Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
An Overview of Traditional Chinese Medicine in the Treatment After Radical Resection of Hepatocellular Carcinoma
Authors Peng Y , Wu X, Zhang Y, Yin Y, Chen X, Zheng D, Wang J
Received 26 May 2023
Accepted for publication 3 November 2023
Published 18 December 2023 Volume 2023:10 Pages 2305—2321
DOI https://doi.org/10.2147/JHC.S413996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Yichen Peng,1,2,* Xia Wu,1,2,* Yurong Zhang,1 Yue Yin,1 Xianglin Chen,2 Ding Zheng,1 Jing Wang1
1The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Hepatobiliary Department, Luzhou, People’s Republic of China; 2Department of Integrated Traditional Chinese & Western Medicine, The Southwest Medical University, Luzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ding Zheng; Jing Wang, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Hepatobiliary Department, No. 182, Chunhui Road, Luzhou, 646000, People’s Republic of China, Tel +86 19937071324, Email [email protected]; [email protected]
Abstract: According to the Barcelona Clinic Liver Cancer (BCLC) system, radical resection of early stage primary hepatocellular carcinoma (HCC) mainly includes liver transplantation, surgical resection, and radiofrequency ablation (RFA), which yield 5-year survival rates of about 70– 79%, 41.3– 69.5%, and 40– 70%, respectively. The tumor-free 5-year rate for HCC patients undergoing radical resection only reach up to 13.7 months, so the prevention of recurrence after radical resection of HCC is very important for the prognosis of patients. The traditional Chinese medicine (TCM) takes the approach of multitarget and overall-regulation to treat tumors, it can also independently present the “component-target-pathway” related to a particular disease, and its systematic and holistic characteristics can provide a personalized therapy based on symptoms of the patient by treating the patient as a whole. TCM as postoperative adjuvant therapy after radical resection of HCC in Barcelona Clinic liver cancer A or B stages, and the numerous clinical trials confirmed that the efficacy of TCM in the field of HCC has a significant effect, not only improving the prognosis and quality of life but also enhancing patient survival rate. However, with the characteristics of multi-target, multi-component, and multi-pathway, the specific mechanism of Chinese medicine in the treatment of diseases is still unclear. Because of the positive pharmacological activities of TCM in combating anti-tumors, the mechanism studies of TCM have demonstrated beneficial effects on the regulation of immune function, chronic inflammation, the proliferation and metastasis of liver cancer cells, autophagy, and cell signaling pathways related to liver cancer. Therefore, this article reviews the mechanism of traditional Chinese medicine in reducing the recurrence rate of HCC after radical resection.
Keywords: traditional Chinese medicine, radical resection, hepatocellular carcinoma, recurrence
According to estimates from the global cancer statistics by the International Agency for Research on Cancer, there were 905,677 new cases and 830,180 liver cancer deaths worldwide in 2020. Primary liver cancer is expected to rank as the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide in 2020.1,2 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, as the safety of surgical techniques for hepatectomy improves, as well as the advancement of systemic treatments and the greater availability of local treatments, early-stage HCC patients are more eligible to receive curative therapies, such as surgery and ablation, that significantly improve overall survival (OS).3,4 At present, the strategies of radical resection of HCC are diversified, including liver resection, liver transplantation, and ablation surgery. However, the overall prognosis for HCC is poor, with a 5-year recurrence rate around 70% either to local recurrence or distant metastasis.5 There is a multicenter study of 734 patients, among 303 patients who developed late HCC recurrence 2 years, the recurrence was associated with sex, cirrhosis, and several aggressive tumor characteristics of the initial HCC.6,7 Liver transplantation is the most effective method for the treatment of HCC. However, due to the limited liver source, it cannot satisfy most patients. Therefore, the current controversial issues mainly focus on the effectiveness and recurrence between hepatectomy and ablation.8 The surgical resection (SR) in treating small hepatocellular carcinoma (SHCC) has higher long-term survival rate and a lower long-term recurrence rate, and radiofrequency ablation (RFA) led to a lower complication rate than SR.9 With long-term use, chemotherapeutic drugs, such as sorafenib, have additional issues such as toxicity and/or drug inefficacy. As a result, both current ablation therapies or hepatectomy are inevitable for the recurrence, and further research to find better methods for preventing recurrence of HCC is necessary.
As a complementary and alternative medicine treatment, Traditional Chinese Medicine (TCM), especially herbal medicine, has an extensive history and been widely used in cancer treatment in China.10,11 Pharmacological research has shown that TCM has been used as a two-way immune regulator with anti-inflammatory,12 immunoregulatory,13 and anti-angiogenic effects.14 A large number of clinical trials confirmed that the efficacy of TCM in the field of HCC, not only improving the prognosis and quality of life but also enhancing patient survival rate.15 The results of several randomized controlled studies have shown that the oral Chinese medicine combined with targeted therapy and immunotherapy can significantly enhance the therapeutic efficacy and reduce the adverse reactions caused by targeted therapy in patients with HCC.16,17 The poor prognosis for radical resection of HCC has led scientists and physicians to search for novel treatment options to improve patient survival. TCM gives new horizons for improving the outcomes of malignancies. In this paper, we describe the mechanism of TCM in the treatment and prevention of recurrence after radical resection of HCC.
Risk Factors for Early Recurrence After Radical Resection of HCC
Hepatectomy represents the first-line treatment for patients with resectable hepatocellular carcinoma (HCC).18 However, it has been reported that the 5-year recurrence rate of HCC is as high as 50% and 70%.19 The main risk factors for recurrence in early-stage HCC patients after radical resection are ascites, higher Child-Pugh score, larger tumor size, low platelet count, liver cirrhosis, elevated a-fetoprotein (AFP) level, and high serum hepatitis B virus (HBV) load.20–22 The factors for recurrence after radical resection of HCC could be divided into three categories: tumor, host, and surgical factors. a) tumor factor: microscopic venous invasion as well as macroscopic portal vein involvement are both major risk factors as metastasis via portal venous system.23 Large tumors, especially tumors larger than 5 cm, significantly increase the risk of postoperative recurrence.24 The larger diameter of the tumor, the greater the possibility of vascular invasion and distant metastasis, which increases the risk of surgery, causing liver metastasis and recurrence and affecting the survival of patients. In addition, the nuclear DNA content in tumor cells has been shown to be closely related to the malignant potential of the neoplasm, and DNA aneuploidy was thought to have prognostic significance in many kinds of malignant tumors.25 Proliferating cell nuclear antigen (PCNA) is an accessory protein of DNA polymerase delta, its expression is related to DNA synthesis and cell replication, the sign of the G1/S phase of the cell cycle, and can be evaluated by immunohistochemical studies.26 (b) Surgical factors: the type of resection (anatomic vs non-anatomic), the extent of resection margin and perioperative blood transfusion are the three most extensive surgical factors impacting postoperative recurrence. But also some studies shown that the type of resection (anatomic vs non-anatomic) is not considered a distinct risk factor for early (2 year) tumor recurrence in patients with HCC and preserved liver function.27 Few studies have argued that intraoperative blood loss and perioperative transfusion not only increase the risk of operative morbidity and mortality but also jeopardize long-term survival, since they actually increase the recurrence rate of the tumor being resected.28,29 Intraoperative procedures for liver cancer, especially large tumors, have been considered to be an important factor leading to the spread of tumor cells in the portal system, which can explain the cause of early recurrence in the liver after resection. (c) Host factors: However, age and gender have also been reported as independent risk factors. The difference in prognosis between men and women among HCC patients may be mainly attributed to estradiol.30
Adjuvant Treatment for Prevention of Recurrence After Radical Operation of HCC
Radical excision is the main surgical treatment for HCC, but the high metastasis and relapse rates after operation severely affect the long-term survival of patients. There are many adjuvant treatment methods to prevent HCC recurrence after radical surgery, such as antiviral therapy, which has potential advantage in terms of reducing the recurrence rate and improving the overall survival (OS) and/or disease-free survival of patients with hepatitis-related HCC.31 In addition, studies believe that preoperative transcatheter arterial chemoembolization (TACE) can also significantly reduce the intrahepatic recurrence rate and improve OS, especially for patients with a higher risk of recurrence.32 Increasing evidence reveals that TACE after radical resection of primary HCC is beneficial for the treatment outcome.33 A randomized controlled trial (RCT) showed that postoperative adjuvant TACE (PATACE) conferred more benefits for HCC patients with high risk of recurrence, such as large tumor diameter (>5 cm), macroscopic vascular invasion, and multiple tumor nodules.34 The efficacy of molecular targeted drugs has brought new hope for patients. Early adjuvant adoptive immunotherapy can significantly improve clinical prognosis. In recent years, sorafenib, lenvatinib, Regorafenib, and Cabozantinib are being explored for prevention of recurrence after radical operation of HCC. Lenvatinib has been approved by the FDA as a first-line treatment for patients with unresectable HCC. Regorafenib has been approved by the FDA as a second-line treatment for patients with advanced HCC who have failed sorafenib. Cabozantinib has been approved by the FDA as a second and third line agent for advanced HCC. Adjuvant lenvatinib therapy after radical resection in patients with China liver cancer staging (CNLC) IIb and/or IIIa HCC resulted in a 1-year recurrence-free survival rate of 50.5% and a median RFS of 16.5 months. In a prospective study of lenvatinib combined with TACE followed by adjuvant therapy for HCC with high-risk recurrence factors, the disease‑free survival (DFS) was 17 months, while the median DFS of TACE in the control group was 9 months.35 Numerous clinical trials are now underway to evaluate the efficacy of immune checkpoint inhibitors for patients with many kinds of cancer, including HCC, and the outcomes of these trials are highly anticipated. In addition, the application of Chinese medicine provides new insights for the treatment of HCC after radical resection. Huaier Granule is a kind of traditional Chinese medicine, which has the function of prolonging the recurrence-free survival period and reducing extrahepatic recurrence.36 A multicenter, randomized, clinical trial involving 39 centers and 1044 patients nationwide demonstrated that the traditional Chinese medicine Huaier Granules as a postoperative adjuvant treatment after radical hepatectomy of HCC in Barcelona Clinic Liver cancer A or B stages had significant RFS prolongation and reduced extrahepatic recurrence.37,38 The TCM plays an important role in reducing the recurrence and metastasis of HCC after radical resection, prolonging the survival time, and improving the quality of patients’ life. The current application of TCM mainly includes Chinese medicine prescriptions and Chinese patent medicine, shown in Table 1 and Table 2. With its unique development concept and its own theoretical system, Chinese medicine has a certain degree of existence in modern research. TCM has the synergistic regulation of multi-components, multiple targets, and multiple pathways, and it is necessary to understand an effective drug derived from TCM or active components for HCC treatment and to discuss their mechanisms of treatment.
