Back to Journals » Patient Preference and Adherence » Volume 17
Advancing Digital Solutions to Overcome Longstanding Barriers in Asthma and COPD Management
Authors Bosnic-Anticevich S , Bakerly ND, Chrystyn H, Hew M, van der Palen J
Received 11 August 2022
Accepted for publication 9 December 2022
Published 28 January 2023 Volume 2023:17 Pages 259—272
DOI https://doi.org/10.2147/PPA.S385857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Sinthia Bosnic-Anticevich,1 Nawar Diar Bakerly,2 Henry Chrystyn,3 Mark Hew,4 Job van der Palen5
1Woolcock Institute of Medical Research, University of Sydney, Sydney, NSW, Australia; 2Manchester Metropolitan University, Manchester, United Kingdom, Salford Royal NHS Foundation Trust, Manchester, UK; 3Inhalation Consultancy Ltd, Leeds, UK; 4Allergy, Asthma, and Clinical Immunology, Alfred Health, Melbourne, VIC, Australia; 5Medical School Twente, Medisch Spectrum Twente, Enschede, the Netherlands, and Section Cognition, Data and Education, University of Twente, Enschede, the Netherlands
Correspondence: Sinthia Bosnic-Anticevich, Woolcock Institute of Medical Research, 431 Glebe Point Road, Glebe, 2037, NSW, Australia, Tel +61 414 015 614, Email [email protected]
Abstract: Maintenance therapy delivered via inhaler is central to asthma and chronic obstructive pulmonary disease (COPD) management. Poor adherence to inhaled medication and errors in inhalation technique have long represented major barriers to the optimal management of these chronic conditions. Technological innovations may provide a means of overcoming these barriers. This narrative review examines ongoing advances in digital technologies relevant to asthma and COPD with the potential to inform clinical decision-making and improve patient care. Digital inhaler devices linked to mobile apps can help bring about changes in patients’ behaviors and attitudes towards disease management, particularly when they build in elements of interactivity and gamification. They can also support ongoing technique education, empowering patients and helping providers maximize the value of consultations and develop effective action plans informed by insights into the patient’s inhaler use patterns and their respiratory health. When combined with innovative techniques such as machine learning, digital devices have the potential to predict exacerbations and prompt pre-emptive intervention. Finally, digital devices may support an advanced precision medicine approach to respiratory disease management and help support shared decision-making. Further work is needed to increase uptake of digital devices and integrate their use into care pathways before their full potential in personalized asthma and COPD management can be realized.
Keywords: asthma, chronic obstructive pulmonary disease, technology, device, data
Introduction – Unmet Needs in the Implementation of Innovative Advances in Inhaler Technology: The Need for an Innovative Approach to Implementation
Together, asthma and chronic obstructive pulmonary disease (COPD) affect almost three quarters of a billion people worldwide.1,2 Despite the availability of effective treatments, poor disease control is common and is associated with reduced quality of life and premature death in patients of all ages.3,4 Inhaled medications play a central role in the management of these conditions for most patients. Global recommendations highlight the importance of adherence to inhaled medication and correct inhalation technique in managing symptoms;3,4 however, despite growing recognition of the unmet needs, both of these continue to pose major challenges in clinical practice.
Innovative technology has the potential to personalize management strategies based on real-time individualized data. A variety of digital strategies have been developed to monitor and improve inhaled medication adherence and technique. A recent Cochrane review by Chan et al investigated the range of digital interventions available to address medication non-adherence, highlighting their potential to improve clinical outcomes in patients with asthma compared with non-digital interventions and usual care.5 Such interventions can range from straightforward text-message reminders and self-management tools (such as mobile applications and online platforms) to more complex digital inhalers composed of add-on or integrated electromechanical sensor(s) and associated microelectronics. While most of the latter are capable of detecting the time and date of inhaler actuations,6,7 more advanced devices can even record clinical-grade inhalation parameters, such as peak inspiratory flow (PIF), inhaled volume, and inhalation duration – a function capable of distinguishing between a physical actuation and an optimal inhalation.8
Digital inhalers can provide greater connectivity between patients and clinicians through the sharing of personalized data. Information on medication adherence and/or technique is transferred between connected devices, such as inhalers and smartphones via mobile application services.6,9 By providing all stakeholders with accurate and objective data, digital technologies may play an important role in overcoming traditionally recurring barriers such as non-adherence in patients with uncontrolled asthma10,11 and should be fundamental to the development of data-driven, personalized patient care.7,9
To realize the full potential of digital inhalers, further innovation is needed to advance research as well as our understanding of respiratory disease and the patient’s role in managing it. Additionally, there is need to inform clinical practice and create suitable infrastructure to successfully incorporate data from digital devices into routine care. Monitoring of any sort cannot benefit patient outcomes unless paired with effective feedback and interventions. Whilst there is an abundance of evidence on what constitutes good respiratory disease management, innovation is required regarding how these digital inhalers, with all their differences and similarities, are integrated into clinical care in the right way, for the right patient, at the right time. This will be our greatest challenge ahead.
