Back to Journals » Journal of Inflammation Research » Volume 17
Association Between Nonthyroidal Illness Syndrome and Disseminated Intravascular Coagulation in Sepsis
Authors Yao Y, Li J, Sun W, Ma C, Ning N, Li J, Ni T, Gui M , Chen Y, Zhao B, Zhang J, Zhu J, Gao C , Sheng H , Mao E
Received 29 September 2023
Accepted for publication 23 January 2024
Published 28 January 2024 Volume 2024:17 Pages 497—506
DOI https://doi.org/10.2147/JIR.S442598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Yi Yao,1,* Juan Li,2,* Wenwu Sun,1,* Chaoping Ma,2,* Ning Ning,1 Jiaoyan Li,1 Tongtian Ni,1 Menglu Gui,1 Ying Chen,1 Bing Zhao,1 Jiyuan Zhang,2 Jiyue Zhu,3 Chengjin Gao,2 Huiqiu Sheng,1 Enqiang Mao1
1Departments of Emergency, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200025, People’s Republic of China; 2Department of Emergency, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 3Department of General Surgery, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiqiu Sheng, Departments of Emergency, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200025, People’s Republic of China, Tel +86-15801971839, Email [email protected] Chengjin Gao, Department of Emergency, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China, Tel +86-18221088783, Email [email protected]
Purpose: Both nonthyroidal illness syndrome (NTIS) and disseminated intravascular coagulation (DIC) are commonly occurred in sepsis. The objective of this study is to evaluate the association between NTIS and DIC, as well as their impacts on the mortality in adults with sepsis.
Patients and methods: A total of 1219 septic patients in two Chinese academic centers from October 2012 and October 2022 were enrolled in analysis. We conduct logistic regression models to analyze the independent risk factors for DIC. Modified Poisson regression models are used to estimate the relative risk (RR) of NTIS on the 28 days mortality in septic patients with DIC. Correlation analysis between thyroid function parameters and coagulation parameters is performed with Pearson coefficient be reported.
Results: DIC is diagnosed on 388 (31.8%) of all the 1219 enrolled septic patients within 72 hours after admission. In multivariate logistic regression models, NTIS (OR 3.19; CI 2.31– 4.46; p< 0.001) is a statistically significant independent risk factor for DIC after adjustment for potential confounders. Twenty-eight days mortality is significantly higher in DIC patients complicated with NTIS compared with the other DIC patients (23.2% vs 14.0%, p=0.024). This result is also robust in different modified Poisson regression models (Model 1: RR 1.46; CI 1.25– 1.70; p< 0.001; Model 2: RR 1.35; CI 1.14– 1.60; p< 0.001; Model 3: RR 1.18; CI 1.02– 1.37; p=0.026). Correlation analysis reveals that the thyroid function parameters of FT3, FT4 and TSH only have weak correlations with coagulation parameters of platelet count, fibrinogen, FDP, D-dimers, PT, APTT and INR in sepsis.
Conclusion: NTIS is an independent risk factor for DIC in adults with sepsis. DIC patients complicated with NTIS have significantly higher severity and higher rate of mortality.