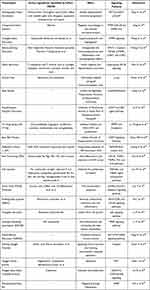 |
Table 1 Traditional Chinese Prescriptions for HCC Treatment and Prevention of Recurrence |
 |
Table 2 Traditional Chinese Herbs and Their Active Ingredients for HCC Treatment and Prevention of Recurrence |
The Mechanism of TCM in Preventing and Treating HCC
Suppression of Proliferation and Cell Cycle Arrest
The critical component of tumor occurrence is tumorigenesis, resulting in loss of programmed cell death through genetic changes.59 Cell cycle regulators are the products of genes that appear to control apoptotic.60 There are two apoptotic pathways: a) mitochondrial-dependent intrinsic pathway, and b) death receptor-mediated extrinsic pathway.99 Furthermore, the tumor suppressor P53 activate various cellular reactions, and the abnormal regulation of p53 leads to cell cycle arrested and apoptosis.61 The uncontrolled progression and proliferation of cell cycle in cancer cells has been known to be a major indicator for tumor development and proliferation, cell cycle arrest has been implicated anticancer effects.100 Studies found that Tetramethylpyrazine (isolated from Ligusticum chuanxiong) induced a significant cell-cycle arrest in the G0/G1 phase during cell cycle progression and apoptosis.78 Studies have also mentioned that TCM can affect tumour progression by influencing cell cycles, including HCC, cervical cancer, nasopharyngeal carcinoma and breast cancer.79 TCM plays a more important role in the G1/S checkpoint pathway and proteins related to the G1/S phase, cyclinD1 is a cell cycle-related protein in G1/S phase, promoting cell from G1 phase into S phase and commence the DNA replication.101 Some researchers have proved that the proliferation of tumor cells and the inhibition of tumor growth may also be caused by apoptosis and G2/M phase arrest. TCM inhibited tumor growth by inducing G2/M cell cycle block, which has a different mechanism of action from Sorafenib.102 Its main targets are serine-threonine kinase Raf-1, vascular endothelial growth factor receptor and platelet-derived growth factor sorafenib, which seem to be effective in prolonging the median survival of patients with liver cancer, but its response rate is very low and may cause drug resistance in some patients.62 Interestingly, the research results show that TCM has a stronger effect on PI3K and PTEN signaling pathways, and induces G2/M phase block in the cell cycle.77,103 In addition, Chinese medicine has advantages in anti-inflammatory and can make up for the shortcomings of Sorafenib.63 The role of traditional Chinese medicine suggests that its combined application with Sorafenib can not only improve the curative effect of liver cancer after radical resection but also reduce the drug resistance to Sorafenib and the rate of tumor recurrence.60,64
Anti-Inflammatory
Most importantly, the Inflammation-mediated liver injury after radical resection of HCC is the key cause of recurrence and distant metastasis.54,90,91 A study has found that the levels of inflammatory factors such as TNF-α, IL-6 and IL-8 in cancer patients before radiotherapy were significantly higher than those in the control group, suggesting that the patients were in an inflammatory response activation state.104 At the cellular and molecular levels, the anti-carcinogenic effects of TCM have been associated with the modulation of multiple inflammatory signal transduction pathways, including Janus kinase (JNK)/signal transducer and activator of transcription 3 (STAT 3) signaling pathways,105 phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (Pi3K/AKT/mTOR)69 and MAPK, AMPK,70 EGFR,72 nuclear factor kappaB (NF-kB) signaling pathway,73 can regulate a variety of inflammatory factors, including TNF-α and IL-6.106,107 The MAPK/p38 signaling pathway not only regulates the expression of pro-inflammatory cytokines but also plays an important role in the activation of cell adhesion, migration, and invasion in HCC patients. The STAT 3 signaling pathway is a pro-inflammatory pathway, which may be triggered by tumor cells. As a tumor suppressor, FOXO negatively regulates the expression of immunosuppressive proteins to promote tumor anti-tumor activity.108 The studies found that Extracts of Qizhu decoction43 and Benzophenones from Anemarrhena asphodeloides Bge109 exhibit anticancer activity via the traditional inflammatory NF-κB signaling pathway.
Traditional Chinese medicine plays an important role in targeting the body’s inflammatory response and immunity, multiple types of cells in the liver, including Kupffer cells and other non-parenchymal cells, can be activated by active ingredients of TCM to release chemokines, cytokines that play a major role in regulating the proliferation of T-cells, and adjusting the proportion of Th1/Th2 cell.104 The active ingredients of TCM to reduce the inflammation after radical resection and their possible mechanisms of action are as follows: a) regulating immune cell function, inhibiting inflammatory response, improving barrier function and modulating related signal transduction pathways.110 b) regulation of the expression of pro-inflammation genes and cytokines and their receptor on immune cells.65 c) promoting anti-inflammation cytokine release, and down-regulate the expression of TLR4/NF-kappaB signaling pathway.54,66 d) stimulating the immune-cell mobilization response in the cancer microenvironment, regulating the infiltration of immune NK cells.67 e) inhibition of signal transducers and transcription response to inflammatory cytokines. f) depletion of immune and inflammatory cells that promote tumor development and progression.71
Immunoregulatory
Immunization to treat HCC has a long history.111 Dunn et al proposed the tumor immune editing theory for the first time and categorized immune interaction with cancer into three stages: elimination by immunosurveillance mechanisms; equilibrium and escape.112 The theory has extended our understanding of interactions between immune system and tumor cells. First, the immune system surveys for and clears most of the malignant cells; When a small number of malignant cells bad evaded the first phase are primed with further editing and enter the “equilibrium” phase, and the equilibrium period can even cover the entire life process of the body in extreme cases; but once the active role of malignant tumor cells breaks this equilibrium state, the tumor cells will successfully “escape” and cause the immune system to lose its effect on the tumor.113 Tumor cells may cause local or systemic immunodeficiency, such as a significant reduction in the number of CD4+ T cells in cancer patients, dendritic cell dysfunction, and higher levels of immunosuppressive factors (such as transforming growth factor-β).56 Immunotherapy is considered a promising cancer treatment method. Chinese medicine emphasizes the importance of enhancing the immune function of cancer patients. Therefore, Chinese medicines for strengthening the body and clearing away heat and detoxification are widely used in the adjuvant treatment of cancer. According to the basic treatment principles of malignant tumors documented in TCM literature, they mainly contained reducing phlegm and resolving masses, promoting blood circulation and detoxification, promoting the body’s resistance, eliminating pathogenic factors, and purging and tonifying in combination, which are widely used in the adjuvant treatment of HCC.114 Herbs for promoting the body’s resistance, such as Ganoderma lucidum,74 Astragalus membranaceus,115 and Ginseng,116 are found to promote cytokines (such as interleukin (IL), tumor necrosis factor (TNF), interferon) and alexins, activating T lymphocytes, B lymphocytes, macrophages, natural killer (NK) cells, and dendritic cells. In the tumor microenvironment, the interaction between the host immune system and tumor cells plays an important role in the pathological progression of tumors. Additional studies demonstrated that Ginsenoside Rg3 exhibited immunomodulatory activity by reducing the levels of the cytokines IFN-gamma and IL-2, rescuing the levels of CD4+ T cells, enhancing the cytotoxic activities of natural killer (NK),16 and inhibiting tumor recurrence by regulating the body’s immunity.
