Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Anhedonia in Depression and Schizophrenia: Brain Reward and Aversion Circuits
Authors Liang S, Wu Y , Hanxiaoran L, Greenshaw AJ, Li T
Received 24 March 2022
Accepted for publication 28 June 2022
Published 7 July 2022 Volume 2022:18 Pages 1385—1396
DOI https://doi.org/10.2147/NDT.S367839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Sugai Liang,1,* Yue Wu,1,* Li Hanxiaoran,1,* Andrew J Greenshaw,2 Tao Li1
1Affiliated Mental Health Centre & Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310013, People’s Republic of China; 2Department of Psychiatry, University of Alberta, Edmonton, AB, T6G 2B7, Canada
*These authors contributed equally to this work
Correspondence: Tao Li, Affiliated Mental Health Centre & Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310013, Zhejiang, People’s Republic of China, Tel +86 571 85126501, Fax +86 571 85121532, Email [email protected]
Abstract: Anhedonia, which is defined as markedly diminished interest or pleasure, is a prominent symptom of psychiatric disorders, most notably major depressive disorder (MDD) and schizophrenia. Anhedonia is considered a transdiagnostic symptom that is associated with deficits in neural reward and aversion functions. Here, we review the characteristics of anhedonia in depression and schizophrenia as well as shared or disorder-specific anhedonia-related alterations in reward and aversion pathways of the brain. In particular, we highlight that anhedonia is characterized by impairments in anticipatory pleasure and integration of reward-related information in MDD, whereas anhedonia in schizophrenia is associated with neurocognitive deficits in representing the value of rewards. Dysregulation of the frontostriatal circuit and mesocortical and mesolimbic circuit systems may be the transdiagnostic neurobiological basis of reward and aversion impairments underlying anhedonia in these two disorders. Blunted aversion processing in depression and relatively strong aversion in schizophrenia are primarily attributed to the dysfunction of the habenula, insula, amygdala, and anterior cingulate cortex. Furthermore, patients with schizophrenia appear to exhibit greater abnormal activation and extended functional coupling than those with depression. From a transdiagnostic perspective, understanding the neural mechanisms underlying anhedonia in patients with psychiatric disorders may help in the development of more targeted and efficacious treatment and intervention strategies.
Keywords: anhedonia, reward pathway, aversion circuit, depression, schizophrenia
Introduction
Anhedonia, which is characterized by a loss of interest or pleasure, reflects deficits in hedonic capacity and is closely related to the constructs of reward valuation, decision making, anticipation, and motivation.1 Anhedonia is considered a transdiagnostic symptom that is associated with deficits in reward and aversion processing and is especially present in patients with major depressive disorder (MDD) and schizophrenia.2–4 In addition, anhedonia is linked to greater severity of clinical symptoms, poorer treatment response, and poorer clinical outcomes in patients with these two disorders.5–7 Anhedonia is a multidimensional construct that includes anticipatory anhedonia (inability to anticipate rewards), consummatory anhedonia (impairments in hedonic response to rewards), and motivational anhedonia (diminished motivation to pursue rewards).8 The constructs of anhedonia have common and dissociable neural underpinnings.9
Anhedonia is a core feature of MDD.10 Patients with MDD have deficits in motivation for rewards owing to low anticipatory pleasure and reduced ability to modulate behavior in response to intermittent rewards.11,12 In contrast, anhedonia in patients with schizophrenia is one of the cardinal negative symptoms and is independent of positive, disorganized, and depressive dimensions.13 As defined in the “Schizophrenia Spectrum Anhedonia Paradox”, hedonic capacity is impaired in individuals with schizotypy and youth at clinical high-risk, whereas it appears intact in patients with schizophrenia. Moreover, a higher frequency of anhedonia is detected during the chronic phase of schizophrenia than during the early phase of illness.14 Anhedonia in schizophrenia may stem from deficits in integrating and maintaining representations of hedonic values, which results in impairments in anticipatory pleasure and goal-directed behavior.15
Previous findings suggest that a common neural basis and genetic factors underlie anhedonia, which transcends the disorder categories of depression and schizophrenia.16–18 Consistent with the Research Domain Criteria initiative focused on transdiagnostic dimensions of psychopathology, there is a crucial need for a more detailed approach to investigate specific properties at the symptom level.19 A better understanding of the precise psychopathological and neurobiological underpinnings that are highly relevant to anhedonia is a necessary step to develop more effective treatment plans for psychiatric disorders.15 The transnosographic method is a promising approach for revealing the overall neurobiological framework that contributes to clinical symptomology and may help improve targeted treatment strategies.20 Notably, neuroimaging can be a powerful tool for investigating the neurobiological mechanisms of anhedonia. Numerous studies have provided evidence for the neurobiological reward and aversion systems underlying anhedonia.19,21–23 Investigating the reward and aversion pathways of the brain may help improve our understanding of the neurological substrates underlying the clinical transdiagnostic symptomatology of anhedonia. In this context, developing interventions to treat anhedonia across different psychiatric disorders could be targeted according to the shared neural abnormalities of critical brain circuits.23,24
Anhedonia is a prominent symptom in patients with MDD and schizophrenia. The characteristics of anhedonia across these two disorders, as well as anhedonia-related reward and aversion neural circuits, have not been extensively explored from a transdiagnostic perspective. Therefore, this review aimed to summarize the neurobiological mechanisms underlying anhedonia based on the reward and aversion neural pathways in depression and schizophrenia. Furthermore, we highlight recent findings of aversion circuits associated with anhedonia in these two disorders.