Keywords: sepsis, low T3 syndrome, nonthyroidal illness syndrome, thyroid function, disseminated intravascular coagulation
Introduction
Sepsis is an infection induced life-threatening syndrome, which impacts millions of people around the world each year. Sepsis accounts for about 6% of adult hospitalization in high-income countries, and the overall mortality rate remains as high as 15%–23%.1,2
Sepsis is a systemic illness that involves, but not limited to circulatory, respiratory, coagulation or endocrine system. The dysregulated inflammatory processes induced by infection compromise multiple systems leading to multiple organ dysfunction syndrome (MODS) and potentially life-threatening consequences.3 Disseminated intravascular coagulation (DIC) in sepsis, defined as a coagulation disorder induced by infection, is characterized by endothelial dysfunction and systemic thrombin generation.4 The prevalence of DIC in septic patients is as about 30%–50%. The percentage is relatively high because the inflammatory reaction and coagulation process closely interact with each other. Complication with DIC indicates unfavorable prognosis and higher mortality for septic patients.5,6
Current precision medicine in the field of intensive care medicine highlights stratifying patients and finding phenotypes with best host-response patterns.7,8 This concept is of great importance in identification and management of DIC in septic patients. For example, anticoagulation treatment is very important but is also controversial in the treatment of DIC, because conflicting conclusions were obtained from a series of clinical studies.9,10 Further analysis suggests that treatment for DIC should concentrate on the very severely ill patients,11 which emphasizes the precise stratification for DIC patients. Risk stratification is of particular importance, because high-risk patients may get benefit from earlier treatment, whereas low-risk patients may get benefit from avoiding unnecessary interventions. Although coagulation markers such as platelets, prothrombin time (PT), activated partial thromboplastin time (APTT), and D-dimers have been widely acknowledged to diagnose DIC on sepsis, these biomarker are not satisfied in severity stratification. Nonthyroidal illness syndrome (NTIS), also called “low T3 syndrome” or “euthyroid sick syndrome”, is the most common endocrine dysfunction in sepsis with a reported rate of about 42% and well known as a negative predictor for outcomes.12 The conclusion of a meta-analysis revealed that either low triiodothyronine or thyroxine was associated with the increased in-hospital mortality in adult septic patients.13 This conclusion was also supported in another study using data from a large critical care database.14 However, even though thyroid function is commonly tested for septic patients, the association between NTIS and DIC in sepsis was not taken seriously in previous studies.
Given that NTIS represents a specific group of patients with higher illness severity and unique pathophysiological characteristics, the objective of the study is therefore to evaluate the association between NTIS and DIC, as well as its impact on the outcomes in adult septic patients, in order to provide evidence for precise intervention in future studies.
Methods
Participants
This retrospective study was conducted in Ruijin Hospital, Shanghai Jiaotong University School of Medicine, and Xinhua Hospital, Shanghai Jiaotong University School of Medicine. The protocol was approved by the institutional ethics board of each hospital, and the informed consent was waived because of the non-interventional, retrospective design of the study. Data analysis was performed in accordance with the 1964 Helsinki Declaration and its later amendments.
Consecutive adult patients (age≥18 years) with a diagnosis of sepsis on admission between May 2015 and May 2022 (Ruijin Hospital, Shanghai Jiaotong University School of Medicine), and between October 2012 and October 2022 (Xinhua Hospital, Shanghai Jiaotong University School of Medicine) were screened. Patients exclusion criteria included: (1) incomplete coagulation function test; (2) without thyroid function test on admission; (3) history of heamatological or coagulation system diseases; (4) history of cirrhosis; (5) using anticoagulants before coagulation function test.
Data Collection
The clinical variables are extracted from the paper-based or electronic medical records for each patient. Baseline demographic information includes age, sex, comorbidities (hypertension, diabetes, and chronic kidney diseases). Infection source is classified into respiratory system source, gastrointestinal tract source, urogenital tract source, and others (including skin and soft tissue infection, bone and joint infection, and meningitis). Laboratory indicators collected within 24 hours after admission include white blood cell count, alanine aminotransferase, total bilirubin, creatinine, lactate, C-reactive protein, procalcitonin, free triiodothyronine (FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH). Acute Physiology and Chronic Health Evaluation II (APACHE II) scores and Sequential Organ Failure Assessment (SOFA) scores are collected within 24 hours after admission. Coagulation parameters (including fibrinogen, fibrin/fibrinogen degradation products (FDP), D-dimers, PT, APTT, and international normalized ratio (INR)) are obtained within 72 hours and DIC is diagnosed on the worst results. Thyroid dysfunctions are classified into euthyroid, history of hypothyroidism, history of hyperthyroidism, NTIS, and others. The rate of 28 days in-hospital mortality is reported. Patients discharged any earlier than 28 days were considered survivors.