Inhibiting Epithelial–Mesenchymal Transition (EMT) of HCC
The TCM has been used for the prevention of recurrence and metastasis of HCC after radical resection for attenuating toxicity and prolonging the survival of HCC patients after surgery.57 The occurrence of tumor Epithelial–Mesenchymal Transition (EMT) is closely related to the tumor migration and invasion. EMT is a key biological process in cancer progression and recurrence, the loss of cell–cell interaction and apical-basal polarity, as well as the enhancement of cell motility, drug resistance allows tumor cells to escape from the preinvasive neoplasm, invasion to distant regions and tissues.55 E-cadherin is one of the tight junction and adhesion proteins, heavily involved in the responsible for cell adhesion, and polarized distribution of intramembrane proteins, including the Na-K ATPase.117 The lack of E-cadherin leads to the decline of cell adhesion function, thereby detaching the cells and promoting the proliferation, invasion, and metastasis of cancer cells. Furthermore, studies have observed Dulcitol,68 a natural product extracted from euonymus alatus was reported that it could induce apoptosis of C6 glioma cells and increasing E-cadherin. Furthermore, the Rad-p53 (recombinant human adenovirus p53 is the first commercial product for gene therapy) and curcumin promote HepG 2 cell apoptosis inhibit epithelial–mesenchymal transition (EMT) and block the progression of G2/M cells.84,118,119 In addition, TCM inhibits the process of EMT and hinders the metastasis of HCC cells in vivo by down-regulating the expression of transforming growth factor beta receptor 2 (TGF-β2R) and Smad family member 3 (Smad 3), suggesting TCM may regulate Smad related pathways to inhibit EMT in HCC.39,120 Transforming growth factor β1 (TGF-β1) binds to TGF beta1 receptors type I and II (TbetaRI and TbetaRII) stimulates the phosphorylation of Smad 2/3 and activation of this Smad-dependent intracellular signaling pathway mediated by Smad-4. Certain studies have noted that the compounds in TCM inhibit tumor growth via regulating TGF-beta/Amad signaling pathways. Further mechanistic study Smad pathways control cellular proliferation, differentiation, and apoptosis.45,121 Some herbs have the ability to block cellular adhesion and invasion by impacting EMT to mesenchymal-epithelial-transition (MET) phenotypic interconversions.122,123 According to previous studies, TGF-β, growth factors including epidermal growth factor (EGF) and hepatocyte growth factor (HGF) trigger downstream signals of EMT through the MAPK and PI3K/Akt pathways.124 TMC can inhibit the activation of Akt, ERK, JNK, and p38MAPK, effectively prevent EMT of liver cancer cells, providing a reasonable explanation for its inhibition of liver cancer cell migration and invasion.98 Further understanding of the mechanism of action of traditional Chinese medicine in preventing metastasis and recurrence after radical resection of liver cancer provides new ideas.
Induces the Differentiation of Myeloid-Derived Suppressor Cells (MDSCs)
TCM is widely used as an anti-inflammatory, anti-viral and anti-cancer agent and thus can be applied in radical resection of liver cancer. The curcumin, as an active ingredient in turmeric, which acts against a variety of cancers (non-small cell lung cancer, colorectal cancer, liver cancer, and human renal clear cell carcinoma), is anti-inflammatory, choleretic, and exerts anti-oxidant effects, without any obvious toxicity in the long term.125 Studies suggested that the TCM could increase the number of effector T cells, reduce the infiltration of FOXP3+Tregs, inhibit cytokine-induced apoptosis of effector T cells and expression of tumor immunosuppressive cytokine, mediating M2 TAM transformation to M1 TAM, and change the tumor microenvironment and kill the tumor cells. Some studies have hypothesized that TCM in combination with α-PD-L1 antibody can provide a favorable environment for immune cells response against cancer.126,127 Recently, curcumin derivatives were described to inhibit cancer cell proliferation by blocking the activation and expansion of MDSC mediated by STAT3 and NF-κB signaling pathways.128 In-depth research on the pathogenesis of cancer has shown that inflammation and immune microenvironment are the key factors for HCC growth, spread and metastasis after radical resection of HCC.42 The tumor microenvironment is filled with regulatory T cells (Tregs), M2 macrophages (TAMs) and myeloid inhibitory cells (MDSCs). MDSCs are significantly found in peripheral blood and solid tumors, maintaining an immune suppressive network in the tumor microenvironment. Previous studies have demonstrated that inhibiting MDSCs in tumors may weaken tumor defense mechanisms and inhibit tumor progression, including lung cancer, malignant melanoma, and liver cancer. The cancer cell-derived GM-CSF is dispensable for the tuning of the tumor microenvironment, with worse prognosis and modulation of inflammation and immunity through recruitment and differentiation of MDSCs. Studies have found that the TCM significantly inhibits the levels of M-CSF, GM-CSF and a variety of inflammatory factors, which provides evidence for the inhibitory effect of TCM on MDSCs and its subsequent anti-liver cancer activity, and TLR4/NF-kB/Myd88 signaling pathway enhances the immunosuppressive function of MDSC.129
Decreasing Telomerase Activity in Precancerous Lesions of Liver
Telomerase is an enzyme, elongates one chain of the telomeric DNA, and compensates for the replication-associated telomere shortening.46,130 The telomerase is an attractive therapeutic target, that cancer cells maintain telomeres through activation of the telomerase enzyme and achieve unlimited replication capacity and immortality, complete cancerous transformation.131 Telomerase has been observed in more than 85% of known human tumors and is considered a promising tumor marker. Researchers have found that TCM such a total alkaloids of mistletoe,132 and mistletoe polysaccharides reduce the telomerase activity of liver cancer cells and reduce the telomerase activity of liver precancerous lesions in rats.133 Signal transducer and activator of transcription 3 (STAT3) is an oncogenic transcription factor, is a member of STAT protein family, an important transcription factor of the JAK/STAT signal pathway,88,95 belonging to the seven member STAT gene family, playing a key role in many processes such as cell growth and apoptosis. STAT 3 Activate Fos, Cyclin-D, CDC25A, c-Myc or Pim1 and other genes involved in the cell cycle process, up-regulate Bcl-2 (B cell CLL/lymphoma-2), BCLXL, and β2-macroglobulin to resist apoptosis Gene.44,134 Many studies have found that the TCM reduces the telomerase activity related to significantly reducing P-STAT 3/T-STAT 3, and P-ERK/T-ERK.98
Mitochondrial Apoptosis
Liver cancer is a complex interaction among multi-gene, multi-target complex process involving multiple signaling pathways.135 There has been a focus on a mechanistic approach for the development of TCM by targeting the induction of apoptotic cell death since apoptosis136, or programmed cell death.137 Surgical resection of tumors is currently the predominant treatment for HCC, and the multi-kinase inhibitor sorafenib (Nexavar) is an oral drug and a multi-target signal transduction inhibitor, is the first-line drug approved by the Food and Drug Administration (FDA) in 2006 for the treatment of advanced liver cancer.40,138 In terms of adverse reactions, HCC patients administered sorafenib mainly included gastrointestinal, physical or skin disease (eg, hand-foot skin reaction, weight loss, hypertension, and diarrhea).139 Therefore, it is urgent to explore safely and effectively drugs to induce hepatocyte apoptosis. Chinese herbal medicine and unique biomedical and pharmaceutical resources have been widely used in the prevention and treatment of hepatocellular carcinoma, which can effectively alleviate clinical symptoms, improve immune function, delay the progression of tumors, and improve the quality of life.75,140 Modern pharmacology studies have shown that the total saponins obtained from radix Astragalus membranaceus could be established as an effective chemotherapeutic agent to suppress cancer cells growth through promoting tumor cells apoptosis and inhibiting tumor angiogenesis and migration.51 Huanglian Jiedu Decoction and its composition have therapeutic effect on liver cancer, effectively induced hepatocarcinoma cell cycle arrest, and reduced HepG 2 cell anti-apoptosis and expression