This narrative review provides a snapshot of the intersection between digital inhaler capabilities and the potential impact they can have on respiratory disease management, based on empirical evidence identifying the challenges, and global strategies driving the framework for the management of chronic respiratory diseases. It also highlights the needs, key gaps and unanswered questions associated with implementing digital inhalers into real-world practice.
The Opportunities
Digital Inhalers Can Address the Fundamental Need for Accurate and Objective Information About Medication Use
Adherence to prescribed controller medications in asthma and COPD is often suboptimal and associated with poorer clinical outcomes.12 This extends to inhaler technique since critical errors in technique are common and can result in apparently “adherent” patients failing to obtain benefit from their prescribed medication.13,14 For instance, the CRITIKAL study found that failure to generate sufficient inspiratory effort is associated with uncontrolled asthma and increased exacerbation rates,14 likely owing to the impact of this error on delivered dose to the lungs.15 Misconceptions about medication use can ensue, with consequential implications for disease management. Hence, better insights into patients’ medication adherence and inhaler technique may provide opportunities to inform clinical decisions and improve care.16
The Opportunity to More Accurately Evaluate and Improve Medication Adherence
Self-reported adherence rates are notoriously unreliable.17–20 Various attempts have been made to address this issue including use of dose counters, prescription refill data and biomarker measurements, such as fractional exhaled nitric oxide. However, each of these approaches has confounding factors and significant limitations such as not providing information about whether doses were inhaled or were simply discharged, and fractional exhaled nitric oxide levels being influenced by a variety of intrinsic and extrinsic factors other than asthma.10,21,22
Simple digital solutions such as dose counters attempt to track adherence rates of patients with obstructive airway diseases in the real world;23 however, these methods are unlikely to accurately reflect actual adherence behaviors of patients.24 In recognition of the importance of both adherence (related to when a patient takes their medication) as well as inhaler technique (how a patient takes their medication), the term “actual adherence” has been coined, which considers the attempted rate of adherence, the interval between doses and the inhaler technique error rate. Compared with standalone measures, such as data from dose counters, prescription refills, claims data, and/or self-reporting, actual adherence data might inform a more complete assessment of medicine use and could be more reflective of changes in the quality of life and lung function of patients with asthma.11,25 Furthermore, objective data from digital inhalers often show significantly lower actual adherence among patients with respiratory diseases compared with standard measures (Table 1).11,24,26–29 For instance, actual adherence data in one study of patients with COPD indicated that less than 25% used their inhaler correctly and on time.28
 |
Table 1 Adherence Measured by Different Electronic Monitoring Devices |
It is also important to appreciate that medication non-adherence can be categorized into two types, unintentional and intentional, that require different remedial approaches.30 Unintentional non-adherence is often responsive to patient education, treatment simplification, or the use of a reminder system. Intentional non-adherence is typically more complex and challenging since strategies focused on unintentional non-adherence are ineffective in these contexts.31
Using real-time electronic medication monitoring technology to initiate support calls or automatic reminders can help reduce unintentional medication non-adherence in patients with asthma and COPD. Vasbinder et al showed that sending tailored SMS reminders, warning of a missed or likely to be missed dose, to children with asthma resulted in better inhaled corticosteroid (ICS) adherence, but no improvement in disease control, quality-adjusted life years (QALY) or exacerbation rates.32 A similar conclusion was reported in another study, entailing sending personalized reminders together with additional support calls (if medication was missed or if rescue medication use doubled). Adherence improved but did not significantly change quality of life in patients with asthma and COPD. However, although there was also no observed effect on time to next exacerbation, the intervention group did show a trend toward a 39% decline in exacerbation frequency.33
Interventions addressing the more complex challenge of intentional non-adherence require additional elements.34 For instance, the gamification of disease management systems – with the intention of replacing the “chore” of routine medication with that of a captivating game – has demonstrated positive results in young people with asthma.35 Elias et al reported improvements in adherence after gamifying the use of a handheld spirometer via a mobile phone-based application. Patient satisfaction rates were high and none of those enrolled reported a preference for the spirometer alone over the novel intervention.35 Similar results were reported for a sensor and companion app containing game features; adolescents with asthma were interested in continued use of the technology, noting that they thought the intervention improved asthma control.36 These results highlight the potential positive impact game features could have on adherence when incorporated into medication regimens.
In a literature review published by Mosnaim et al, digital interventions utilized in asthma management were identified and compared.37 It was reported that “generalized” interventions (defined as noninteractive, non-patient specific digital education material) demonstrated improvements in medication adherence; however, these did not translate to improvements in asthma burden. This may be a result of overestimated adherence caused by intentional non-adherence, such as the deliberate firing of inhalers to increase adherence measurements.32 In contrast, Mosnaim reported that interactive interventions (most often composed of an asthma management platform alone or in combination with a digital inhaler) that involved patient-specific data and bi-directional communication demonstrated improvements in both medication adherence and disease impairment.37 These results highlight the superior impact of initiatives that provide personalized feedback when compared with those providing patients with more generalized content. The preference and needs of each patient should always be considered when initiating such interventions; incorporating interactive elements, such as feedback on inhaler technique, in a way which best suits the patient, may be vital in translating improvements in adherence to symptom burden in patients with respiratory diseases.37,38
The Opportunity to Improve Inhaler Technique in Real Time
Poor technique is associated with adverse disease outcomes.14 Patients are often unaware that they are using their inhaler incorrectly, and many healthcare professionals (HCPs) who care for asthma and COPD patients lack the required skill to properly demonstrate optimal technique.39,40 Furthermore, improvements in technique achieved through training are often short-lived.41 Personalized community pharmacist-delivered inhaler training, informed by data recorded by a digital device, might give rise to more sustained improvements in clinical outcomes,42 although data on such interventions are limited at present.