Brain Reward and Aversion Circuits
Reward and aversion are two major components of motivational control.25 Impaired reward and aversion processing is relevant to anhedonia.1,25,26 Anhedonia comprises behavioral deficits in three reward processing subtypes: reward liking, reward wanting, and reward learning.9 Previous findings have indicated that reward processing involves the prefrontal cortex (PFC), anterior cingulate cortex (ACC), striatum, ventral pallidum, ventral tegmental area (VTA), substantia nigra (SN), amygdala, and insula.1,26 Moreover, abnormal dopaminergic and glutamatergic functions within the VTA and nucleus accumbens (NAc), which are key regions of the reward neural pathway, are associated with anhedonia.27 Low ventral striatum activation during reward anticipation related to anhedonia can predict the transition to depression in previously healthy adolescents.28 In addition, inflammation and cytokines may reduce neural activity and dopamine release in reward-related corticostriatal regions in association with anhedonia and reduced motivation.29,30 Furthermore, early and chronic life adversities may downregulate mesolimbic dopamine signaling and frontostriatal activity linked with anhedonia.21,31
Aversion is derived from an internal feeling of avoidance behavior to avoid potentially harmful stimuli.32 Emerging evidence indicates that the key brain regions of the aversion pathway include the lateral habenula (LHb), medial PFC (mPFC), ventral pallidum, periaqueductal gray matter, VTA, amygdala, and insula.33–36 The LHb is regarded as an anti-reward center of the brain, which is involved in aversion and mood regulation via the modulation of the dopamine and serotonin systems.37,38 During aversive events, healthy individuals exhibit increased activity of the habenula–VTA pathway, coupling of the putamen and mPFC, and reduced activity between the SN and external segment of the globus pallidus.36 Previous findings from rodents have suggested that glutamatergic neurons in the lateral hypothalamic area mediate aversive signals via the projections to the LHb.33 Moreover, in rats, photostimulation of dopamine terminals in the mPFC induced increased signal-to-noise ratio in neurons projecting from the mPFC to the dorsal periaqueductal gray in response to aversive stimuli.34 Additionally, chronic stress has been shown to trigger social aversion in mice via glucocorticoid receptors in VTA dopaminoceptive neurons.31 It has been shown that in mice, the activity of neurons that project from the anterior insula to the basolateral amygdala is responsible for forming and retrieving aversive taste memory.39 The anterior insula is involved in encoding signals of aversive cues and outcomes in humans,40 and the sublenticular/extended amygdala is involved in the processing of both aversive and positive stimuli.41 Attenuated amygdala processing of positive stimuli has been shown to be related to greater anhedonia in patients with MDD.42
Reward and Aversion Circuits and Anhedonia in MDD
MDD is a disorder with considerable heterogeneity with a broad constellation of presentations and symptoms. Anhedonia, which is defined as reduced interest or pleasure in all or almost all previously enjoyed activities, is a hallmark symptom of MDD.10 It affects more than half of individuals with current MDD.13,43 In patients with MDD, anhedonia is characterized by impairments in anticipatory pleasure and integration of reward-related information from past experiences22 and is related to greater severity of clinical symptoms and poorer treatment response.5,7 Anhedonia has emerged as a key dimension that predicts recovery and is related to a longer time to remission, especially in young adults with depression.5
Reward Circuits and Anhedonia in MDD
Previous research has demonstrated that structural and functional alterations in the reward pathways of the brain are highly relevant to anhedonia and aberrant reward-related perception and memory in depression.9,44 The key brain areas of the reward pathway related to anhedonia in depression include the ventral and dorsal striatum, ventromedial PFC (vmPFC), orbitofrontal cortex (OFC), and ACC.30,45,46 Structural magnetic resonance imaging (MRI) studies have reported that anhedonia in depression is associated with reduced gray matter volume in the OFC and caudate nucleus.18,19 In addition, diffusion tensor imaging studies have reported that abnormal structural connectivity of the reward network is related to anhedonia in patients with depression.47,48 Microstructural alterations in the segment of the superolateral medial forebrain bundle connecting the VTA with the medial OFC are related to anhedonia and depression severity.47 Moreover, aberrant white matter microstructural integrity of the cingulum and uncinate fasciculus is negatively correlated with anhedonia in patients with depression.49–51 Furthermore, disrupted structural connectivity between the bilateral anterior thalamic radiation and the left corticospinal tract is significantly linked to the severity of anticipatory anhedonia in MDD patients.48
Previous findings suggest that the frontostriatal and mesocorticolimbic circuit systems are involved in anhedonia-related reward processing in patients with depression.9,16,52 Resting-state functional MRI (fMRI) research has revealed that a biotype of depression that is characterized by hyperconnectivity of the frontostriatal and thalamic networks is associated with anhedonia and psychomotor retardation.53 In addition, reduced functional connectivity (FC) and regional homogeneity within the ventral striatum and vmPFC are correlated with greater anhedonia in MDD patients,30,54 and decreased functional coupling between NAc subregions and frontoparietal areas is also linked to anhedonia in patients with depression.45 Moreover, the constructs of anhedonia in depression appear to have dissociated neural underpinnings; increased intrinsic function of the left dorsal ACC and reduced cortical thickness of the left rostral ACC and lateral OFC are respectively correlated with anticipatory and consummatory anhedonia.55