of protein Bcl-x.41 TCM can upregulate Bax expression by downregulating Bcl-2 expression in tumor cells, modulating the PI3K/AKT or STAT3 signaling pathway.50 Otherwise, TCM inhibited tumor proliferation, migration, and invasion of HCC after HCC surgery through inducing cyclin D1 and cyclin-dependent kinase (CDK1), Bcl-2, MMP-2, MMP-9, and other protein expression, enhance the expression of bax, cleaved Caspase-3, and P21.141–143 However, mitochondria still play a central and multifunctional role in the proliferation and growth of malignant tumor cells, which indicates the therapeutic potential in targeting mitochondria.144 Furthermore, the components of TCM induce apoptosis by up-regulating the expression of tumor suppressor genes p21Cip1/WAF1, p53, the pro-apoptotic protein Bax, activating Caspase apoptotic signals, and down-regulating the expression of the anti-apoptotic proteins Bcl-2, Bcl-XL. P53 is closely associated with cancer inhibition, playing a crucial role in a variety of intracellular and extracellular regulatory mechanisms, activating the target proteins, such as Cdk4, the Cdk inhibitor P21, and cyclinD1, to induce cell cycle arrest and apoptosis.145,146 Bcl-2 is the most important anti-apoptotic members in the Bcl-2 family, binding to the pro-apoptotic proteins Bax (Bcl-2-associated X protein) to protect cells against apoptosis by maintaining the integrity of the mitochondrial membrane.147 Bax is thought to homo-oligomerize and form pores in the outer mitochondrial membrane, leading to the increase of mitochondrial outer-membrane permeabilization (MOMP) and release of apoptogenic mitochondrial intermembrane proteins, such as cytochrome c, binding to the adaptor protein APAF-1 and causing the aggregation and activation of the initiator caspase-9.148,149 The anti-apoptotic protein bcl-2 hinders the flow of Ca 2+ from the endoplasmic reticulum to the cytoplasm, and combines it with bax to form a bax-bcl-2 heterodimer, thereby preventing the occurrence of cell apoptosis.76 On the one hand, the ratio of bax/bcl-2 is unbalanced, forming a large number of bax-bax homodimers to promote the permeability of the mitochondrial membrane;87 on the other hand, it induces the release of the apoptotic factor cytochrome C (Cyt-C).150 TCM can regulate the tumor cell signaling pathway p53/Bcl-2/Cyt-C/caspase-3 to induce mitochondrial cell apoptosis, thereby exerting an anti-liver cancer effect. 3-kinase/protein kinase B (PI3K/Akt) and mitogen-activated protein kinase (MAPK) are also regulated by mitochondrial signals.89,151,152
Improving the Morphology and Structure of Tumor Blood Vessels
Tumor vessels are structurally and functionally abnormal, lack of a complete basement membrane tightly connected to the cells and a single thin blood vessel wall, leading to an abnormal tumor microenvironment characterized by interstitial hypertension, hypoxia, and acidosis, which in turn hinder delivery and efficacy of anti-tumor treatment.153 After the TCM treatment, studies shown that the tumor blood vessels will appear “normalized” within a certain period of time, making tumor blood vessels became regular, the morphology of the pericytes is regular, and improving the hypoxia and acidosis inside tumor.154 Platelet endothelial cell (EC) adhesion molecule-1 (PECAM-1), also known as cluster of differentiation 31 (CD 31), is an adhesion molecule on the surface of ECs, which accounts for a large part of the connections between actin cytoskeleton of the cells.52,155 CD 31 is implicated in angiogenesis, leukocyte migration, and T cell activation has been demonstrated on immortalized PnMECs. Adenocarcinoma cells and tumor tissue sections showed strong positive expression of CD 31. The study shown that the tumor vascular endothelial cells in the APS-curcumin combination are arranged neatly and closely connected with the carrier group. Pericytes, an integral component of mature blood vessels, wrapping around endothelial cell tubes to provide structural support and generate functional, mature blood vessels and modulate endothelial cell proliferation, survival, and function.58,83,156 Compared with vascular cells in normal tissues, pericytes in tumor exhibit altered morphology and epigenetic programming, showing defective blood vessel supporting functions. Clinical data have previously shown that low pericyte coverage is related to reduced patient survival.157 NG2 is a typical marker of perivascular cells, which contributes to the stabilization of microvessels, the regulation of capillary blood flow, and angiogenesis.158 In pericytes, the expression of NG2 plays an important role in the localization of pericytes to the endothelial layer and the interaction with endothelial cells. Studies have found that the combined application of Chinese medicine has a synergistic effect on tumor blood vessel maturation.53,82 In summary, traditional Chinese medicine can improve the morphology and structure of tumor blood vessels and promote the maturation of tumor blood vessels, which provides a possibility for the normalization of tumor blood vessels in the treatment of liver cancer, and brings a breakthrough for effective anti-cancer treatment.48
Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) stress is recognized as a regulator of homeostasis regarding the accumulation of misfolded proteins in the ER, and maintaining protein balance, which is called protective unfolded protein response (UPR).159 The main function of UPR is to regulate protein balance through translation attenuation and up-regulation of genes encoding ER chaperone proteins and secretion mechanisms to improve the protein folding ability of ER.160 However, continuous or intense endoplasmic reticulum stress will drive these unfolded proteins to the cytoplasm, where they are degraded by the ubiquitin-proteasome system (UPS). Once UPS fails, death-related protein kinase (Dapk) will be triggered, and death-related protein kinase (Dapk) is the upstream integrator of apoptosis and autophagy.161 Liu et al161 found that Polygonum bistorta aqueous extract (PB) can cause endoplasmic reticulum stress, thereby inhibiting the activity of autophagosomes and proteasomes, inducing Hep3B cell apoptosis. The relationship between autophagy and apoptosis is complex.162 The accumulation of autophagy will lead to a large amount of cell degradation, leading to cell apoptosis, and autophagy will be negatively regulated by cell apoptosis.97 In the process of proteasome formation, the interaction of growth factors, cytoskeleton, and adhesion molecules is considered to be a strategy to change cell migration and adhesion.86 In fact, extracts from natural products have the ability to regulate cancer cells. The increase in reactive oxygen species can cause protein damage and degradation, but cancer cells may eliminate reactive oxygen species to fight stress by enhancing their antioxidant capacity. Chinese herbal medicines may reduce the antioxidant capacity of cancer cells, thereby increasing the growth rate of tumors.96
Conclusion
Under the guidance of the theory of traditional Chinese medicine, which reflects philosophical principles and embodies large dialectical thought, TCM believes that the human body is a dynamic and complex system, focusing on the influence of the internal and external environments of the human body. After radical resection of HCC, the internal and external environments of patients themselves are destroyed. Modern studies have found that Chinese medicine has passed Immunity, inflammation, and cell proliferation are regulated to reduce the recurrence rate of patients with HCC. However, under the background of modern medicine, there are certain difficulties in the application of traditional Chinese medicine after radical resection of HCC. Therefore, further research should consider investigating the underlying mechanisms of traditional Chinese medicine used in reducing the recurrence of patients with HCC after radical resection to determine which type of TCM or adjunctive treatment is the most effective for improving the survival rate of patients.
Funding