Definition
The diagnosis of sepsis is defined as (SOFA) score ≥2 points consequent to the infection (Sepsis-3). Septic shock is defined as sepsis with persisting hypotension requiring vasopressors to maintain mean arterial pressure ≥65 mm Hg and having a serum lactate level >2 mmol/L despite adequate volume resuscitation.15 The diagnosis of DIC is defined according to the International Society on Thrombosis and Haemostasis (ISTH) diagnostic criteria for overt DIC.16 Patients with ISTH overt DIC scores ≥5 is diagnosed of DIC. NTIS in this study is defined as a low serum FT3 level (<2.63pmol/L) without an elevated TSH level (<4.94mIU/mL).17
Data Statistics
Categorical data will be described in terms of frequency or ratio. Continuous variables will be described using median and interquartile range. Categorical variables will be compared using χ2 test or Fisher's exact test. Continuous variables will be compared using the t-test for normally distributed variables or Wilcoxon rank-sum test for non-normally distributed variables. We implement multiple predictive mean matching imputations using chained equation and created 5 independent datasets to compute missing data. Variables with missing data more than 20% are deleted.
Survival curve between septic patients with or without DIC is plotted using the Kaplan–Meier method. The associations between NTIS and DIC are estimated using logistic regression models. Variables in univariate logistic regression model with p <0.15 are included in multivariate logistic regression model. The effect of NTIS on the 28 days mortality in septic patients with DIC is analyzed. Considering the high 28 days mortality in septic patients with DIC, we used modified Poisson regression model with robust (sandwich) estimation of variance to calculate the relative risk (RR), instead of logistic regression model with odds ratio (OR). Baseline information includes age, sex, and comorbidities are adjusted. Moreover, total bilirubin represents liver function; creatinine represents renal function; lactate represents oxygen debt; C-reactive protein represents inflammation reaction degree; procalcitonin represents infection severity, and APACHE II score represent the overall condition. These variables are also adjusted in the models. The correlations between thyroid function parameters and coagulation parameters are reported as Pearson correlation coefficients and shown in correlation-based heatmap. Sensitivity analysis is conducted on the raw data to evaluate the associations between NTIS and DIC, and the effect of NTIS on the 28 days mortality in septic patients with DIC. Statistical analyses are performed using R software (version 4.2.1). A two-sided significance level less than 0.05 is defined as statistical significance.
Results
Patients’ Characteristics
A total of 2665 patients diagnosed with sepsis on admission in two centers are included. After screening, 1219 septic patients are enrolled. Figure 1 shows the screening flowchart. Among them, DIC is diagnosed in 388 (31.8%) patients within 72 hours after admission, and 165 (13.5%) patients died within 28 days. Remarkable laboratory differences are found at the levels of alanine aminotransferase, creatinine, C-reactive protein and procalcitonin. All the coagulation parameters are significantly worse in patients with DIC, except for INR (median 1.2 vs 1.2, p=0.082). Patients with DIC have a significantly lower FT3 level (median 2.1 pmol/L vs 2.7 pmol/L, p<0.001), and FT4 level (median 12.1 pmol/L vs 13.9 pmol/L, p<0.001), but no difference on TSH level (median 0.9μIU/mL vs 1.1μIU/mL, p=0.944). APACHEII scores are higher (median 13 vs 11, p<0.001), more patients suffer septic shock (37.4% vs 22.0%, p<0.001), and more patients dies within 28 days (19.3% vs 10.8%, p<0.001) in DIC group (Supplementary Figure 1). There is no difference on the history of hypothyroidism (3.4% vs 5.7%, p=0.083) or hyperthyroidism (3.1% vs 3.0%, p=0.936) between groups, but much more patients suffer NTIS in DIC group (57.7% vs 27.9%, p<0.001) (Table 1).