Task fMRI research has demonstrated that when encountering pleasurable stimuli, the aberrant connectivity between the posterior vmPFC and the mesolimbic reward system is negatively correlated with anhedonia in patients with MDD.56 When individuals with depression receive an unexpected reward, they exhibit abnormal frontostriatal hypoactivation, especially in the OFC and ventral striatum.46 Moreover, reduced activation in the ventral striatum during reward anticipation is correlated with anhedonia and depression severity in patients with MDD.16,52 Reward anticipation is assessed by measuring prediction error, which is defined as the response to the discrepancy between anticipated and received rewards.57 NAc activity is associated with an inverse correlation between reward anticipation and prediction error in healthy controls, and a lower correlation may predict greater anhedonia in individuals with MDD.58 In addition, reduced neural reward prediction-error signaling in the medial OFC and ventral striatum is inversely correlated with anhedonia severity, which reflects reward processing deficits in MDD.59 During reward processing tasks, reward liking and reward wanting in depression is associated with striatal hypoactivation, alongside mPFC and dorsolateral PFC hyperactivation and OFC hypoactivation, whereas reward learning is related to blunted frontostriatal sensitivity to positive feedback.9 Mapping activation and connectivity patterns of reward networks may help understand the neural basis of reward deficits associated with anhedonia in patients with MDD.23 The identification of brain functional circuits linked to anhedonia may enable a better understanding of the heterogeneity of MDD and help track one of its core symptoms.56
Aberrant metabolite status of neurotransmitters in reward processing regions has also been implicated in reward deficits and anhedonia.54,60,61 In depression, dysfunction of the ACC in the reward neural circuitry is highly relevant to anhedonia.60,62,63 Reduced glutamine and γ-aminobutyric acid (GABA) levels in the pregenual ACC are associated with anhedonia in adolescents with depression.60,62 Additionally, glutamine/glutamate imbalance in the rostral ACC is associated with anhedonia in depression patients.63 Moreover, a subtype of depression, characterized by increased peripheral inflammation and glutamate level in the left basal ganglia, has been reported to be associated with anhedonia and reduced network integrity within reward processing regions.54 Neuroinflammation and oxidative stress likely contribute to reductions in glutathione in the occipital cortex, which results in glutamate and dopamine dysregulation; this, in turn, affects the reward circuitry and induces anhedonia in patients with MDD.61 Thus, reward deficits, alongside functional and neurochemical alterations within and beyond the reward circuitry, may give rise to anhedonia in depression patients.
Aversion Circuits and Anhedonia in MDD
The neural basis of anhedonia is closely related to dysfunctional aversion circuits. LHb is a key brain structure for mediating behavioral responses to aversive stimuli.64 In rodents, increased expression of a specific calcium protein kinase in the LHb mediates depressive behaviors, such as anhedonia and despair behavior.65 Stimulation of LHb neurons establishes connections with distinct subpopulations of VTA neurons and triggers aversion-associated behavior in mice; thus, the dysregulation of the LHb–VTA pathway may be a key mechanism underlying aversion processing deficits and depression pathogenesis.4 Additionally, individuals with MDD have larger habenula volumes and greater left habenula activation, which correlate with the severity of depressive symptoms and anhedonia.66
The brain circuits that mediate aversive processing in depression patients include the PFC, amygdala, and caudate.42,67,68 Patients with MDD show greater amygdala activation in response to negative than positive facial expressions, and reduced amygdala responsiveness to positive stimuli is associated with higher physical anhedonia scores.42 Patients who have recovered from depression have abnormal neural responses, whereby activation in the caudate nucleus while viewing aversive stimuli is increased, and neural responses in the PFC to both pleasant and aversive conditions are diminished.68 Moreover, adolescents at a high risk of depression show attenuated neural responses to aversive stimuli, with a decrease in activation of the vmPFC and pregenual ACC.67 In line with the emotion context insensitivity theory of depression, blunted aversion observed both before depression onset and during the residual phase may be a trait marker of the illness.67–69
Treatments for Anhedonia in Patients with MDD
Anhedonia and cognitive deficits are typically resistant to first-line antidepressant treatments.7 Previous clinical studies have suggested that selective serotonin reuptake inhibitors are ineffective for anhedonia.5,70 Vortioxetine is a multimodal-acting antidepressant that may be effective in ameliorating anhedonia, especially in female patients with MDD.71 Ketamine may rapidly alleviate anhedonia in depression patients because of its direct effect on mitochondrial energy metabolism.72 In addition, kappa-opioid receptor antagonists that target the ventral striatum, one of the core hubs of the reward system, may improve the rate of reward learning and alleviate anhedonia.73 Bupropion is a dopaminergic and noradrenergic reuptake inhibitor and has been offered as a treatment for reward-related deficits and blunted affect in patients with MDD and may increase neural responses to anticipation, effort and consummation of rewards, and aversive stimuli.74 However, despite the growing number of studies on treatments, the efficacy of interventions remains unsatisfactory. Besides, new psychological treatments such as positive affect treatment and positive affect stimulation and sustainment that target the Positive Valence Systems are providing promise for anhedonia.75 Abnormal neural responses to rewards and aversion that are associated with anhedonia may be potential targets for intervention and prevention strategies for depression. Moreover, understanding the neural substrates of anhedonia in depression patients is vital for identifying neurobiological treatment markers.