This work was funded by Scientific Research Project of National Traditional Chinese Medicine Clinical Research Base Construction Unit, Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University (Affiliated Traditional Chinese Medicine Hospital (2020) No. 33), Joint Project of Science and Technology Department of Sichuan Province – Luzhou Municipal People ‘s Government – Southwest Medical University (22ZDYF3796), Sichuan Provincial Administration of Traditional Chinese Medicine for Traditional Chinese Medicine Research (Sichuan Traditional Chinese Medicine (2021) No.13), Luzhou Government-Southwest Medical University Science and Technology Strategic Cooperation Project (2021LZXNYD-Z08).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ouyang J, Wang Z, Yuan K, et al. Adjuvant lenvatinib plus PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy: a retrospective landmark analysis. J Hepatocell Carcinoma. 2023;10:1465–1477. doi:10.2147/JHC.S424616
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018;25(1):1073274817744621. doi:10.1177/1073274817744621
4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
5. Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27(1):85–102. doi:10.1016/j.cld.2022.08.004
6. Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi:10.1001/jamasurg.2018.4334
7. Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27(8):933–940. doi:10.1097/MEG.0000000000000383
8. Tao C, Wu F, Wang H, et al. Clinical benefits of neoadjuvant radiotherapy on the postoperative recurrence of centrally located hepatocellular carcinoma: a real-world evidence based on Phase II clinical trial. J Hepatocell Carcinoma. 2023;10:753–764. doi:10.2147/JHC.S403287
9. Wicks JS, Dale BS, Ruffolo L, et al. Comparable and complimentary modalities for treatment of small-sized HCC: surgical resection, radiofrequency ablation, and microwave ablation. J Clin Med. 2023;12(15):5006. doi:10.3390/jcm12155006
10. Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes Dis. 2019;7(3):370–379. doi:10.1016/j.gendis.2019.10.016
11. Li Y, Li Y, Zhang J, et al. Current perspective of traditional Chinese Medicines and active ingredients in the therapy of hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:41–56. doi:10.2147/JHC.S346047
12. Dai Y, Gao S, Liu X, et al. Effect of aidi injection plus TACE on hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:9196409. doi:10.1155/2018/9196409
13. Hong XC, Liang QL, Luo XB, et al. Clinical study of XiangShaLiuJunZi decoction combined with S-1 as maintenance therapy for stage III or IV gastric carcinoma and colorectal carcinoma. Medicine. 2020;99(19):e20081. doi:10.1097/MD.0000000000020081
14. Huang L, Li H, Xie D, Shi T, Wen C. Personalizing Chinese medicine by integrating molecular features of diseases and herb ingredient information: application to acute myeloid leukemia. Oncotarget. 2017;8(26):43579–43591. doi:10.18632/oncotarget.16983
15. Zheng Y, Zhang W, Xu L, Zhou H, Yuan M, Xu H. Recent progress in understanding the action of natural compounds at novel therapeutic drug targets for the treatment of liver cancer. Front Oncol. 2022;11:795548. doi:10.3389/fonc.2021.795548
16. Sun MY, Song YN, Zhang M, Zhang CY, Zhang LJ, Zhang H. Ginsenoside Rg3 inhibits the migration and invasion of liver cancer cells by increasing the protein expression of ARHGAP9. Oncol Lett. 2019;17(1):965–973. doi:10.3892/ol.2018.9701
17. Zhang H, Tang QF, Sun MY, et al. ARHGAP9 suppresses the migration and invasion of hepatocellular carcinoma cells through up-regulating FOXJ2/E-cadherin. Cell Death Dis. 2018;9(9):916. doi:10.1038/s41419-018-0976-0
18. Yu T, Ye X, Wen Z, et al. Intraoperative indocyanine green retention test of left hemiliver in decision-making for patients with hepatocellular carcinoma undergoing right hepatectomy. Front Surg. 2021;8:709017. doi:10.3389/fsurg.2021.709017
19. Shi F, Fu Y, Wang J, et al. Trametenolic acid B triggers HSP90AA4P and autophagy in HepG2/2.2.15 cells by proteomic analysis. ACS Omega. 2020;5(22):13042–13051. doi:10.1021/acsomega.0c00962
20. Shakiba E, Ramezani M, Sadeghi M. Evaluation of serum interleukin-6 levels in hepatocellular carcinoma patients: a systematic review and meta-analysis. Clin Exp Hepatol. 2018;4(3):182–190. doi:10.5114/ceh.2018.78122
21. Jiang T, Zhang XS, Pan F, et al. The ratio of preoperative alpha-fetoprotein level to total tumor volume as a prognostic factor of hepatocellular carcinoma after liver transplantation. Medicine. 2021;100(26):e26487. doi:10.1097/MD.0000000000026487
22. Huang D, Yang B, Yao Y, et al. Autophagic inhibition of Caveolin-1 by compound phyllanthus urinaria l. activates ubiquitination and proteasome degradation of β-catenin to suppress metastasis of hepatitis B-associated hepatocellular carcinoma. Front Pharmacol. 2021;12:659325. doi:10.3389/fphar.2021.659325
23. Isik B, Gonultas F, Sahin T, Yilmaz S. Microvascular venous invasion in hepatocellular carcinoma: why do recurrences occur? J Gastrointest Cancer. 2020;51(4):1133–1136. doi:10.1007/s12029-020-00487-9
24. He S, Fan X, Ma H, et al. Effect of prophylactic TACE on 5-year survival of patients with hepatocellular carcinoma after hepatectomy. Oncol Lett. 2019;18(2):1824–1830. doi:10.3892/ol.2019.10517
25. Bansal C, Gupta A, Kumar A, Srivastava A. Morphometric evaluation and clinical correlations in pediatric malignant small round cell tumors. Indian J Med Paediatr Oncol. 2014;35(4):267–270. doi:10.4103/0971-5851.144987
26. Pan J, Zhang J, Hsia S-M. Research progress of PCNA in reproductive system diseases. Evid Based Complement Alternat Med. 2021;2021:2391917. doi:10.1155/2021/2391917
27. Eltawil KM, Kidd M, Giovinazzo F, Helmy AH, Salem RR. Differentiating the impact of anatomic and non-anatomic liver resection on early recurrence in patients with Hepatocellular Carcinoma. World J Surg Oncol. 2010;8(1):43. doi:10.1186/1477-7819-8-43
28. Zeng ZM, Mo N, Zeng J, et al. Advances in postoperative adjuvant therapy for primary liver cancer. World J Gastrointest Oncol. 2022;14(9):1604–1621. doi:10.4251/wjgo.v14.i9.1604
29. Kim SH, Son SY, Park YS, Ahn SH, Park DJ, Kim HH. Risk factors for anastomotic leakage: a retrospective cohort study in a single gastric surgical unit. J Gastric Cancer. 2015;15(3):167–175. doi:10.5230/jgc.2015.15.3.167
30. Wang R, Liu Y, Sun H, et al. Estradiol is significantly associated with prognosis in non-surgical liver cancer patients: from bench to bedside. Aging. 2021;13(3):3483–3500. doi:10.18632/aging.202280
31. Lin WC, Lin YS, Chang CW, et al. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS One. 2020;15(5):e0233212. doi:10.1371/journal.pone.0233212
32. Wei ZQ, Zhang YW. Transcatheter arterial chemoembolization followed by surgical resection for hepatocellular carcinoma: a focus on its controversies and screening of patients most likely to benefit. Chin Med J. 2021;134(19):2275–2286. doi:10.1097/CM9.0000000000001767
33. Gao Z, Du G, Pang Y, et al. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high-risk factors. Medicine. 2017;96(33):e7426. doi:10.1097/MD.0000000000007426
34. Zhu GQ, Wang K, Wang B, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. Cancer Manag Res. 2018;11:63–79. doi:10.2147/CMAR.S186150
35. Zhou J, Sun H, Huang Z, et al. Adjuvant lenvatinib after radical resection in patients with hepatocellular carcinoma(HCC): preliminary analysis of a prospective, multi‐center, single‐arm study. J Clin Oncol. 2022;40(S16):e16158. doi:10.1200/JCO.2022.40.16_suppl.e16158
36. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi:10.1136/gutjnl-2018-315983
37. Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15(2):155–169. doi:10.1007/s11684-021-0848-3
38. Niu Y, Shan L, Gao H, et al. Huaier suppresses the hepatocellular carcinoma cell cycle by regulating minichromosome maintenance proteins. Onco Targets Ther. 2020;13:12015–12025. doi:10.2147/OTT.S279723
39. Yang X, Feng Y, Liu Y, et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine. 2021;87:153575. doi:10.1016/j.phymed.2021.153575
40. Yang Y, Sun M, Yao W, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J ImmunotherCancer. 2020;8(1):e000317. doi:10.1136/jitc-2019-000317
41. Wang N, Feng Y, Tan HY, et al. Inhibition of eukaryotic elongation factor-2 confers to tumor suppression by a herbal formulation Huanglian-Jiedu decoction in human hepatocellular carcinoma. J Ethnopharmacol. 2015;164:309–318. doi:10.1016/j.jep.2015.02.025
42. Cheng C, Shou Q, Lang J, et al. Gehua jiecheng decoction inhibits diethylnitrosamine-induced hepatocellular carcinoma in mice by improving tumor immunosuppression microenvironment. Front Pharmacol. 2020;11:809. doi:10.3389/fphar.2020.00809
43. Wan LF, Shen JJ, Wang YH, et al. Extracts of Qizhu decoction inhibit hepatitis and hepatocellular carcinoma in vitro and in C57BL/6 mice by suppressing NF-κB signaling. SCI Rep. 2019;9(1):1415. doi:10.1038/s41598-018-38391-9