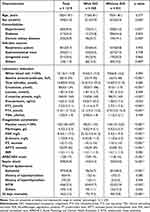 |
Table 1 Characteristics Between Septic Patients with or Without DIC |
 |
Figure 1 Screening flowchart. Abbreviation: DIC, disseminated intravascular coagulation. |
Association Between NTIS and DIC in Sepsis
Of all the 1219 patients, the distribution of ISTH overt DIC scores is as follows: 330 of them score between 0 and 2; 501 of them score between 3 and 4; 350 of them score between 5 and 6; and 38 of them score between 7 and 8. Figure 2 shows the incidence of NTIS in the groups with different DIC scores. The NTIS percentage is increasing gradually with the DIC scores (15.4%, 36.1%, 55.7% and 76.3%, respectively). Logistic regression model is developed to estimate the risk factors for DIC. Variables in univariate logistic regression model with p <0.15 are included in multivariate logistic regression model. Finally, in multivariate logistic regression model, the following confounders are included: age, sex, hypertension, chronic kidney diseases, infection source classified as others, white blood cell count, alanine aminotransferase, total bilirubin, creatinine, C-reactive protein, procalcitonin, APACHE II score, and thyroid dysfunctions. In multivariate model, NTIS (OR 3.19; CI 2.31–4.46; p<0.001) is a statistically significant independent risk factor for DIC compared with euthyroid or the other thyroidal dysfunctions (Table 2). The results are similar in sensitivity analysis on raw data (Supplementary Table 2).
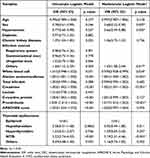 |
Table 2 Analysis of Risk Factors for DIC in Sepsis Using Logistic Regression Models |
 |
Figure 2 Association between NTIS and DIC in sepsis. Abbreviations: DIC, disseminated intravascular coagulation; NTIS, nonthyroidal illness syndrome. |
Impact of NTIS on the 28 Days Mortality in Septic Patients with DIC
A total of 388 patients with DIC are divided into two groups: patients with both NTIS and DIC (n=224); and patients with DIC, but without NTIS (n=164). The patients’ characteristics between two groups are shown in Supplementary Table 1. DIC patients with NTIS have higher APACHEII score (median 15 vs 11, p<0.001) and 28 days mortality rate (23.2% vs 14.0%, p=0.024) compared with the DIC patients without NTIS. Modified Poisson regression models are adjusted for several risk factors, including age, sex, comorbidities, total bilirubin, creatinine, lactate, C-reactive protein, procalcitonin, and APACHE II score. The results in different models reveal that after adjustment for potential confounders, the 28 days mortality is still significantly higher in NTIS group (Model 1: RR 1.46; CI 1.25–1.70; p<0.001; Model 2: RR 1.35; CI 1.14–1.60; p<0.001; Model 3: RR 1.18; CI 1.02–1.37; p=0.026) (Table 3). The results are similar in sensitivity analysis on raw data (Supplementary Table 3).
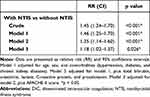 |
Table 3 Impact of NTIS on the 28 Days Mortality in Septic Patients with DIC Estimated Using Modified Poisson Regression Models |
Correlations Between Thyroid Function Parameters and Coagulation Parameters in Sepsis
The correlation analysis between thyroid function parameters and coagulation parameters in sepsis are shown in Figure 3. The thyroid function parameters of FT3, FT4 and TSH do not have strong correlations with coagulation parameters of platelet count, fibrinogen, FDP, D-dimers, PT, APTT and INR in sepsis. There is only significantly weak correlation between FT3 and FDP (Pearson coefficient −0.17); FT3 and D-dimers (Pearson coefficient −0.18); FT3 and PT (Pearson coefficient −0.16); FT3 and APTT (Pearson coefficient −0.19); and weak correlation between FT4 and D-dimers (Pearson coefficient −0.15); FT4 and APTT (Pearson coefficient −0.16).
Discussion
To our knowledge, this is the first study to investigate the association between NTIS and DIC in sepsis. It is found that, after adjustment for potential confounders, NTIS is an independent risk factor for DIC in sepsis. Moreover, DIC patients with NTIS have higher incidence of death than those without NTIS, which indicates the higher severity and worse prognosis in this subgroup.