Reward and Aversion Circuits and Anhedonia in Schizophrenia
Anhedonia is considered a biological vulnerability marker of schizophrenia spectrum pathology.13 In schizophrenia, anhedonia has been reported to vary in prevalence from 41%, using questionnaires, to more than 80%, when assessed via clinical interviews.76,77 Anhedonia has a detrimental effect on the functioning and quality of life in individuals with schizophrenia.13 The negative symptoms of schizophrenia, particularly anhedonia and avolition, may reflect a difficulty in representing the value of rewarding experiences in cognition and working memory.78 Investigating the neural correlates of anhedonia in patients with schizophrenia may provide valuable insight into the pathophysiology of negative symptoms.79
The anhedonia phenotype in schizophrenia reflects a degree of “disorganization” within the reward system due to disrupted cognition and aberrant stimulus processing.22 In schizophrenia, there is a synergistic interaction between deficits of neurocognition and reward processing.80 Anhedonia in schizophrenia may be driven more by neurocognitive impairments in representing reward valuation than by hedonic deficits in response to savoring rewards in the moment.21 In addition, motivational deficits in schizophrenia are linked to a compromised ability to form adaptive representations of expected value.17 While effort expenditure deficits in depression are associated with reduced reward responsivity.2 From the perspective of consummatory anhedonia, patients with schizophrenia appear to have unaltered reward liking, which is in contrast to those with depression.43
Reward Circuits and Anhedonia in Schizophrenia
Anhedonia in patients with schizophrenia is associated with dysregulation of the dopaminergic system and neural reward circuitry.17,80,81 Previous reports have shown that disruption of microstructural integrity of the cingulum bundle, which mediates dorsal limbic system integration, is related to anhedonia in individuals with psychosis.82,83 Additionally, fractional anisotropy values of the superior longitudinal fasciculus II, a major frontoparietal white matter tract, are correlated with trait anhedonia in patients with schizophrenia.83 It has also been reported that aberrant properties of fiber tracts connecting the medial OFC to the rostral ACC are associated with greater anhedonia in patients with schizophrenia.84 Similar to the functional coupling observed in patients with schizophrenia, healthy controls with social anhedonia show decreased FC between the retrosplenial cortex, a region of default mode network (DMN), and the right fusiform gyrus.85 Moreover, anhedonia is negatively correlated with the basal cerebral blood volume of the OFC in individuals at clinical high risk of psychosis.86 Furthermore, a positron emission tomography study found that medial prefrontal default-mode hypoactivity is correlated with physical anhedonia in schizophrenia patients.87
Previous studies have revealed that anhedonia in patients with schizophrenia is attributed to the dysregulation of the frontostriatal circuit and mesocortical and mesolimbic circuit systems.17,22,46,79 Reduced OFC and putamen/ventral striatum activity during reward anticipation is linked to greater anhedonia and depressive symptoms in patients with schizophrenia.79,88 Additionally, activation of the ACC and mPFC during receipt of an unexpected reward predicts task-related motivation, which is associated with the severity of anhedonia in patients with schizophrenia.46 The motivational deficits of schizophrenia are thought to result from a reduced ability to differentiate between signal gains and instances of loss-avoidance, which are associated with the dysfunction of the frontostriatal pathway, including the vmPFC, dorsal ACC, anterior insula, and ventral striatum.17 Furthermore, patients with schizophrenia exhibit an inverse correlation between anhedonia-asociality and posterior cingulate and precuneus activity, a key part of the DMN, during an auditory oddball task.89 Dysfunction of the striatum, cortex, and limbic regions and impaired integration of the reward networks may also lead to anhedonia in patients with schizophrenia.80
Aversion Circuits and Anhedonia in Schizophrenia
Aversion circuits play an important role in the development of anhedonia in patients with schizophrenia. Patients with first-episode schizophrenia exhibit more prominent impairments in emotion–behavior coupling for aversion-avoidance behavior than healthy controls.90 In addition, schizophrenia patients show increased aversion to angry faces during a reward learning task using emotional face stimuli.91 Moreover, patients with schizophrenia exhibit relatively strong aversive emotions toward stimuli considered pleasant or neutral by others.92 In a neuroimaging study, schizophrenia patients have lower gray matter volume in the bilateral habenula and enhanced functional coupling between the right habenula and subcortical regions related to the dopaminergic reward pathways, which include the left ventral striatum, caudate, and putamen.93 Aberrant habenula activity in response to unexpected negative outcomes has also been shown to be associated with the mediation of feedback-processing deficits in patients with schizophrenia.64,94
In patients with schizophrenia, ventral limbic regions show attenuated deactivation in response to target versus aversive events, and activation of the ACC in response to aversive images is inversely correlated to the severity of anhedonia and avolition symptoms.95 Additionally, functional coupling of the striatal-amygdala network is positively correlated with the severity of anhedonia-asociality and negatively associated with oxytocin receptor-gene methylation in female patients with schizophrenia.96 Furthermore, patients with schizophrenia exposed to unpleasant odors fail to activate limbic regions, which include the insula, parahippocampal gyrus, and NAc, and abnormalities in these regions are associated with the neural substrates of anhedonia in patients with schizophrenia.97
Treatments for Anhedonia in Patients with Schizophrenia
Clinical research findings indicate that patients with schizophrenia are less responsive than those with depression to treatments for anhedonia.13 For schizophrenia, atypical antipsychotics are superior to typical antipsychotics in reducing negative symptoms, such as anhedonia; however, none of them have achieved the threshold for clinically significant improvement.98 Intermittent theta-burst stimulation over the dorsomedial PFC has also been shown to have little effect on ameliorating anhedonia in patients with schizophrenia.99 Thus, anhedonia in schizophrenia patients cannot be effectively treated with current treatments, which highlights the crucial need for more effective interventions.22 Anhedonia is considered a trait-marker of schizophrenia and is highly relevant to the dysfunction of reward and aversion systems.22,91,95 Therefore, elucidating the neurobiological mechanisms underlying anhedonia may help in the identification of potential treatment targets for schizophrenia.