44. Chou ST, Hsiang CY, Lo HY, et al. Exploration of anti-cancer effects and mechanisms of Zuo-Jin-Wan and its alkaloid components in vitro and in orthotopic HepG2 xenograft immunocompetent mice. BMC Complement Altern Med. 2017;17(1):121. doi:10.1186/s12906-017-1586-6
45. Liang S, Zou Y, Gao J, et al. The Chinese Medicine, Jiedu Recipe, inhibits the epithelial mesenchymal transition of hepatocellular carcinoma via the regulation of Smad2/3 dependent and independent pathways. Evid Based Complement Alternat Med. 2018;2018:5629304. doi:10.1155/2018/5629304
46. Yu X, Lin XJ, Wang S, et al. Antitumor Efficacy of Huqizhengxiao (HQZX) decoction based on inhibition of telomerase activity in nude mice of hepatocarcinoma xenograft. Integr Cancer Ther. 2018;17(4):1216–1224. doi:10.1177/1534735418785999
47. Li Y, Li Y, Zou Z, Li Y, Xie H, Yang H. Yin Yang Gong Ji pill is an ancient formula with antitumor activity against hepatoma cells. J Ethnopharmacol. 2020;248:112267. doi:10.1016/j.jep.2019.112267
48. Chen HW, Shen AL, Liu LY, Peng J, Chu JF. Bear Bile Powder Inhibits Growth of hepatocellular carcinoma via suppressing STAT3 signaling pathway in mice. Chin J Integr Med. 2020;26(5):370–374. doi:10.1007/s11655-019-3010-1
49. Fan D, Liu C, Li L, et al. Deciphering antitumor mechanism of Pien Tze Huang in mice of hepatocellular carcinoma based on proteomics. J Immunol Res. 2020;2020:4876251. doi:10.1155/2020/4876251
50. Lan HY, An P, Liu QP, et al. Aidi injection induces apoptosis of hepatocellular carcinoma cells through the mitochondrial pathway. J Ethnopharmacol. 2021;274:114073. doi:10.1016/j.jep.2021.114073
51. Cai Y, Du Q, Deng TH, et al. Huxie Huaji ointment induced apoptosis of liver cancer cells in vivo and in vitro by activating the mitochondrial pathway. Evid Based Complement Alternat Med. 2021;2021:9922059. doi:10.1155/2021/9922059
52. Xia J, Rong L, Sawakami T, et al. Shufeng Jiedu Capsule and its active ingredients induce apoptosis, inhibit migration and invasion, and enhances doxorubicin therapeutic efficacy in hepatocellular carcinoma. Biomed Pharmacother. 2018;99:921–930. doi:10.1016/j.biopha.2018.01.163
53. Li M, Zhang M, Zhang ZL, et al. Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncol Res. 2017;25(2):233–239. doi:10.3727/096504016X14742891049073
54. Li X, Yu H, Gong Y, Wu P, Feng Q, Liu C. Fuzheng Xiaozheng prescription relieves rat hepatocellular carcinoma through improving anti-inflammation capacity and regulating lipid related metabolisms. J Ethnopharmacol. 2022;284:114801. doi:10.1016/j.jep.2021.114801
55. Feng XY, Chen BC, Li JC, et al. Gansui-Banxia Decoction extraction inhibits MDSCs accumulation via AKT /STAT3/ERK signaling pathways to regulate antitumor immunity in C57bl/6 mice. Phytomedicine. 2021;93:153779. doi:10.1016/j.phymed.2021.153779
56. Chen X, Cao Z, Zhang Y, et al. Fuzheng Qingjie granules inhibit growth of hepatoma cells via inducing mitochondria-mediated apoptosis and enhancing immune function. Integr Cancer Ther. 2017;16(3):329–338. doi:10.1177/1534735416654761
57. Shen Y, Yang F, Peng H, et al. Anti-tumor effect of Yanggan Huayu granule by inducing AKT-mediated apoptosis in hepatocellular carcinoma. J Ethnopharmacol. 2022;282:114601. doi:10.1016/j.jep.2021.114601
58. Hu B, Zhang T, An HM, Zheng JL, Yan X, Huang XW. Herbal formula YGJDSJ inhibits Anchorage-independent growth and induces anoikis in hepatocellular carcinoma Bel-7402 cells. BMC Complement Altern Med. 2018;18(1):17. doi:10.1186/s12906-018-2083-2
59. Wu L, Zhao J, Cai H, et al. Dahuang Zhechong Pill combined with doxorubicin induces cell death through regulating energy metabolism in human hepatocellular carcinoma cells. Evid Based Complement Alternat Med. 2017;2017:6279576. doi:10.1155/2017/6279576
60. Wu HC, Lay IS, Shibu MA, et al. Zanthoxylum avicennae extract enhances GSK-3β to attenuate β-catenin via phosphatase 2A to block metastatic effects of HA22T cells and hepatocellular carcinoma xenografted nude mice. Environ Toxicol Int J. 2017;32(9):2133–2143. doi:10.1002/tox.22426
61. Tan ZB, Fan HJ, Wu YT, et al. Rheum palmatum extract exerts anti-hepatocellular carcinoma effects by inhibiting signal transducer and activator of transcription 3 signaling. J Ethnopharmacol. 2019;232:62–72. doi:10.1016/j.jep.2018.12.019
62. Wang D, Yang C, Wang Z, et al. Norcantharidin combined with Coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation. SCI Rep. 2017;7(1):9373. doi:10.1038/s41598-017-09668-2
63. Guo Z, Zhou Y, Yang J, Shao X. Dendrobium candidum extract inhibits proliferation and induces apoptosis of liver cancer cells by inactivating Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019;110:371–379. doi:10.1016/j.biopha.2018.11.149
64. Wang R, Shao X, Yang J, Liu Z, Chew L, Shao Y. Ginkgo biloba extract mechanism inhibits hepatocellular carcinoma through the nuclear factor-κB/p53 signaling pathway. J Environ Pathol Toxicol Oncol. 2020;39(2):179–189. doi:10.1615/JEnvironPatholToxicolOncol.2020034510
65. Yuan P, Li J, Aipire A, et al. Cistanche tubulosa phenylethanoid glycosides induce apoptosis in H22 hepatocellular carcinoma cells through both extrinsic and intrinsic signaling pathways. BMC Complement Altern Med. 2018;18(1):275. doi:10.1186/s12906-018-2201-1
66. Tsai JJ, Chen JH, Chen CH, Chung JG, Hsu FT. Apoptosis induction and ERK/NF-κB inactivation are associated with magnolol-inhibited tumor progression in hepatocellular carcinoma in vivo. Environ Toxicol Int J. 2020;35(2):167–175. doi:10.1002/tox.22853
67. Song YG, Kang L, Tian S, et al. Study on the anti-hepatocarcinoma effect and molecular mechanism of Prunella vulgaris total flavonoids. J Ethnopharmacol. 2021;273:113891. doi:10.1016/j.jep.2021.113891
68. Lin XL, Li K, Yang Z, Chen B, Zhang T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine. 2020;66:153112. doi:10.1016/j.phymed.2019.153112
69. Lu L, Liu S, Dong Q, Xin Y. Salidroside suppresses the metastasis of hepatocellular carcinoma cells by inhibiting the activation of the Notch1 signaling pathway. Mol Med Rep. 2019;19(6):4964–4972. doi:10.3892/mmr.2019.10115
70. Zhang J, Gao Y, Han H, Zou C, Feng Y, Zhang H. Matrine suppresses lung metastasis of human hepatocellular carcinoma by directly targeting matrix metalloproteinase-9. Biochem Biophys Res Commun. 2019;515(1):57–63. doi:10.1016/j.bbrc.2019.04.063
71. Le J, Fu Y, Han Q, et al. Transcriptome analysis of the inhibitory effect of sennoside A on the metastasis of hepatocellular carcinoma cells. Front Pharmacol. 2021;11:566099. doi:10.3389/fphar.2020.566099
72. Ye Y, Song Y, Zhuang J, Wang G, Ni J, Xia W. Anticancer effects of echinacoside in hepatocellular carcinoma mouse model and HepG2 cells. J Cell Physiol. 2019;234(2):1880–1888. doi:10.1002/jcp.27063
73. Sun Y, Tan YJ, Lu ZZ, et al. Arctigenin inhibits liver cancer tumorigenesis by inhibiting gankyrin expression via C/EBPα and PPARα. Front Pharmacol. 2018;9:268. doi:10.3389/fphar.2018.00268
74. Sohretoglu D, Shile H. Ganoderma lucidum polysaccharides as an anti-cancer agent. Anti Cancer Agents Med Chem. 2018;18(5):667–674. doi:10.2174/1871520617666171113121246
75. Mo Z, Cao Z, Yu L, et al. An integrative analysis reveals the potential mechanism between Herbal Medicine Yinchen and immunoregulation in hepatocellular carcinoma. Biomed Res Int. 2020;2020:8886914. doi:10.1155/2020/8886914
76. Qi X, Wang L, Wang H, Yang L, Li X, Wang L. Aconitine inhibits the proliferation of hepatocellular carcinoma by inducing apoptosis. Int J Clin Exp Pathol. 2018;11(11):5278–5289.
77. Li NN, Meng XS, Men WX, Bao YR, Wang S. Total flavonoids from oroxylum indicum induce apoptosis via PI3K/Akt/PTEN signaling pathway in liver cancer. Evid Based Complement Alternat Med. 2018;2018:3021476. doi:10.1155/2018/3021476
78. Bi L, Yan X, Chen W, Gao J, Qian L, Qiu S. Antihepatocellular carcinoma potential of tetramethylpyrazine induces cell cycle modulation and mitochondrial-dependent apoptosis: regulation of p53 signaling pathway in HepG2 cells in vitro. Integr Cancer Ther. 2016;15(2):226–236. doi:10.1177/1534735416637424
79. Liu T, Tian L, Fu X, Wei L, Li J, Wang T. Saffron inhibits the proliferation of hepatocellular carcinoma via inducing cell apoptosis. Panminerva Med. 2020;62(1):7–12. doi:10.23736/S0031-0808.18.03561-9
80. Lei Y, Gan H, Huang Y, et al. Digitoxin inhibits proliferation of multidrug-resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. Oncol Lett. 2020;20(4):71. doi:10.3892/ol.2020.11932
81. Tan HY, Wang N, Tsao SW, Zhang Z, Feng Y Suppression of vascular endothelial growth factor via inactivation of eukaryotic elongation factor 2 by alkaloids in coptidis rhizome in hepatocellular carcinoma. Integr Cancer Ther. 2014;13(5):425–43.