NTIS was first described in 1970s and manifested as changes in the level of serum thyroid hormones without concomitant thyroid diseases.18 In previous reports, it was estimated about 40% critical illnesses in ICU complicated with NTIS.19 This number is close in our study that, 37.4% of all the enrolled septic patients are diagnosed with NTIS on admission. It was well known that NTIS has a negative impact on the prognosis of the disease with a relevant increased risk of death. However, even though the underlying pathophysiological mechanism has been clarified, the question that whether the NTIS represents a beneficial adaptive response to stress or a maladaptive response to illness that requires treatment remains unanswered.20 In this study, NTIS is an independent risk factor for DIC after adjustment for confounders. DIC is an acquired thrombohemorrhagic syndrome marked by extensive uncontrolled activation of coagulation with intravascular micro-thrombosis and progressive depletion of coagulation factor. Generally, patients suffer more severe illnesses have higher chance to be complicated with DIC. In previous studies, it was reported that liver function, specific infection site and delayed use of antibiotics are risk factors for DIC in septic patients.21 The association between DIC and specific hematological cancer or metabolic diseases were also described in some studies but not in septic population.22,23 In this study, we adjusted factors including demographic characteristics, severity, infection indicators and organ function indicators in the multivariate logistic regression model, and found that NTIS is an independent risk factor for DIC in sepsis. Other risk factors include sex, history of hypertension, specific infection site, white blood cell count, alanine aminotransferase level, creatinine level and procalcitonin level. The influence of thyroid function on the coagulation-fibrinolytic system has been appreciated in previous study. Overt hypothyroidism is reported to have an increased risk of bleeding, and overt hyperthyroidism is reported to have an increased risk of thrombosis.24 In a prospective cohort study, Sevim et al reported that bleeding time, PT, APTT and clotting time are increased in patients with overt hypothyroidism compared with euthyroid controls. Moreover, the bleeding time, PT, APTT and clotting time are decreased after overt hypothyroidism was treated with levothyroxine.25 As a contrast, in another retrospective study, patients with overt hyperthyroidism have shortened APTT and higher fibrinogen level.26 However, in our study, thyroid hormone levels are only weakly associated with coagulation parameters such as PT, APTT, and D-dimers. It seems unlikely that the impact of thyroid hormones in “low T3 syndrome” status has a direct impact on coagulation process in sepsis. Another explanation may be the changed fibrinolytic system in NTIS. The fibrinolytic system could automatically counteract the thrombosis as the clot starts to form. However, the activity of antifibrinolytic factors such as PAI-1, thrombin activatable fibrinolysis inhibitor (TAFI) or other prothrombotic mediators may be impaired in sepsis.27 Thyroid hormones are reported to be associated with the activity of PAI-1 and TAFI.28,29 Anti-thrombin III is a potent inhibitor of the coagulation cascade and takes part in the anticoagulatory process in septic patients. Anti-thrombin III level is significantly decreased in the septic animal model, and supplement of T3 could protect septic rats from DIC through increasing level of anti-thrombin III,30 which may be one explanation for the increased risk of DIC in septic patients with NTIS in this study.
It is well recognized that DIC is associated with worse outcomes in septic patients. In our study, DIC patients have significantly higher 28 days mortality compared with those septic patients without DIC (19.3% vs 10.8%). Given that coagulation processes contribute to the thrombotic events in sepsis, it is logical that drugs that could modulate the coagulation processes might influence outcomes. A series of randomized controlled trials with anticoagulant treatment or coagulation inhibitors treatment solely were conducted but failed to prove benefits in sepsis. Vincent et al reported the results of the SCARLET trial, in which septic patients diagnosed with sepsis-associated coagulopathy were treated with recombinant human soluble thrombomodulin, but the difference of 28 days mortality is not significantly different between groups.31 In 2009, Jaimes et al reported the trial results of the effect of heparin on the 28 days mortality in sepsis, but there was no difference on the outcome.10 Meta-analyses and systematic reviews have been published, and interestingly, Wang et al reported that heparin may have therapeutic effects in patients with severe sepsis, but it has no effect on patients with non-severe sepsis.32 Similarly, in the analysis of a multicenter nationwide retrospective cohort study, anticoagulant treatment could effectively reduce mortality only in subgroups of patients with very severely ill.11 In our study, DIC patients with NTIS have both higher APACHEII score (median 15 vs 11) and higher mortality (23.2% vs 14.0%) compared with the other DIC patients. If this specific group of patients could get benefit from anticoagulants or the combination treatment of endocrine and coagulation treatment, it is worth to be considered in future studies.