In 2020, Curran et al reported that the insertion of a flow probe directly into the mouthpiece of a valved holding chamber could allow the real-time analysis of respiratory flow during use, without increasing inhaled resistance or disturbing drug delivery to the lungs.43 This discovery highlighted the potential of accurately and passively measuring inspiratory flow profiles of children and adults. Other such devices can also provide informative data; for instance, a Spacer Data Logger Device, capable of recording adherence and user technique, was effective in identifying incorrect inhaler use and the resulting impact on the dose received in patients with asthma and COPD.44
More sophisticated digital devices capable of providing information on inhalation parameters, without requiring use of a separate spirometer, are a recent development in respiratory care.45 The acoustic sensing INhaler Compliance Assessment (INCA) device is widely considered one of the first add-on digital devices capable of monitoring inhaler technique. Of note, this device was only introduced within the past decade.45,46 Since its development, the INCA device has contributed to important research highlighting the discrepancies between reported and actual adherence rates in patients with asthma and COPD.11
In late 2018, the ProAir® (albuterol) Digihaler®47 (Teva Pharmaceuticals, LLC) became the first FDA-approved inhaler with integrated sensors capable of recording and providing feedback on PIF, time to PIF, inhalation volume and duration, in addition to recording data on adherence.8,48 Using visual displays, via a smartphone app connected to the inhaler by Bluetooth, the data recorded is provided to patients and (optionally) HCPs offering potential opportunities to identify and correct problems with inhaler technique.15 Another example of connected inhaler technology is the Respiro® RS01X device (Amiko, Milan, Italy), whose built-in sensors can provide information on each critical movement during inhaler actuation.45,49–51 Using Bluetooth, the device can be paired with a companion mobile application to provide patients with personalized information, and usage data can be wirelessly uploaded to a provider portal for HCP access. These features may promote disease management and enable the efficient monitoring of large populations of respiratory patients, respectively.52
Several other smart inhalers are either licensed for use or undergoing clinical trial evaluation45,53 This includes the recently approved Enerzair® Breezhaler® (Novartis Pharmaceuticals UK, Ltd),54,55 which, when paired with the Propeller® mobile application, is also available to support clinicians and patients with asthma in monitoring and recording inhaler device handling.54 The key features of digital inhaler devices are summarized in Table 2. Costs for use are country-specific and depend on local reimbursement strategies, and are thus considered outside the scope of this review. Despite numerous advances in inhaler technology, there remains a fundamental need to enhance respiratory care through improved inhaler training, for both patients and HCPs – a challenge that will require effective implementation of the inhaler technology available, as well as the appropriate use of the information they provide.
 |
Table 2 Digital Inhaler Devices Currently Licensed for Use or Undergoing Clinical Trial Evaluation |
Advancing Clinical Decision-Making Through a Personalized Data-Driven Approach
Current guidelines emphasize the importance of a proactive and personalized approach to asthma and COPD management;3,4 however, the concept of personalized management is not explored in detail in the guidelines. Hence, it is not surprising that best practices are often not realized in real-world clinical settings,56 with limiting factors including lack of time, knowledge, and experience on the part of HCPs.57 To develop suitable personalized action plans, clinicians require accurate insights into patient behavior and disease status, as well as the ability to interpret these and make appropriate judgements.3,4 Until very recently, decision-making has unavoidably depended on subjective and often unreliable data. Innovations providing real-world data may in the future guide personalized solutions. This is where digital inhalers have the potential to have a significant impact. Innovation is required in how best to use them both from the patient and the healthcare provider perspective, considering the data that they can capture.
The Capability to Incorporate Insights on Patient Behavior into Treatment Decision-Making
Digital inhalers allow the passive collection of otherwise unobtainable data relating to factors that influence patient adherence behavior. For example, using Bluetooth-enabled inhaler devices, researchers have been able to conclude that contextual factors, such as boredom and symptoms, were associated with altered (in this instance, increased) adherence behavior in children with asthma.58 Additionally, distinct behavioral phenotypes have been identified in patients with respiratory diseases. Following the assessment of inhaler use frequency and proficiency in patients with COPD, three equally sized clusters corresponding to distinct patterns of patient behavior were identified. These included two with high error rates but different levels of inhaler use (high versus low), and a third with overall good adherence and technique. Factors that were predictive of cluster assignment included age, cognition, comorbidities, and poor lung function.59 These categories were also identified by Cushen et al, but with the addition of a fourth cluster containing users with both low error rates and low inhaler use27 (Figure 1).