82. Shi L, Zhang G, Zheng Z, Lu B, Ji L. Andrographolide reduced VEGFA expression in hepatoma cancer cells by inactivating HIF-1: the involvement of JNK and MTA1/HDCA. Chem Biol Interact. 2017;273:228–236. doi:10.1016/j.cbi.2017.06.024
83. Tang D, Zhang S, Shi X, et al. Combination of astragali polysaccharide and curcumin improves the morphological structure of tumor vessels and induces tumor vascular normalization to inhibit the growth of hepatocellular carcinoma. Integr Cancer Ther. 2019;18:1534735418824408. doi:10.1177/1534735418824408
84. Qu J, Lu W, Chen M, et al. Combined effect of recombinant human adenovirus p53 and curcumin in the treatment of liver cancer. EXP Ther Med. 2020;20(5):18. doi:10.3892/etm.2020.9145
85. Tian S, Liao L, Zhou Q, et al. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol Lett. 2021;21(4):286. doi:10.3892/ol.2021.12547
86. Hu P, Ke C, Guo X, et al. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Dig Liver Dis. 2019;51(1):120–126. doi:10.1016/j.dld.2018.06.012
87. Zhang G, Zeng X, Zhang R, et al. Dioscin suppresses hepatocellular carcinoma tumor growth by inducing apoptosis and regulation of TP53, BAX, BCL2 and cleaved CASP3. Phytomedicine. 2016;23(12):1329–1336. doi:10.1016/j.phymed.2016.07.003
88. Li F, Wang J, Wu N, Zhang H, Li Z, Wei N. H1, a derivative of tetrandrine, enhances the efficacy of 5-FU in Bel7402/5-FU cells via suppressing STAT3/MCL-1 and inducing PUMA. Biochem Biophys Res Commun. 2019;520(1):93–98. doi:10.1016/j.bbrc.2019.09.082
89. Yu Y, Qian L, Du N, Liu Y, Zhao X, Zhang X. Ganoderma lucidum polysaccharide enhances radiosensitivity of hepatocellular carcinoma cell line HepG2 through Akt signaling pathway. Exp Ther Med. 2017;14(6):5903–5907. doi:10.3892/etm.2017.5340
90. Tian YD, Lin S, Yang PT, et al. Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. 2019;10(20):4947–4953. doi:10.7150/jca.30286
91. Zhang CY, Jiang ZM, Ma XF, et al. Saikosaponin-d inhibits the hepatoma cells and enhances chemosensitivity through SENP5-dependent inhibition of Gli1 SUMOylation under Hypoxia. Front Pharmacol. 2019;10:1039. doi:10.3389/fphar.2019.01039
92. Yu L, Jiang B, Chen Z, et al. Cytisine induces endoplasmic reticulum stress caused by calcium overload in HepG2 cells. OncolRep. 2018;39(3):1475–1484. doi:10.3892/or.2018.6200
93. Deng LJ, Lei YH, Quan JY, et al. 1β-OH-arenobufagin induces mitochondrial apoptosis in hepatocellular carcinoma through the suppression of mTOR signaling pathway. J Ethnopharmacol. 2021;266:113443. doi:10.1016/j.jep.2020.113443
94. Yu Z, Feng H, Sun X, et al. Bufalin suppresses hepatocarcinogenesis by targeting β-catenin/TCF signaling via cell cycle-related kinase. Sci Rep. 2018;8(1):3891. doi:10.1038/s41598-018-22113-2
95. Liu K, Tian T, Zheng Y, et al. Scutellarin inhibits proliferation and invasion of hepatocellular carcinoma cells via down-regulation of JAK2/STAT3 pathway. J Cell Mol Med. 2019;23(4):3040–3044. doi:10.1111/jcmm.14169
96. Gao Y, Chu S, Shao Q, et al. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. 2017;51(1):1–13. doi:10.1080/10715762.2016.1234710
97. Qian YY, Li WY, Yan Y, et al. Celastrus orbiculatus extracts inhibit human hepatocellular carcinoma growth by targeting mTOR signaling pathways. Chin J Integr Med. 2019;25(11):845–852. doi:10.1007/s11655-019-3035-5
98. Hsu WC, Ramesh S, Shibu MA, et al. Platycodin D reverses histone deacetylase inhibitor resistance in hepatocellular carcinoma cells by repressing ERK1/2-mediated cofilin-1 phosphorylation. Phytomedicine. 2021;82:153442. doi:10.1016/j.phymed.2020.153442
99. Guo Z, Chen W, Dai G, Huang Y. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4. Int J Mol Med. 2020;45(1):141–150. doi:10.3892/ijmm.2019.4391
100. Qi F, Wang J, Zhao L, Cai P, Tang W, Wang Z. Cinobufacini inhibits epithelial-mesenchymal transition of human hepatocellular carcinoma cells through c-Met/ERK signaling pathway. Biosci Trends. 2018;12(3):291–297. doi:10.5582/bst.2018.01082
101. Shan L, Li Y, Jiang H, et al. Huaier restrains proliferative and migratory potential of hepatocellular carcinoma cells partially through decreased Yes-Associated Protein 1. J Cancer. 2017;8(19):4087–4097. doi:10.7150/jca.21018
102. Han H, Wang L, Liu Y, et al. Combination of curcuma zedoary and kelp inhibits growth and metastasis of liver cancer in vivo and in vitro via reducing endogenous H2S levels. Food Funct. 2019;10(1):224–234. doi:10.1039/c8fo01594e
103. Zhao J, Lin W, Cao Z, et al. Total alkaloids of Rubus aleaefolius Poir. Inhibit the STAT3 signaling pathway leading to suppression of proliferation and cell cycle arrest in a mouse model of hepatocellular carcinoma. Oncol Rep. 2013;30(3):1309–1314. doi:10.3892/or.2013.2585
104. Wang XB, Wu DJ, Chen WP, Liu J, Ju YJ. Impact of radiotherapy on immunological parameters, levels of inflammatory factors, and clinical prognosis in patients with esophageal cancer. J Radiat Res. 2019;60(3):353–363. doi:10.1093/jrr/rrz006
105. Sena P, Mancini S, Bertacchini J, Carnevale G, Pedroni M, Roncucci L. Autoimmunity profiles as prognostic indicators in patients with colorectal cancer versus those with cancer at other sites: a prospective study. Cancers. 2021;13(13):3239. doi:10.3390/cancers13133239
106. Chen PC, Chen CC, Ker YB, Chang CH, Chyau CC, Hu ML. Anti-metastatic effects of antrodan with and without cisplatin on Lewis lung carcinomas in a Mouse Xenograft Model. Int J Mol Sci. 2018;19(6):1565. doi:10.3390/ijms19061565
107. Klimaszewska-Wiśniewska A, Grzanka D, Czajkowska P, et al. Cellular and molecular alterations induced by low‑dose fisetin in human chronic myeloid leukemia cells. Int J Oncol. 2019;55(6):1261–1274. doi:10.3892/ijo.2019.4889
108. Su YS, Kuo MZ, Kuo YT, et al. Diterpenoid anthraquinones as chemopreventive agents altered microRNA and transcriptome expressions in cancer cells. Biomed Pharmacother. 2021;136:111260. doi:10.1016/j.biopha.2021.111260
109. Wu DL, Liao ZD, Chen FF, et al. Benzophenones from Anemarrhena asphodeloides Bge. Exhibit anticancer activity in HepG2 cells via the NF-κB signaling pathway. Molecules. 2019;24(12):2246. doi:10.3390/molecules24122246
110. Shi J, Wang L, Lu Y, et al. Protective effects of sea buckthorn pulp and seed oils against radiation-induced acute intestinal injury. J Radiat Res. 2017;58(1):24–32. doi:10.1093/jrr/rrw069
111. Yin Z, Chen D, Liang S, Li X. Neoadjuvant therapy for hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:929–946. doi:10.2147/JHC.S357313
112. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002 Nov;3(11):991–8
113. Zheng S, Asnani M, Thomas-Tikhonenko A, et al. Escape from ALL-CARTaz: leukemia immunoediting in the age of chimeric antigen receptors. Cancer Journal. 2019;25(3):217–222. doi:10.1097/PPO.0000000000000381
114. Yu D, Guang YA. Clinical effects of Xihuang Pill combined with chemotherapy in patients with advanced colorectal cancer. Evid Based Complement Altern Med. 2017;2017:5936086. doi:10.1155/2017/5936086
115. Xia D, Li W, Tang C, Jiang J. Astragaloside IV, as a potential anticancer agent. Front Pharmacol. 2023;14:1065505. doi:10.3389/fphar.2023.1065505
116. Hu J, Jiao J, Wang Y, et al. Effect of extract from ginseng rust rot on the inhibition of human hepatocellular carcinoma cells in vitro. Micron. 2019;124:102710. doi:10.1016/j.micron.2019.102710
117. Gerardi G, Rivero-Pérez MD, Cavia-Saiz M, et al. Wine Pomace Product Inhibit Listeria monocytogenes invasion of intestinal cell lines Caco-2 and SW-480. Foods. 2021;10(7):1485. doi:10.3390/foods10071485
118. Gong G, Liu Q, Deng Y, et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol. 2020;149:639–650. doi:10.1016/j.ijbiomac.2020.01.251
119. Liu JS, Huo CY, Cao HH, et al. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway [published correction appears in Phytomedicine. 2021 Nov;92:153731]. Phytomedicine. 2019;61:152843. doi:10.1016/j.phymed.2019.152843
120. Ma L, Jiang H, Xu X, et al. Tanshinone IIA mediates SMAD7-YAP interaction to inhibit liver cancer growth by inactivating the transforming growth factor beta signaling pathway. Aging. 2019;11(21):9719–9737. doi:10.18632/aging.102420
121. Wen B, Wei YT, Mu LL, Wen GR, Zhao K. The molecular mechanisms of celecoxib in tumor development. Medicine. 2020;99(40):e22544. doi:10.1097/MD.0000000000022544
122. Di W, Li F, He L, et al. A transcription factor DAF-5 functions in Haemonchus contortus development. Parasit Vectors. 2021;14(1):529. doi:10.1186/s13071-021-05036-2
123. Sheng J, Zou X, Cheng Z, et al. Recent advances in herbal medicines for digestive system malignancies. Front Pharmacol. 2018;9:1249. doi:10.3389/fphar.2018.01249
124. Pretzsch E, Bösch F, Neumann J, et al. Mechanisms of metastasis in colorectal cancer and metastatic organotropism: hematogenous versus peritoneal spread. J Oncol. 2019;2019:7407190. doi:10.1155/2019/7407190
125. El Khoury D, Matar R, Touma T. Curcumin and endometrial carcinoma: an old spice as a novel agent. Int J Womens Health. 2019;11:249–256. doi:10.2147/IJWH.S194262
126. Yang YSH, Li ZL, Shih YJ, et al. Herbal medicines attenuate PD-L1 expression to induce anti-proliferation in obesity-related cancers. Nutrients. 2019;11(12):2979. doi:10.3390/nu11122979
127. Shao Y, Zhu W, Da J, et al. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017;10:2675–2683. doi:10.2147/OTT.S130653
128. Tu SP, Jin H, Shi JD, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res. 2012;5(2):205–215. doi:10.1158/1940-6207.CAPR-11-0247
129. Cheng-En H, Gan J, Zhang R-D, et al. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46(2):156–164. doi:10.3109/00365521.2010.516450
130. Helgadottir H, Rocha Trocoli Drakensjö I, Girnita A. Personalized medicine in malignant melanoma: towards patient tailored treatment. Front Oncol. 2018;8:202. doi:10.3389/fonc.2018.00202
131. Wang Z, Wu X. Abnormal function of telomere protein TRF2 induces cell mutation and the effects of environmental tumor-promoting factors (Review). Oncol Rep. 2021;46(2):184. doi:10.3892/or.2021.8135
132. Lyu SY, Choi SH, Park WB. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch Pharmacal Res. 2002;25(1):93–101. doi:10.1007/BF02975269
133. Yang P, Jiang Y, Pan Y, et al. Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression. Sci Rep. 2019;9(1):6428. doi:10.1038/s41598-019-41444-2
134. Park S-Y, Lee C-J, Choi J-H, et al. The JAK2/STAT3/CCND2 Axis promotes colorectal cancer stem cell persistence and radioresistance. J Exper Clin Cancer Res. 2019;38(1):399. doi:10.1186/s13046-019-1405-7
135. uang C, Xiang Y, Chen S, et al. Dermokine contributes to epithelial-mesenchymal transition through increased activation of signal transducer and activator of transcription 3 in pancreatic cancer. Cancer Sci. 2017;108(11):2130–2141. doi:10.1111/cas.13347
136. Wang B, Min W, Lin S, et al. Saikosaponin-d increases radiation-induced apoptosis of hepatoma cells by promoting autophagy via inhibiting mTOR phosphorylation. Int J Med Sci. 2021;18(6):1465–1473. doi:10.7150/ijms.53024
137. Zhang B, Shi D, Zhang X, Liang G, Liu W, Qiao S. FK866 inhibits the epithelial-mesenchymal transition of hepatocarcinoma MHCC97-H cells. Oncol Lett. 2018;16(6):7231–7238. doi:10.3892/ol.2018.9541
138. Shamay Y, Shah J, Işık M, et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat Mater. 2018;17(4):361–368. doi:10.1038/s41563-017-0007-z
139. Tang W, Chen Z, Zhang W, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5(1):87. doi:10.1038/s41392-020-0187-x
140. Abdul-Hafez A, Mohamed T, Omar H, Shemis M, Uhal BD. The renin angiotensin system in liver and lung: impact and therapeutic potential in organ fibrosis. J Lung Pulm Respir Res. 2018;5(1):00160.