There are several limitations in this study. First, although the data in this study were prospectively collected, this remains a retrospective study, and missing data is inevitable. Even though we do our best to fill the missing value with multiple imputations, the bias still exists. Second, there are several scoring systems to diagnose DIC, but there is no gold standard so that misdiagnosis of DIC is probably inevitable We choose ISTH diagnostic criteria for diagnosing DIC in this study, because it provides high specificity in diagnosing DIC comparing with the other scoring systems.33 Third, there are many drugs that could influence the thyroid function test results in sepsis, such as dopamine and glucocorticoids, which could reduce TSH levels and the peripheral conversion of FT4 to FT3.34 We did not exclude patients using these drugs when diagnosing NTIS, because these drugs are necessary for patients with severe sepsis. So the patients diagnosed with NTIS in this study may not definitely satisfy the diagnosis of NTIS, but reflect the real situation in real world. Fourth, the long-term survival data is absent in this study.
Conclusions
In conclusion, this is the first study that reports NTIS as an independent risk factor for DIC in adults with sepsis. DIC patients with NTIS have higher rate of 28 days mortality than those without NTIS. Future study on DIC should pay attention on this subgroup of patients for severity stratification or precise treatment.
Data Sharing Statement
The data will be shared upon request from the corresponding authors.
Ethics Approval and Consent to Participate
This study was approved by the institutional ethics board of Ruijin Hospital, Shanghai Jiaotong University School of Medicine and Xinhua Hospital, Shanghai Jiaotong University School of Medicine. As a non-interventional retrospective study, informed consent was waived by the Ethical committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine and Xinhua Hospital, Shanghai Jiaotong University School of Medicine. The study was performed in accordance with the 1964 Helsinki Declaration and its later amendments.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funds, grants, or other support was received.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in us hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi:10.1001/jama.2017.13836
2. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. New Engl J Med. 2017;376:2235–2244. doi:10.1056/NEJMoa1703058
3. Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34:77–84. doi:10.1097/ACO.0000000000000963
4. Simmons J, Pittet J-F. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28(2):227–236. doi:10.1097/ACO.0000000000000163
5. Gando S, Shiraishi A, Yamakawa K, et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182–188. doi:10.1016/j.thromres.2019.04.025
6. Levi M. The coagulant response in sepsis. Clinics Chest Med. 2008;29:627–642. doi:10.1016/j.ccm.2008.06.006
7. Wong HR. Intensive care medicine in 2050: precision medicine. Intensive Care Med. 2017;43:1507–1509. doi:10.1007/s00134-017-4727-y
8. Papathanakos G, Andrianopoulos I, Xenikakis M, et al. Clinical sepsis phenotypes in critically ill patients. Microorganisms. 2023;2023:11.