 |
Figure 1 Four clusters of adherence behaviors as identified and reported by Cushen et al.27 |
Meanwhile, in patients with asthma, three clusters of adherence (representing poor, moderate and good) were identified and explored for stability. It was reported that the most accurate cluster prediction was approximated in a decision tree that used the percentage of prescribed doses taken during the study. In data unseen to the training model, this method had an accuracy of 84%.60 Going forward, such behavioral phenotypes should be taken into consideration – despite the demonstrated heterogeneity within patient behaviors, knowledge of these patterns could aid the development and selection of adherence interventions.59,60
Digital inhalers may also aid the identification of appropriate patient populations who require escalation of therapy. The GINA and GOLD guidelines recommend that adherence and inhaler technique be assessed prior to initiating or stepping-up treatment.3,4 However, van Boven et al reported that more than 80% of patients (98/120) commencing GINA step 5 therapies for severe asthma had poor adherence to ICS/long-acting beta agonist therapy over the previous 12 months.61 In a study of preschool-age children with severe wheeze by Bingham et al,62 50% (24/48) had suboptimal adherence with ICS, while Lee et al63 reported that at least 50% of 69 patients with difficult asthma eligible for novel therapies, such as biologics and thermoplasty, were nonadherent with their ICS or ICS/long-acting beta agonist therapy medication. These studies highlight an unmet need for better identification of medication adherence in patients with respiratory disease.61–63 In contrast, Sulaiman et al11 reported that of 146 patients, 40 (27%) had uncontrolled asthma despite having actual adherence rates of greater than 80% (reflecting good inhaler technique as well as good adherence) (Figure 2) – such patients are likely appropriate candidates for add-on biologic therapy.11 Digital inhalers could therefore assist clinical decision-making through both the prevention of unnecessary escalation to biologics in patients with poor adherence/inhaler technique,64 and the identification of patients who may benefit most from novel or step-up therapies. However, studies are needed to confirm these benefits.
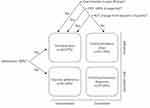 |
Figure 2 Clinical outcomes at the end of a 3-month study. After 3 months, patient data on peak expiratory flow (PEF), asthma control (Asthma Control Test [ACT] and Asthma Quality of Life Questionnaire) and inhaler adherence (from the INCA device) were combined to provide an assessment of each patient’s asthma, as suggested by Global Initiative for Asthma. These included: 1) assess adherence (patient is uncontrolled and adherence is poor); 2) review comorbidities (patient is uncontrolled, adherence is good and PEF is stable); 3) step-up therapy (patient is uncontrolled, adherence is good and PEF is unstable); 4) consider reducing therapy if the patient has both good control and PEF >80%. Results of the decision tool are shown and indicate that after the monitored adherence program, 40 (27%) patients needed additional medication as the next step. This material has not been reviewed prior to release; therefore the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content. Reproduced with permission of the ©ERS 2022. European Respiratory Journal Jan 2018, 51(1):1701,126; DOI: 10.1183/13993003.01126–2017.11 |
The Opportunity to Implement a Risk Identification and Reduction Approach
Exacerbations of asthma and COPD are often preceded by predictable changes in patient behaviors (short-acting beta2-agonist use) and inhalation parameters (PIF, inhalation volume and inhalation duration).65,66 Devices capable of accurately measuring these parameters, including continuous short-acting beta2-agonist overreliance, might potentially enable the recognition of changes in inhaler technique and lung physiology that precede a clinical exacerbation.67,68 Applying machine learning techniques to the objective and personalized data obtained from digital inhalers could potentially enable the accurate prediction – and therefore guide pre-emptive treatment of – impending exacerbations in a way that has not previously been possible.
Consequently, several efforts have been made to develop a system capable of predicting impending exacerbations of respiratory diseases.69–71 In 2016, Killane et al compared exacerbation prediction models using two sources of inhaler medication adherence data: dose counter and electronic monitoring. While reported adherence was significantly higher with the dose counter than that reported by electronic monitoring, the relative risk measures produced by the predictive model were not significantly different between the two sources.71 In another study, adherence data were collected and used to calculate entropy (used as a measure of irregular inhaler use over this period), defined in terms of transitional probabilities between different levels of adherence. Patients with difficult-to-treat asthma underwent electronic monitoring of ICS over 50 days. A significant association between entropy and asthma exacerbations at all levels of severity, including requiring hospitalization, was found.70 These data highlight the need to identify the optimum metrics to develop a predictive model.
A predictive model of impending asthma exacerbations was created by applying machine learning techniques to data downloaded from the ProAir Digihaler together with clinical and demographic information. The generated model predicted an impending exacerbation over the following 5 days with high diagnostic accuracy (a receiver operating characteristic area under curve of 0.83 [out of a maximum 1]).67 The most significant factors contributing to the model were features based on the number of inhalations during the 4 days prior to prediction.67 A similar model for prediction of COPD exacerbations achieved a receiver operating characteristic area under curve value of 0.77, with features based on baseline inhalation parameters the most significant factors contributing to the model.68 While more data are needed, these initial models represent first steps in the development of predictive systems capable of causing a meaningful shift from reactive to proactive care within respiratory medicine.