Transdiagnostic Brain Alterations in MDD and Schizophrenia
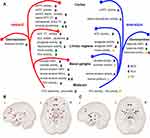
Anhedonia is recognized as a transdiagnostic symptom of depression and schizophrenia and is linked to deficits in the reward and aversion systems (Figure 1). Anhedonia shares common neurobiological alterations of the frontostriatal network and mesocorticolimbic circuits for reward and aversion processing in both patients with MDD and schizophrenia.1,22,24,46,100,101 A transdiagnostic meta-analysis reported that consummatory anhedonia is associated with decreased activation of the ventral basal ganglia region in both disorders, and anticipatory anhedonia is linked to areas of the frontostriatal circuitry, which include the ventral striatum, dorsal ACC, middle frontal gyrus, and medial frontal gyrus.24 Additionally, in MDD and schizophrenia patients, reward deficits are associated with hypoconnectivity between the NAc and the DMN and hyperconnectivity between the NAc and the cingulo-opercular network (CON); moreover, reward responsivity impairments are associated with DMN hyperconnectivity and diminished connectivity between the DMN and CON.102 Volumetric abnormalities in the putamen and cerebellum have been reported to negatively correlate with anhedonia scores in both MDD and schizophrenia patients, which suggests that volumetric alterations within the putamen–cerebellum network mediate reward-related goal-directed behaviors in both disorders.103 Inflammation and cytokines may affect dopamine neurotransmission, which mediates several aspects of anhedonic behavior.29 In addition to the dysfunction of dopaminergic signaling, dysregulation of glutamate and serotonin are also involved in anhedonia.58,80,89 Furthermore, anhedonia has shared genetic influences across multiple diagnostic categories, and the genetic risk of anhedonia also influences brain structure, especially regions associated with reward and pleasure processing.18,104 Thus, the shared genetic and inflammation factors may contribute to the common alterations in the reward and aversion pathways, which results in the manifestation of anhedonia in patients with depression and schizophrenia.
Dissociable or disorder-specific alterations in brain pathways are also linked to anhedonia among different clinical diagnostic categories.23 The spatial distribution of reward processing regions differs between depression and schizophrenia patients during reward learning; patients with MDD exhibit reduced prediction error signaling in the striatum and midbrain, whereas those with schizophrenia show reduced prediction signaling in the dorsal striatum, thalamus, and limbic regions.105 Moreover, abnormal prediction error encoding in MDD patients gives rise to anhedonia symptoms by attenuating reward learning events, whereas disturbed signal encoding in schizophrenia patients contributes to psychotic symptoms by driving aberrant salience toward external and internal stimuli.105,106 Depression and schizophrenia likely reflect illness-specific neural valuation and incentive salience formation associated with reward and aversion processing.105,106 Both depression and schizophrenia groups show reduced activation in the mPFC in response to unexpected rewards, with activation being significantly more aberrant in schizophrenia patients than in depression patients.46 The severity of depressive symptoms in patients with schizophrenia negatively correlates with ventral striatum activation during the receipt of a reward, which indicates that impaired hedonic reward processing contributes to the development of depressive symptoms in schizophrenia.81,88 Furthermore, from a cellular perspective, dopaminergic neurons are reclassified according to specific projection subtypes and thus may contribute uniquely to the processing of rewards and aversion.32 In addition to the heterogeneous cellular structure, dopaminergic, GABAergic, and glutamatergic neurons engage in complex interactions to modulate network activity.35 Thus, adaptations of both dopaminergic and glutamatergic functions within the VTA and NAc may differ in directionality according to cell type and stress paradigm.27
Future Directions and Conclusion
Anhedonia is a prominent symptom of depression and schizophrenia. A transnosographic approach is a promising method for revealing the overall neurobiological framework on and beyond reward and aversion circuits relevant to anhedonia.20 Taking into account the heterogeneity of individual differences, identifying biologically homogenous subtypes tied to multi-faceted anhedonia will be a method to differentiate depression from schizophrenia and improve the early diagnosis and treatment in these two disorders.54 In addition, to define symptomology related neuroimaging biomarkers, it is significant to investigate the shared brain circuits and specific alterations between anhedonia and other negative symptoms and depressive symptoms in psychiatric disorders, especially in schizophrenia. Moreover, the future studies require to define the neuroimaging biomarkers and peripheral phenotype features which have the ability to represent anhedonia and suggest specific treatment options, such as medication, transcranial magnetic stimulation, transcranial direct current stimulation and psychotherapy.
Previous research has reported that environmental stressors contribute to the development of anhedonia, and it also holds a significant role to disentangle the influences of key dimensions of stress on specific aspects of reward processing in schizophrenia and depression.31,107 Besides, neuroinflammation and peripheral inflammation associated with glutamate and dopamine dysregulation that affects the reward circuitry and induces anhedonia.54,61 Inflammation cytokines interact with tryptophan kynurenine pathway and also affect the formation of neopterin and tetrahy-drobiopterin, which are associated with dysregulation of neurotransmitters and may impede the function of neural circuits related to anhedonia.29 Future research is needed to better understand how inflammation and cytokines regulates reward and aversion circuits through its effects on the dopamine and glutamate systems and leading to anhedonia in schizophrenia and depression. In addition, genetic predisposition to anhedonia also influences brain structure and function associated with reward and aversion processing.18,104 The future studies will enhance the possibility of combining genetic factors, peripheral measures and neuroimaging-based biomarkers relevant to anhedonia in the pathophysiology of depression and schizophrenia.
In this review, we summarized characteristics of anhedonia in depression and schizophrenia, as well as anhedonia-related reward and aversion neural circuits across these two disorders. Symptom-based studies to identify a biomarker may help understand the precise mechanisms of the discrepancies of anhedonia and the underlying reward and aversion pathway deficits in both disorders. The dysfunction of the frontostriatal and mesocorticolimbic circuit systems involved in reward and aversion processing play a critical role in the development of anhedonia in both patients with depression and schizophrenia. Establishing transdiagnostic and/or specific neurobiological alterations in anhedonia and investigating circuit dysregulation in depression and schizophrenia may facilitate the development of more targeted and effective treatment and intervention strategies.