141. Su J, Liao D, Su Y, et al. Novel polysaccharide extracted from Sipunculus nudus inhibits HepG2 tumour growth in vivo by enhancing immune function and inducing tumour cell apoptosis. J Cell Mol Med. 2021;25(17):8338–8351. doi:10.1111/jcmm.16793
142. Wu R, Liang Y, Xu M, et al. Advances in chemical constituents, clinical applications, pharmacology, pharmacokinetics and toxicology of Erigeron breviscapus. Front Pharmacol. 2021;12:656335. doi:10.3389/fphar.2021.656335
143. Wang Z, Liu F, Yu JJ, Jin JZ. β-Bourbonene attenuates proliferation and induces apoptosis of prostate cancer cells. Oncol Lett. 2018;16(4):4519–4525. doi:10.3892/ol.2018.9183
144. Liu Z, Ren B, Wang Y, et al. Sesamol induces human hepatocellular carcinoma cells apoptosis by impairing mitochondrial function and suppressing autophagy. Sci Rep. 2017;7(1):45728. doi:10.1038/srep45728
145. Choudhary HB, Mandlik SK, Mandlik DS. Role of p53 suppression in the pathogenesis of hepatocellular carcinoma. World J Gastrointest Pathophysiol. 2023;14(3):46–70. doi:10.4291/wjgp.v14.i3.46
146. Li H, Ruan WJ, Liu LQ, et al. Impact of Taurine on the proliferation and apoptosis of human cervical carcinoma cells and its mechanism. Chin Med J. 2019;132(8):948–956. doi:10.1097/CM9.0000000000000162
147. Wang QS, Gao LN, Zhu XN, et al. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics. 2019;9(21):6239–6255. doi:10.7150/thno.35972
148. Shu Y, Yang Y, Zhao Y, et al. Melittin inducing the apoptosis of renal tubule epithelial cells through upregulation of Bax/Bcl-2 expression and activation of TNF-α signaling pathway. Biomed Res Int. 2019;2019:9450368. doi:10.1155/2019/9450368
149. Londono C, Osorio C, Gama V, Alzate O. Mortalin, apoptosis, and neurodegeneration. Biomolecules. 2012;2(1):143–164. doi:10.3390/biom2010143
150. Zhou C, Luo Y, Lei Z, Wei G. UHPLC-ESI-MS analysis of purified flavonoids fraction from stem of dendrobium denneaum paxt. and its preliminary study in inducing apoptosis of HepG2 cells. Evid Based Complement Alternat Med. 2018;2018:8936307. doi:10.1155/2018/8936307
151. Yu B, Wang AH, Zhou K, Chai LJ, Liu L. Molecular pathway of psoralidin-induced apoptosis in HepG2 cell line. Chin J Integr Med. 2019;25(10):757–762. doi:10.1007/s11655-016-2251-5
152. Wang X, Peng P, Pan Z, Fang Z, Lu W, Liu X. Psoralen inhibits malignant proliferation and induces apoptosis through triggering endoplasmic reticulum stress in human SMMC7721 hepatoma cells. Biol Res. 2019;52(1):34. doi:10.1186/s40659-019-0241-8
153. Tan J, Sun X, Wang S, et al. Evaluation of angiogenesis and pathological classification of extrahepatic cholangiocarcinoma by dynamic MR imaging for E-Healthcare. J Healthc Eng. 2021;2021:8666498. doi:10.1155/2021/8666498
154. Liu M, Li H, Wang X, Jing L, Jiang P, Li Y. Experimental study of the vascular normalization window for tumors treated with apatinib and the efficacy of sequential chemotherapy with apatinib in lung cancer-bearing mice and patients. Cancer Med. 2020;9(8):2660–2673. doi:10.1002/cam4.2923
155. Raineri EJM, Yedavally H, Salvati A, van Dijl JM. Time-resolved analysis of Staphylococcus aureus invading the endothelial barrier. Virulence. 2020;11(1):1623–1639. doi:10.1080/21505594.2020.1844418
156. Sneddon JB, Tang Q, Stock P, et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell. 2018;22(6):810–823. doi:10.1016/j.stem.2018.05.016
157. Zhang L, Wang Y, Rashid MH, et al. Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis. Oncotarget. 2017;8(31):51591–51607. doi:10.18632/oncotarget.18196
158. Zhang H, Wu Z, Hu D, et al. Immunotherapeutic targeting of NG2/CSPG4 in solid organ cancers. Vaccines. 2022;10(7):1023. doi:10.3390/vaccines10071023
159. Li C-F, Pan Y-K, Gao Y, et al. Autophagy protects HUVECs against ER stress-mediated apoptosis under simulated microgravity. Apoptosis. 2019;24(9–10):812–825. doi:10.1007/s10495-019-01560-w
160. Chen Z, Zhang J, Xue H, et al. Nitidine chloride is a potential alternative therapy for glioma through inducing endoplasmic reticulum stress and alleviating epithelial-mesenchymal transition. Integr Cancer Ther. 2020;19:1534735419900927. doi:10.1177/1534735419900927
161. Liu Y-H, Weng Y-P, Lin H-Y, et al. Aqueous extract of Polygonum bistorta modulates proteostasis by ROS-induced ER stress in human hepatoma cells. Sci Rep. 2017;7(1):41437. doi:10.1038/srep41437
162. Wen D, Liu W-L, Lu Z-W, et al. SNHG9, a papillary thyroid cancer cell exosome-enriched lncRNA, inhibits cell autophagy and promotes cell apoptosis of normal thyroid epithelial cell Nthy-ori-3 through YBOX3/P21 pathway. Front Oncol. 2021;11:647034. doi:10.3389/fonc.2021.647034
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
CT-Based Radiomics for the Recurrence Prediction of Hepatocellular Carcinoma After Surgical Resection
Wang F, Chen Q, Zhang Y, Chen Y, Zhu Y, Zhou W, Liang X, Yang Y, Hu H
Journal of Hepatocellular Carcinoma 2022, 9:453-465
Published Date: 23 May 2022
A Machine Learning Model Based on Health Records for Predicting Recurrence After Microwave Ablation of Hepatocellular Carcinoma
An C, Yang H, Yu X, Han Z, Cheng Z, Liu F, Dou J, Li B, Li Y, Li Y, Yu J, Liang P
Journal of Hepatocellular Carcinoma 2022, 9:671-684
Published Date: 28 July 2022
Recurrent Hepatocellular Carcinoma: Patterns, Detection, Staging and Treatment
Papaconstantinou D, Tsilimigras DI, Pawlik TM
Journal of Hepatocellular Carcinoma 2022, 9:947-957
Published Date: 3 September 2022
Delayed Hepatocellular Carcinoma Recurrence After Liver Transplantation: Comprehensive Clinical Characterization of Case Series
Wong TH, Ho CM, Hsu HH, Wu YM, Ho MC, Lee PH, Hu RH
Journal of Hepatocellular Carcinoma 2022, 9:1081-1091
Published Date: 17 October 2022
Clinical Benefits of Neoadjuvant Radiotherapy on the Postoperative Recurrence of Centrally Located Hepatocellular Carcinoma: A Real-World Evidence Based on Phase II Clinical Trial
Tao C, Wu F, Wang H, Wang L, Liu Y, Wu A, Zheng L, Wang Y, Chen B, Rong W, Wu J
Journal of Hepatocellular Carcinoma 2023, 10:753-764
Published Date: 16 May 2023