Support Patient Empowerment and Shared Decision-Making
Shared decision-making is increasingly becoming an important part of clinical practice, representing a profound shift in medical culture. Involving patients in the clinical decision-making process may be associated with increased patient satisfaction and improved disease outcomes.72 Research confirms that the benefits of shared decision-making may be enhanced by the provision of useful information on a patient’s disease status and management, particularly if this can be sourced from objective data.31,73 Smart inhalers have clear potential to provide valuable insights here; however, there are important additional considerations around their use. For instance, some can provide feedback immediately, whereas others require data to be downloaded. Different devices may be convenient for a particular patient. Indeed, shared decision-making involves engaging the patient according to their individual preferences.72 Limited studies have indicated that patients welcome the use of smart inhalers with feedback;74–76 however, their usefulness has been doubted in patients who used their inhalers only when they had symptoms.76 Clearly, more studies are needed in this area.
Where to from Here?
The Need for a Consensus Framework for Implementation of Digital Systems
Technological innovation is already enabling many patients with chronic conditions to take more control of their disease management and achieve better disease outcomes. However, while these products are becoming increasingly available, they differ and population-level infrastructure capable of interpreting the volume of patient data captured and supporting widespread use of digital devices within health services is lacking.77 Lessons from other therapy areas could provide invaluable insights into the facilitators of and barriers to integrating digital technologies/inhalers into clinical practice. For example, after acknowledging the considerable benefits of digital glucose monitoring in diabetes care, focus groups with practicing endocrinologists highlighted that the data generated increased provider workload and responsibilities more than anticipated. In particular, insurance and reimbursement coverage of the devices and associated visits were considered burdensome.78 Similarly, in asthma care, Kan et al reported concerns from healthcare providers regarding the anticipated additional workload that is likely to accompany the use of digital device data in routine practice, and that extra employees would be required to manage this increase.79
Other reports have emphasized the need for sound cybersecurity within digital systems.80 In diabetes care, this has been addressed through the development of DTSec, a cybersecurity standard, containing both performance and assurance requirements, for connected diabetes devices.80,81 The need to streamline data and reports from a variety of digital systems is also important for successful application.78,82 The integration of data into electronic medical records would likely also improve the efficiency of associated medical appointments for both patients and clinicians alike.79,82 Thus, digital solutions have great potential to offer advanced and targeted care should a consensus framework for implementation be established.
Knowledge and Practice Gaps – Will Improvements in Adherence and Technique Translate to Clinical and Economic Outcomes?
While digital devices may improve patient adherence to inhaler medication and their inhalation technique, there are conflicting reports of whether such changes could translate into clinical and economic outcomes. One study in pediatric patients with asthma32 and another in patients with COPD,73 reported conflicting results. Each study investigated the clinical and economic outcomes of an adherence-enhancing intervention involving real-time dose alerts. Although both interventions resulted in improved adherence, only Alshabani et al reported an improvement in clinical and economic outcomes, through a reduction in COPD-related healthcare utilization.73 Whereas, Vasbinder et al reported numerically higher costs but no identifiable improvement in clinical outcomes in the asthma population.32 The latter outcome may in part be due to incorrect inhaler technique, highlighting the need for future studies to focus on novel interventions that not only consider adherence, but also other clinically meaningful factors.
Evidence on the effect of digital devices on clinical outcomes is currently lacking; however, some potentially insightful studies are ongoing. These include the Maximizing Adherence and Gaining New Information for Your COPD (MAGNIFY) primary care cluster randomized trial in COPD, designed to investigate whether adherence support can improve COPD outcomes in real life in the UK (https://opri.sg/magnify/).83 In children with asthma, ongoing studies include the Improving Adherence by Guiding Inhalation via Electronic Monitoring (IMAGINE) I trial using the Respiro® add-on sensor,84 and a pilot study of the HeroTracker electronic monitoring device linked to BreatheSmart mobile app.85 In adults and adolescents with asthma, the CONNECT study program (NCT03890666 and NCT04677959) is exploring the effects of the albuterol and (in CONNECT2) the fluticasone/salmeterol Digihaler on asthma control.
The cost-effectiveness of an adherence-enhancing intervention in patients with COPD was assessed using a health-economic model with data from INCA trials, and Irish national economic and epidemiological information.86 Using digital device data, patients were separated into four clusters of inhaler adherence behavior: 1) regular use, good technique; 2) regular use, frequent critical technique errors; 3) irregular use, good technique; and 4) irregular use, frequent critical technique errors. Compared with Cluster 1, patients in Cluster 3 were significantly more likely to visit the emergency department during the 12-month follow-up period, and significantly greater death rates were seen in Cluster 4 during this period (Figure 3). Post-intervention, Clusters 2 and 4 gained one QALY per €6520 and €3935 invested, respectively – values substantially lower than the Irish threshold of €45,000/QALY. Notably, rather than incurring additional costs, the adherence-enhancing intervention in Cluster 3 resulted in annual cost-savings of €845 per person86 suggesting that identifying patients most likely to benefit from adherence-enhancing interventions could be fundamental in maintaining treatment cost-effectiveness. However, translating such reports to real-world implementation is challenging because reimbursement incentives for HCPs to engage with the technologies are currently lacking – this issue is one deserving of the time and effort required to reach a solution.