Acknowledgments
The literature on this subject is extensive, and the authors apologize for any omissions of relevant papers. This review was supported in part by grants from the Project for Hangzhou Medical Disciplines of Excellence, the Key Project for Hangzhou Medical Disciplines (No. 2021042001 and No. 2021012901 to TL), and the Science & Technology Development Project of Hangzhou (No. 202004A11 to TL and No. A20210013 to SGL).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi:10.1016/j.tins.2011.11.005
2. Culbreth AJ, Moran EK, Barch DM. Effort-cost decision-making in psychosis and depression: could a similar behavioral deficit arise from disparate psychological and neural mechanisms? Psychol Med. 2018;48(6):889–904. doi:10.1017/S0033291717002525
3. He Z, Jiang Y, Gu S, et al. The aversion function of the limbic dopaminergic neurons and their roles in functional neurological disorders. Front Cell Dev Biol. 2021;9:713762. doi:10.3389/fcell.2021.713762
4. Lammel S, Lim BK, Ran C, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi:10.1038/nature11527
5. McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51(4):404–411. doi:10.1016/j.jaac.2012.01.011
6. Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(S1):35–53. doi:10.1017/S1092852914000601
7. Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand. 2001;103(2):122–130. doi:10.1034/j.1600-0447.2001.103002122.x
8. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi:10.1016/j.neubiorev.2010.06.006
9. Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. 2020;20(4):816–841. doi:10.3758/s13415-020-00804-6
10. American Psychiatric Association D, Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Vol 5. Washington, DC: American psychiatric association; 2013.
11. Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121(1):51–60. doi:10.1037/a0024945
12. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi:10.1097/YCO.0000000000000122
13. Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8(2):22. doi:10.1186/1744-859X-8-22
14. Strauss GP, Cohen AS. The schizophrenia spectrum anhedonia paradox. World Psychiatry. 2018;17(2):221–222. doi:10.1002/wps.20529
15. Pizzagalli DA. The “anhedonia paradox” in schizophrenia: insights from affective neuroscience. Biol Psychiatry. 2010;67(10):899–901. doi:10.1016/j.biopsych.2010.02.022
16. Hägele C, Schlagenhauf F, Rapp M, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232(2):331–341. doi:10.1007/s00213-014-3662-7
17. Waltz JA, Xu Z, Brown EC, Ruiz RR, Frank MJ, Gold JM. Motivational deficits in schizophrenia are associated with reduced differentiation between gain and loss-avoidance feedback in the striatum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(3):239–247. doi:10.1016/j.bpsc.2017.07.008
18. Ward J, Lyall LM, Bethlehem RAI, et al. Novel genome-wide associations for anhedonia, genetic correlation with psychiatric disorders, and polygenic association with brain structure. Transl Psychiatry. 2019;9(1):327. doi:10.1038/s41398-019-0635-y
19. Enneking V, Krüssel P, Zaremba D, et al. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44(5):883–889. doi:10.1038/s41386-018-0283-6
20. Guessoum SB, Le Strat Y, Dubertret C, Mallet J. A transnosographic approach of negative symptoms pathophysiology in schizophrenia and depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109862. doi:10.1016/j.pnpbp.2020.109862
21. Nusslock R, Alloy LB. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3–16. doi:10.1016/j.jad.2017.02.001
22. Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH. Anhedonia in depression and schizophrenia: a transdiagnostic challenge. CNS Neurosci Ther. 2018;24(7):615–623. doi:10.1111/cns.12854
23. Sharma A, Wolf DH, Ciric R, et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry. 2017;174(7):657–666. doi:10.1176/appi.ajp.2016.16070774
24. Zhang B, Lin P, Shi H, et al. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 2016;10(3):920–939. doi:10.1007/s11682-015-9457-6
25. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi:10.1016/j.neuron.2010.11.022
26. Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi:10.1016/j.neuron.2015.02.018
27. Fox ME, Lobo MK. The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry. 2019;24(12):1798–1815. doi:10.1038/s41380-019-0415-3
28. Stringaris A, Vidal-Ribas Belil P, Artiges E, et al. The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172(12):1215–1223. doi:10.1176/appi.ajp.2015.14101298
29. Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: inflammatory mediators of anhedonia. Neurosci Biobehav Rev. 2016;64:148–166. doi:10.1016/j.neubiorev.2016.02.017
30. Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–1365. doi:10.1038/mp.2015.168
31. Barik J, Marti F, Morel C, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339(6117):332–335. doi:10.1126/science.1226767
32. Verharen JPH, Zhu Y, Lammel S. Aversion hot spots in the dopamine system. Curr Opin Neurobiol. 2020;64:46–52. doi:10.1016/j.conb.2020.02.002
33. Lazaridis I, Tzortzi O, Weglage M, et al. A hypothalamus-habenula circuit controls aversion. Mol Psychiatry. 2019;24(9):1351–1368. doi:10.1038/s41380-019-0369-5
34. Vander Weele CM, Siciliano CA, Matthews GA, et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature. 2018;563(7731):397–401. doi:10.1038/s41586-018-0682-1
35. Wulff AB, Tooley J, Marconi LJ, Creed MC. Ventral pallidal modulation of aversion processing. Brain Res. 2019;1713:62–69. doi:10.1016/j.brainres.2018.10.010
36. Hennigan K, D’Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 2015;35(1):198–208. doi:10.1523/JNEUROSCI.0927-14.2015
37. Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12(1):77–84. doi:10.1038/nn.2233
38. Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci. 2011;14(9):1209–1216. doi:10.1038/nn.2902
