conclusion
The ATHENA trial was intended to assess the safety of oliceridine in a broad-based group of adult patients experiencing moderate to severe pain following surgical procedures or non-surgical medical conditions. Although the study was not powered for safety or efficacy, oliceridine IV was generally safe and well tolerated in this heterogeneous population of patients with moderate to severe acute pain for whom a parenteral opioid was warranted. Most of the patients enrolled in the ATHENA trial received oliceridine for the management of moderate to severe post-surgical acute pain. Thus, the findings in the patients with non-surgical medical conditions are limited to the patients characterized in this study and not generalizable to larger non-surgical populations. The low incidence of respiratory AEs, and PONV, along with rapid and sustained analgesia allowed for titration to a dose that provided analgesia and favorable tolerability. Oliceridine, a novel centrally acting G-protein pathway-selective µ-opioid receptor agonist may represent a potential new treatment option for patients requiring IV opioid therapy.
Data Availability
Data from the ATHENA trial are held by Trevena Inc. and are not publicly available. Access to specific data sets, protocols, and reports will be considered on the basis of proposed quality and alignment with the aims of the original study.
Acknowledgements
The authors would like to thank all investigators and participants of the trial. The manuscript was drafted under direction from the authors with medical writing support provided jointly by Kanaka Sridharan, M.S. R.Ph, an employee at Trevena Inc., and Donna McGuire of Engage Scientific Solutions (funded by Trevena Inc for development of figures). Authors would also like to thank Mark Demitrack, Chief Medical Officer, Trevena, Inc; for the thoughtful review and comments during the development of the manuscript. This study was sponsored by Trevena, Inc. At the time of preparation and submission of this manuscript, Oliceridine, an investigational IV opioid, was not yet approved by the FDA or any other governmental agencies.
Disclosure
Sergio D Bergese, Marek Brzezinski, Gregory B Hammer, Timothy L Beard, Peter H Pan, Sharon E Mace and Richard D Berkowitz received research grant funding for the ATHENA study and have served as a consultant for Trevena, Inc. At the time of conduct of the study, and submission of the manuscript to the journal, Sergio D Bergese was affiliated with the Ohio State University Wexner Medical Center; that was the participating site for the trial. Harold S Minkowitz is a consultant for Trevena, Inc. and received research funding from Trevena, Inc., AcelRx Pharmaceuticals, Cara Therapeutics, Durect, Heron Therapeutics, Innocoll, and Pacira Pharmaceuticals. Linda Wase and Kristina Cochrane are full-time employees of Trevena, Inc. and own stock in Trevena, Inc. Ashraf S Habib has received research support from Trevena, Inc., Pacira Pharmaceuticals, BioQ Pharma and Halyard Health and is a consultant for Trevena, Inc. The authors report no other conflicts of interest in this work.
References
1. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi:10.2147/JPR.S144066. eCollection 2017.
2. Stein C. New concepts in opioid analgesia. Expert Opin Investig Drugs. 2018;27(10):765–775. doi:10.1080/13543784.2018.1516204
3. Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care. 2011;17(Suppl 11):S276–S287.
4. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. doi:10.1097/ALN.0b013e31823c1030
5. Wardhan R, Chelly J. Recent advances in acute pain management: understanding the mechanisms of acute pain, the prescription of opioids, and the role of multimodal pain therapy. F1000Research. 2017;6:2065. doi:10.12688/f1000research
6. DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. doi:10.1124/jpet.112.201616
7. Chen XT, Pitis P, Liu G, et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi:10.1021/jm4010829
8. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi:10.1126/science.286.5449.2495
9. DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi:10.1146/annurev.physiol.69.022405.154749
10. Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195–1201. doi:10.1124/jpet.105.087254
11. Singla NK, Skobieranda F, Soergel DG, et al. APOLLO-2: a randomized, placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the mu-opioid receptor, for management of moderate to severe acute pain following abdominoplasty. Pain Pract. 2019;19(7):715–731. doi:10.1111/papr.12801
12. Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the micro-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res. 2019;12:927–943. doi:10.2147/JPR.S171013
13. American Society of Anesthesiologists Clinical Information [Internet]. Schaumburg, IL: ASA physical status classification system; [Approved 2014 Oct, 15] American Society of Anesthesiologists. 2017;Available from: https://www.asahq.org/resources/clinical-information/asa-physicalstatus-classification-system.
14. Moline B, Roberts M, Houser J. Validity and interrater reliability of the Moline-Roberts pharmacologic sedation scale. Clin Nurse Spec. 2012;26(3):140–148. doi:10.1097/NUR.0b013e3182503fd6
15. Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293–308. doi:10.3109/00952998709001515
16. Lintzeris N, Bammer G, Rushworth L, Jolley DJ, Whelan G. Buprenorphine dosing regime for inpatient heroin withdrawal: a symptom-triggered dose titration study. Drug Alcohol Depend. 2003;70(3):287–294. doi:10.1016/S0376-8716(03)00015-2
17. Singla N, Minkowitz HS, Soergel DG, et al. A randomized, phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res. 2017;10:2413–2424. doi:10.2147/JPR.S137952
18. Memtsoudis SG, Poeran J, Zubizarreta N, et al. Association of multimodal pain management strategies with perioperative outcomes and resource utilization: a population-based study. Anesthesiology. 2018;128(5):891–902. doi:10.1097/ALN.0000000000002132
19. Shafi S, Collinsworth AW, Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153(8):757–763. doi:10.1001/jamasurg.2018.1039
20. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi:10.1097/00000542-199909000-00022
21. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi:10.1213/ANE.0000000000000002
22. Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol. 2014;722:67–78. doi:10.1016/j.ejphar.2013.09.074
23. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317–325. doi:10.2217/pmt.14.19
24. Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93(2):212–223. doi:10.1093/bja/aeh180
25. Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659–665. doi:10.1097/ALN.0000000000000564
26. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747–758. doi:10.1093/bja/aen094
27. Zuckerman M, Greller HA, Babu KM. A review of the toxicologic implications of obesity. J Med Toxicol. 2015;11(3):342–354. doi:10.1007/s13181-015-0488-6
28. Kramer NM, Gazelka HM, Thompson VH, Batsis JA, Swetz KM. Challenges to safe and effective pain management in patients with super obesity: case report and literature review. J Pain Symptom Manage. 2018;55(3):1047–1052. doi:10.1016/j.jpainsymman.2017.11.005
29. Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging. 2008;3(2):273–278. doi:10.2147/CIA.S1847
30. Nagele P, Pal S, Brown F, Blood J, Miller JP, Johnston J. Postoperative QT interval prolongation in patients undergoing noncardiac surgery under general anesthesia. Anesthesiology. 2012;117(2):321–328. doi:10.1097/ALN.0b013e31825e6eb3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.