 |
Figure 3 Proportional contributions to all-cause clinical outcomes according to adherence cluster over a 12-month follow-up period. Proportional contribution of each adherence cluster to all-cause clinical outcomes over the 12-month follow-up period (adjusted for the number of participants per cluster). Reported differences are the absolute differences in the proportion of events attributable to cluster 3 vs cluster 1 for emergency department and hospital admission and cluster 4 vs cluster 1 for death. ^ denotes P = 0.05, *P < 0.05. Notes: Reproduced from van Boven JFM, Cushen B, Sulaiman I et al. Personalising adherence-enhancing interventions using a smart inhaler in patients with COPD: an exploratory cost-effectiveness analysis. NPJ Prim Care Respir Med. 2018;28(1):24. Creative commons license is https://creativecommons.org/licenses/by/4.0/.86 |
Conclusions
The terms “adherence” and “inhaler technique” are widely used when discussing inhaler use by patients with asthma and COPD. In fact, these terms are facades for what are highly complex and individualized patient behaviors, attitudes, and approaches to disease management, which for decades have not been addressed satisfactorily. Major advances in technology have been successfully incorporated into inhaler devices, providing a suite of digital solutions with the potential to revolutionize disease management and outcomes. Digital inhaler devices linked to mobile apps can help bring about changes in patients’ behaviors and attitudes towards the management of their asthma or COPD, particularly when they build in elements of interactivity and gamification. They can also support ongoing technique education, empowering patients and helping providers maximize the value of consultations and develop effective action plans informed by objective insights into the patient’s inhaler use patterns and their respiratory health. And, when combined with innovative techniques such as machine learning, they have the potential to predict exacerbations and prompt pre-emptive intervention. Finally, digital inhalers have the potential to support an advanced precision-medicine approach to respiratory disease management and drive shared decision-making. However, there is a risk that consumers and health care providers may feel bombarded by the abundance of choice and capabilities of these technologies. To overcome this barrier and realize the potential of digital inhalers, an informed, strategic approach that respects user preferences is needed. Integration into management guidelines, suitable reports and strategies, and support for uptake in real-life practice are all needed. Only then will we be able to use data captured by digital devices to deliver personalized disease management.
Abbreviations
COPD, chronic obstructive pulmonary disease; DTSec, diabetes technology security; EMD, electronic monitoring device; HCP, healthcare professional; ICS, inhaled corticosteroids; IMAGINE, Improving Adherence by Guiding Inhalation via Electronic Monitoring; INCA, INhaler Compliance Assessment; MAGNIFY, Maximising Adherence and Gaining New Information for Your COPD; PIF, peak inspiratory flow; QALY, quality-adjusted life year; SMS, short message service.
Ethics Approval and Informed Consent
This article is a review and analysis of previously published studies and does not include any new studies on human or animal subjects performed by any of the authors.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Connie Lathe and Ian Grieve of Ashfield MedComms, an Inizio company, and funded by Teva Pharmaceuticals, LLC.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study (including development of study design, conduct of the research, and medical writing services) was funded by Teva Pharmaceuticals, LLC.
Disclosure
SB-A reports grant and personal fee for advisory board contribution and/or expert lectures/educational activities from GSK, AstraZeneca, Mylan, Viatris, Boehringer Ingelheim, Sanofi; NDB reports grants, personal fees, and non-restrictive educational fees from Teva, GSK, Chiesi, AZ, and Boehringer Ingelheim; HC has received grants and personnel fees from several pharmaceutical companies that market inhaled products. These include Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Innovata Biomed, Meda, Menarini, Mundipharma, Napp Pharmaceuticals, Nemera, NorPharma, Novartis, Orion, Sanofi, Teva, Trudell Medical International, UCB and Zentiva; MH reports grants, speaker fees and consultancies from GSK, AZ, Novartis, Sanofi and Teva outside of the submitted work and paid to his employer Alfred Health; JvdP reports grants and personal fees from Chiesi, Boehringer Ingelheim, Teva, and GSK, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Asthma Network. The Global Asthma Report 2018; 2018. Available from: http://globalasthmareport.org/s .
2. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05.020415
3. Global Initiative for Asthma. Global Strategy for Asthma Management & Prevention; 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdfs .
4. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdfs .
5. Chan A, De Simoni A, Wileman V, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Sys Rev. 2022;6(6):Cd013030. doi:10.1002/14651858.CD013030.pub2
6. Chan AHY, Pleasants RA, Dhand R, et al. Digital inhalers for asthma or chronic obstructive pulmonary disease: a scientific perspective. Pulm Ther. 2021;7(2):345–376. doi:10.1007/s41030-021-00167-4
7. Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018;52(5):1801147. doi:10.1183/13993003.01147-2018
8. Chrystyn H, Saralaya D, Shenoy A, et al. Investigating the accuracy of the Digihaler, a new electronic multidose dry-powder inhaler, in measuring inhalation parameters. J Aerosol Med Pulm Drug Deliv. 2022;35(3):166–177. doi:10.1089/jamp.2021.0031
9. Chrystyn H, Audibert R, Keller M, Quaglia B, Vecellio L, Roche N. Real-life inhaler adherence and technique: time to get smarter! Respir Med. 2019;158:24–32. doi:10.1016/j.rmed.2019.09.008
10. Lindsay JT, Heaney LG. Non-adherence in difficult asthma and advances in detection. Expert Rev Respir Med. 2013;7(6):607–614. doi:10.1586/17476348.2013.842129
11. Sulaiman I, Greene G, MacHale E, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126. doi:10.1183/13993003.01126-2017
12. Brennan V, Mulvey C, Costello RW. The clinical impact of adherence to therapy in airways disease. Breathe. 2021;17(2):210039. doi:10.1183/20734735.0039-2021
13. Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22. doi:10.1038/s41533-017-0016-z
14. Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler Errors in the CRITIKAL Study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081.e1079. doi:10.1016/j.jaip.2017.01.004
15. Chrystyn H, Merchant R, Safioti G, et al. The Digihaler®: a new approach to manage asthma and COPD. Respiratory Drug Delivery. 2020;1:249–260.
16. Hollenbach JP, Cushing A, Melvin E, McGowan B, Cloutier MM, Manice M. Understanding clinicians’ attitudes toward a mobile health strategy to childhood asthma management: a qualitative study. J Asthma. 2017;54(7):754–760. doi:10.1080/02770903.2016.1263649
17. Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(6):1051–1057. doi:10.1016/S0091-6749(96)80190-4
18. Otsuki M, Eakin MN, Rand CS, et al. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics. 2009;124(6):1513–1521. doi:10.1542/peds.2008-2961
19. Krishnan JA, Bender BG, Wamboldt FS, et al. Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. J Allergy Clin Immunol. 2012;129(1):112–118. doi:10.1016/j.jaci.2011.10.030
20. Mosnaim G, Li H, Martin M, et al. The impact of peer support and mp3 messaging on adherence to inhaled corticosteroids in minority adolescents with asthma: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013;1(5):485–493. doi:10.1016/j.jaip.2013.06.010
21. Rupani H, Kent BD. Using fractional exhaled nitric oxide measurement in clinical asthma management. Chest. 2022;161(4):906–917. doi:10.1016/j.chest.2021.10.015
22. Melvin E, Cushing A, Tam A, Kitada R, Manice M. Assessing the use of BreatheSmart® mobile technology in adult patients with asthma: a remote observational study. BMJ Open Respir Res. 2017;4(1):e000204. doi:10.1136/bmjresp-2017-000204
23. Guleria R, Korukonda K. Clinical impact of a digital dose counter pressurized metered-dose inhaler on uncontrolled asthma: cross-sectional, observational, surveillance study. Interact J Med Res. 2019;8(2):e13530. doi:10.2196/13530
24. Hesso I, Nabhani Gebara S, Greene G, Co Stello RW, Kayyali R. A quantitative evaluation of adherence and inhalation technique among respiratory patients: an observational study using an electronic inhaler assessment device. Int J Clin Pract. 2020;74(2):e13437. doi:10.1111/ijcp.13437
25. Sulaiman I, Seheult J, MacHale E, et al. A method to calculate adherence to inhaled therapy that reflects the changes in clinical features of asthma. Ann Am Thorac Soc. 2016;13(11):1894–1903. doi:10.1513/AnnalsATS.201603-222OC
26. Stanford RH, Averell CM, Johnson PT, Buysman EK, Carlyle MH. Adherence and usage patterns of inhaled corticosteroids-long-acting beta-agonists by using inhaler-monitoring technology. Allergy Asthma Proc. 2020;41(4):256–264. doi:10.2500/aap.2020.41.200037
27. Cushen B, Sulaiman I, Greene G, et al. The clinical impact of different adherence behaviors in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1630–1633. doi:10.1164/rccm.201712-2469LE
28. Moran C, Doyle F, Sulaiman I, et al. The INCA(TM) (Inhaler Compliance Assessment(TM)): a comparison with established measures of adherence. Psychol Health. 2017;32(10):1266–1287. doi:10.1080/08870446.2017.1290243
29. Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13(4):559–563. doi:10.1111/j.1440-1843.2008.01292.x
30. Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. doi:10.1016/j.rmed.2012.09.017
31. George M. Adherence in asthma and COPD: new strategies for an old problem. Respir Care. 2018;63(6):818–831. doi:10.4187/respcare.05905
32. Vasbinder EC, Goossens LM, Rutten-van Mölken MP, et al. e-Monitoring of Asthma Therapy to Improve Compliance in children (e-MATIC): a randomised controlled trial. Eur Respir J. 2016;48(3):758–767. doi:10.1183/13993003.01698-2015