39. Kayyal H, Yiannakas A, Kolatt Chandran S, Khamaisy M, Sharma V, Rosenblum K. Activity of insula to basolateral amygdala projecting neurons is necessary and sufficient for taste valence representation. J Neurosci. 2019;39(47):9369–9382. doi:10.1523/JNEUROSCI.0752-19.2019
40. Palminteri S, Justo D, Jauffret C, et al. Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron. 2012;76(5):998–1009. doi:10.1016/j.neuron.2012.10.017
41. Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–733. doi:10.1038/sj.npp.1300113
42. Stuhrmann A, Dohm K, Kugel H, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013;38(4):249–258. doi:10.1503/jpn.120060
43. Szczypiński JJ, Gola M. Dopamine dysregulation hypothesis: the common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev Neurosci. 2018;29(7):727–744. doi:10.1515/revneuro-2017-0091
44. Ely BA, Nguyen TNB, Tobe RH, Walker AM, Gabbay V. Multimodal investigations of reward circuitry and anhedonia in adolescent depression. Front Psychiatry. 2021;12:678709. doi:10.3389/fpsyt.2021.678709
45. Liu R, Wang Y, Chen X, Zhang Z, Xiao L, Zhou Y. Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage Clin. 2021;30:102599. doi:10.1016/j.nicl.2021.102599
46. Segarra N, Metastasio A, Ziauddeen H, et al. Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. 2016;41(8):2001–2010. doi:10.1038/npp.2015.370
47. Bracht T, Horn H, Strik W, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord. 2014;155:186–193. doi:10.1016/j.jad.2013.10.048
48. Yang XH, Wang Y, Wang DF, et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res Neuroimaging. 2017;264:29–34. doi:10.1016/j.pscychresns.2017.04.005
49. Dillon DG, Gonenc A, Belleau E, Pizzagalli DA. Depression is associated with dimensional and categorical effects on white matter pathways. Depress Anxiety. 2018;35(5):440–447. doi:10.1002/da.22734
50. Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry. 2012;72(4):296–302. doi:10.1016/j.biopsych.2012.01.022
51. Coloigner J, Batail JM, Commowick O, et al. White matter abnormalities in depression: a categorical and phenotypic diffusion MRI study. Neuroimage Clin. 2019;22:101710. doi:10.1016/j.nicl.2019.101710
52. Takamura M, Okamoto Y, Okada G, et al. Patients with major depressive disorder exhibit reduced reward size coding in the striatum. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):317–323. doi:10.1016/j.pnpbp.2017.07.006
53. Drysdale AT, Grosenick L, Downar J, et al. Erratum: resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(2):264. doi:10.1038/nm0217-264d
54. Haroon E, Chen X, Li Z, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8(1):189. doi:10.1038/s41398-018-0241-4
55. Liu X, Li L, Li M, Ren Z, Ma P. Characterizing the subtype of anhedonia in major depressive disorder: a symptom-specific multimodal MRI study. Psychiatry Res Neuroimaging. 2021;308:111239. doi:10.1016/j.pscychresns.2020.111239
56. Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6(5):e810. doi:10.1038/tp.2016.80
57. Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. doi:10.1152/jn.1998.80.1.1
58. Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289. doi:10.1016/j.pnpbp.2021.110289
59. Rothkirch M, Tonn J, Köhler S, Sterzer P. Neural mechanisms of reinforcement learning in unmedicated patients with major depressive disorder. Brain. 2017;140(4):1147–1157. doi:10.1093/brain/awx025
60. Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66(5):478–486. doi:10.1001/archgenpsychiatry.2009.39
61. Lapidus KA, Gabbay V, Mao X, et al. In vivo (1)H MRS study of potential associations between glutathione, oxidative stress and anhedonia in major depressive disorder. Neurosci Lett. 2014;569:74–79. doi:10.1016/j.neulet.2014.03.056
62. Gabbay V, Mao X, Klein RG, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69(2):139–149. doi:10.1001/archgenpsychiatry.2011.131
63. Colic L, von Düring F, Denzel D, et al. Rostral anterior cingulate glutamine/glutamate disbalance in major depressive disorder depends on symptom severity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(12):1049–1058. doi:10.1016/j.bpsc.2019.04.003
64. Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503. doi:10.1038/nrn2866
65. Li K, Zhou T, Liao L, et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341(6149):1016–1020. doi:10.1126/science.1240729
66. Liu WH, Valton V, Wang LZ, Zhu YH, Roiser JP. Association between habenula dysfunction and motivational symptoms in unmedicated major depressive disorder. Soc Cogn Affect Neurosci. 2017;12(9):1520–1533. doi:10.1093/scan/nsx074
67. Rzepa E, Fisk J, McCabe C. Blunted neural response to anticipation, effort and consummation of reward and aversion in adolescents with depression symptomatology. J Psychopharmacol. 2017;31(3):303–311. doi:10.1177/0269881116681416
68. McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205(4):667–677. doi:10.1007/s00213-009-1573-9
69. Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114(4):627–639. doi:10.1037/0021-843X.114.4.627
70. Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52(11):1469–1478. doi:10.1176/appi.ps.52.11.1469
71. Cao B, Park C, Subramaniapillai M, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17. doi:10.3389/fpsyt.2019.00017
72. Cao B, Zhu J, Zuckerman H, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109–117. doi:10.1016/j.pnpbp.2019.01.002
73. Pizzagalli DA, Smoski M, Ang YS, et al. Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS). Neuropsychopharmacology. 2020;45(10):1656–1663. doi:10.1038/s41386-020-0738-4
74. Dean Z, Horndasch S, Giannopoulos P, McCabe C. Enhanced neural response to anticipation, effort and consummation of reward and aversion during bupropion treatment. Psychol Med. 2016;46(11):2263–2274. doi:10.1017/S003329171600088X