9. Zarychanski R, Doucette S, Fergusson D, et al. Early intravenous unfractionated heparin and mortality in septic shock. Crit Care Med. 2008;36:2973–2979. doi:10.1097/CCM.0b013e31818b8c6b
10. Jaimes F, De La Rosa G, Morales C, et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (The HETRASE Study). Crit Care Med. 2009;37:1185–1196. doi:10.1097/CCM.0b013e31819c06bc
11. Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Critical Care. 2016;20:229. doi:10.1186/s13054-016-1415-1
12. Ning N, Li J, Sun W, et al. Different subtypes of nonthyroidal illness syndrome on the prognosis of septic patients: a two-centered retrospective cohort study. Front Endocrinol. 2023;14:1227530. doi:10.3389/fendo.2023.1227530
13. Kim JG, Shin H, Kim W, et al. The value of decreased thyroid hormone for predicting mortality in adult septic patients: a systematic review and meta-analysis. Sci Rep. 2018;8:14137. doi:10.1038/s41598-018-32543-7
14. Wang Y, Sun F, Hong G, et al. Thyroid hormone levels as a predictor marker predict the prognosis of patients with sepsis. Am J Emergency Med. 2021;45:42–47. doi:10.1016/j.ajem.2021.02.014
15. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–810. doi:10.1001/jama.2016.0287
16. Taylor FB, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi:10.1055/s-0037-1616068
17. Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest. 2021;44:1597–1607. doi:10.1007/s40618-020-01482-4
18. Bermudez F, Surks MI, Oppenheimer JH. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975;41:27–40. doi:10.1210/jcem-41-1-27
19. Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239–244. doi:10.1016/j.metabol.2006.09.020
20. Gheorghita V, Barbu AE, Gheorghiu ML, et al. Endocrine dysfunction in sepsis: a beneficial or deleterious host response? Germs. 2015;5:17–25. doi:10.11599/germs.2015.1067
21. Rinaldi I, Sudaryo MK, Prihartono NA, Hebelka H, Lagerstrand KM. Disseminated intravascular coagulation in sepsis and associated factors. J Clin Med. 2022;12:11. doi:10.3390/jcm12010011
22. Levi M. Disseminated intravascular coagulation in cancer: an update. Semin Thromb Hemost. 2019;45:342–347. doi:10.1055/s-0039-1687890
23. Nogami K, Muraki I, Imano H, et al. Risk of disseminated intravascular coagulation in patients with type 2 diabetes mellitus: retrospective cohort study. BMJ open. 2017;7:e013894.
24. Squizzato A, Romualdi E, Buller HR, et al. Clinical review: thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. J Clin Endocrinol Metab. 2007;92:2415–2420. doi:10.1210/jc.2007-0199
25. Gullu S, Sav H, Kamel N. Effects of levothyroxine treatment on biochemical and hemostasis parameters in patients with hypothyroidism. Europ J Endocrinol. 2005;152:355–361. doi:10.1530/eje.1.01857
26. Lippi G, Franchini M, Targher G, et al. Hyperthyroidism is associated with shortened APTT and increased fibrinogen values in a general population of unselected outpatients. J Thromb Thrombol. 2009;28:362–365. doi:10.1007/s11239-008-0269-z
27. Hayakawa M, Sawamura A, Gando S, et al. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb Res. 2012;130:906–913. doi:10.1016/j.thromres.2012.01.015
28. Lupoli R, Di Minno MN, Tortora A, et al. Primary and secondary hemostasis in patients with subclinical hypothyroidism: effect of levothyroxine treatment. J Clin Endocrinol Metab. 2015;100:2659–2665. doi:10.1210/jc.2015-1726
29. Akinci B, Comlekci A, Ali Ozcan M, et al. Elevated thrombin activatable fibrinolysis inhibitor (TAFI) antigen levels in overt and subclinical hypothyroid patients were reduced by levothyroxine replacement. Endocr J. 2007;54:45–52. doi:10.1507/endocrj.K06-062
30. Chapital AD, Hendrick SR, Lloyd L, et al. The effects of triiodothyronine augmentation on antithrombin III levels in sepsis. Am Surg. 2001;67:253–255. doi:10.1177/000313480106700310
31. Vincent JL, Francois B, Zabolotskikh I, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA. 2019;321:1993–2002. doi:10.1001/jama.2019.5358
32. Wang C, Chi C, Guo L, et al. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Critical Care. 2014;18:563. doi:10.1186/s13054-014-0563-4
33. Takemitsu T, Wada H, Hatada T, et al. Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thromb Haemost. 2011;105:40–44. doi:10.1160/TH10-05-0293
34. Surks MI, Sievert R. Sievert R Drugs and thyroid function. New Engl J Med. 1995;333:1688–1694. doi:10.1056/NEJM199512213332507
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