75. Sandman CF, Craske MG. Psychological Treatments for Anhedonia. Springer; 2021.
76. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17(1):27–49. doi:10.1093/schbul/17.1.27
77. Schürhoff F, Szöke A, Bellivier F, et al. Anhedonia in schizophrenia: a distinct familial subtype? Schizophr Res. 2003;61(1):59–66. doi:10.1016/S0920-9964(02)00237-2
78. Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74(2):130–136. doi:10.1016/j.biopsych.2012.12.022
79. Harvey PO, Armony J, Malla A, Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J Psychiatr Res. 2010;44(11):707–716. doi:10.1016/j.jpsychires.2009.12.008
80. Robison AJ, Thakkar KN, Diwadkar VA. Cognition and reward circuits in schizophrenia: synergistic, not separate. Biol Psychiatry. 2020;87(3):204–214. doi:10.1016/j.biopsych.2019.09.021
81. Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia–relationship to apathy and depression. Schizophr Res. 2010;118(1–3):154–161. doi:10.1016/j.schres.2009.11.007
82. Hovington CL, Bodnar M, Chakravarty MM, Joober R, Malla AK, Lepage M. Investigation of white matter abnormalities in first episode psychosis patients with persistent negative symptoms. Psychiatry Res. 2015;233(3):402–408. doi:10.1016/j.pscychresns.2015.06.017
83. Lee JS, Han K, Lee SK, Seok JH, Kim JJ. Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neurosci Lett. 2014;579:7–11. doi:10.1016/j.neulet.2014.07.001
84. Ohtani T, Bouix S, Hosokawa T, et al. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: a DTI study. Schizophr Res. 2014;157(1–3):190–197. doi:10.1016/j.schres.2014.05.016
85. Yang ZY, Zhang RT, Li Y, et al. Functional connectivity of the default mode network is associated with prospection in schizophrenia patients and individuals with social anhedonia. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:412–420. doi:10.1016/j.pnpbp.2019.02.008
86. Cressman VL, Schobel SA, Steinfeld S, et al. Anhedonia in the psychosis risk syndrome: associations with social impairment and basal orbitofrontal cortical activity. NPJ Schizophr. 2015;1(1):15020. doi:10.1038/npjschz.2015.20
87. Park IH, Kim JJ, Chun J, et al. Medial prefrontal default-mode hypoactivity affecting trait physical anhedonia in schizophrenia. Psychiatry Res. 2009;171(3):155–165. doi:10.1016/j.pscychresns.2008.03.010
88. Arrondo G, Segarra N, Metastasio A, et al. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol. 2015;6:1280. doi:10.3389/fpsyg.2015.01280
89. Shaffer JJ, Peterson MJ, McMahon MA, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol Neuropsychiatry. 2015;1(4):191–200. doi:10.1159/000440979
90. Lui SS, Liu AC, Chui WW, et al. The nature of anhedonia and avolition in patients with first-episode schizophrenia. Psychol Med. 2016;46(2):437–447. doi:10.1017/S0033291715001968
91. Evans S, Shergill SS, Chouhan V, Bristow E, Collier T, Averbeck BB. Patients with schizophrenia show increased aversion to angry faces in an associative learning task. Psychol Med. 2011;41(7):1471–1479. doi:10.1017/S0033291710001960
92. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36(1):143–150. doi:10.1093/schbul/sbn061
93. Zhang L, Wang H, Luan S, et al. Altered volume and functional connectivity of the habenula in schizophrenia. Front Hum Neurosci. 2017;11:636. doi:10.3389/fnhum.2017.00636
94. Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr Bull. 2006;32(3):417–421. doi:10.1093/schbul/sbj083
95. Dichter GS, Bellion C, Casp M, Belger A. Impaired modulation of attention and emotion in schizophrenia. Schizophr Bull. 2010;36(3):595–606. doi:10.1093/schbul/sbn118
96. Bang M, Kang JI, Kim SJ, et al. Reduced DNA methylation of the oxytocin receptor gene is associated with anhedonia-asociality in women with recent-onset schizophrenia and ultra-high risk for psychosis. Schizophr Bull. 2019;45(6):1279–1290. doi:10.1093/schbul/sbz016
97. Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286(4):427–435. doi:10.1001/jama.286.4.427
98. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892–899. doi:10.1093/schbul/sbu170
99. Bodén R, Bengtsson J, Thörnblom E, Struckmann W, Persson J. Dorsomedial prefrontal theta burst stimulation to treat anhedonia, avolition, and blunted affect in schizophrenia or depression – a randomized controlled trial. J Affect Disord. 2021;290:308–315. doi:10.1016/j.jad.2021.04.053
100. Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136(3):1126–1134. doi:10.1016/j.jad.2011.09.048
101. Volman SF, Lammel S, Margolis EB, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33(45):17569–17576. doi:10.1523/JNEUROSCI.3250-13.2013
102. Sharma A, Wolf D, Ciric R, et al. Connectome-wide analysis reveals common dimensional reward deficits across mood and psychotic disorders. Neuropsychopharmacology. 2016;41:S531–S532.
103. Schaub AC, Kirschner M, Schweinfurth N, et al. Neural mapping of anhedonia across psychiatric diagnoses: a transdiagnostic neuroimaging analysis. Neuroimage Clin. 2021;32:102825. doi:10.1016/j.nicl.2021.102825
104. Ren H, Fabbri C, Uher R, et al. Genes associated with anhedonia: a new analysis in a large clinical trial (GENDEP). Transl Psychiatry. 2018;8(1):150. doi:10.1038/s41398-018-0198-3
105. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(6):1751–1764. doi:10.1093/brain/awr059
106. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi:10.1176/appi.ajp.160.1.13
107. Stanton CH, Holmes AJ, Chang SWC, Joormann J. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42(1):23–42. doi:10.1016/j.tins.2018.09.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.