33. Gregoriano C, Dieterle T, Breitenstein A-L, et al. Does a tailored intervention to promote adherence in patients with chronic lung disease affect exacerbations? A randomized controlled trial. Respir Res. 2019;20(1):273. doi:10.1186/s12931-019-1219-3
34. McGillicuddy JW, Gregoski MJ, Weiland AK, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trial. JMIR Res Protoc. 2013;2(2):e32. doi:10.2196/resprot.2633
35. Elias P, Rajan NO, McArthur K, Dacso CC. InSpire to promote lung assessment in youth: evolving the self-management paradigms of young people with asthma. Med 2.0. 2013;2(1):e1. doi:10.2196/med20.2014
36. Cushing A, Manice MP, Ting A, Parides MK. Feasibility of a novel mHealth management system to capture and improve medication adherence among adolescents with asthma. Patient Prefer Adherence. 2016;10:2271–2275. doi:10.2147/PPA.S115713
37. Mosnaim G, Safioti G, Brown R, et al. Digital health technology in asthma: a comprehensive scoping review. J Allergy Clin Immunol Pract. 2021;9(6):2377–2398. doi:10.1016/j.jaip.2021.02.028
38. Unni E, Gabriel S, Ariely R. A review of the use and effectiveness of digital health technologies in patients with asthma. Ann Allergy Asthma Immunol. 2018;121(6):680–691.e681. doi:10.1016/j.anai.2018.10.016
39. Bosnic-Anticevich S, Callan C, Chrystyn H, et al. Inhaler technique mastery and maintenance in healthcare professionals trained on different devices. J Asthma. 2018;55(1):79–88. doi:10.1080/02770903.2017.1310227
40. Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44(8):593–598. doi:10.1080/02770900701554334
41. Klijn SL, Hiligsmann M, Evers S, Román-Rodríguez M, van der Molen T, van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1
42. O’Dwyer S, Greene G, MacHale E, et al. Personalized biofeedback on inhaler adherence and technique by community pharmacists: a cluster randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8(2):635–644. doi:10.1016/j.jaip.2019.09.008
43. Curran E, Porée T, Rubin BK. Real-time analysis of the respiratory flow through a valved holding chamber. J Aerosol Med Pulm Drug Deliv. 2020;33(4):205–213. doi:10.1089/jamp.2019.1563
44. O’Callaghan C, Smith NJ, Barry PW, Denyer J. Development of an intelligent spacer data logger system. J Aerosol Med Pulm Drug Deliv. 2017;30(6):444–450. doi:10.1089/jamp.2017.1375
45. Mehta PP. Dry powder inhalers: a concise summary of the electronic monitoring devices. Ther Deliv. 2021;12(1):1–6. doi:10.4155/tde-2020-0091
46. D’Arcy S, MacHale E, Seheult J, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLoS One. 2014;9(6):e98701. doi:10.1371/journal.pone.0098701
47. Teva Pharmaceuticals. Digihaler inhalers. 2022. Available from: https://www.digihaler.com/#digihaler-inhalerss .
48. Food & Drug Administration. ProAir® Digihaler® Prescribing Information; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205636Orig1s012lbl.pdfs .
49. Braido F, Paa F, Ponti L, Canonica GW. A new tool for inhalers’ use and adherence monitoring: the Amiko® validation trial. Int J Eng Res Sci. 2016;2(10):159–166.
50. Rogueda P, Grinovero M, Ponti L, Purkins G, Croad O Telehealth ready: performance of the Amiko Respiro Sense connected technology with Merxin DPIs; 2018. Available from: https://ddl-conference.com/wp-content/uploads/2018/10/22.Rogueda.poster.pdfs .
51. Sloots J, Bakker M, van der Palen J, et al. Adherence to an eHealth self-management intervention for patients with both COPD and heart failure: results of a pilot study. Int J Chron Obstruct Pulmon Dis. 2021;16:2089–2103. doi:10.2147/COPD.S299598
52. Amiko. Amiko Respiro; 2022. Available from: https://amiko.io/s .
53. Dhruve H, Jackson DJ. Assessing adherence to inhaled therapies in asthma and the emergence of electronic monitoring devices. Eur Respir Rev. 2022;31(164):210271. doi:10.1183/16000617.0271-2021
54. Novartis. Enerzair® Breezhaler®. Summary of product characteristics; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/enerzair-breezhaler-epar-product-information_en.pdfs .
55. European Medicines Agency. Enerzair® Breezhaler®. EPAR; 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/enerzair-breezhaler#product-information-sections .
56. Kouri A, Boulet LP, Kaplan A, Gupta S. An evidence-based, point-of-care tool to guide completion of asthma action plans in practice. Eur Respir J. 2017;49:5. doi:10.1183/13993003.02238-2016
57. Gupta S, Kaplan A. Solving the mystery of the yellow zone of the asthma action plan. NPJ Prim Care Respir Med. 2018;28(1):1. doi:10.1038/s41533-017-0067-1
58. Teufel II RJ, Patel SK, Shuler AB, et al. Smartphones for real-time assessment of adherence behavior and symptom exacerbation for high-risk youth with asthma: pilot study. JMIR Pediatr Parent. 2018;1(2):e8. doi:10.2196/pediatrics.9796
59. Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343. doi:10.1164/rccm.201604-0733OC
60. Tibble H, Chan A, Mitchell EA, et al. A data-driven typology of asthma medication adherence using cluster analysis. Sci Rep. 2020;10(1):14999. doi:10.1038/s41598-020-72060-0
61. van Boven JFM, Koponen M, Lalic S, et al. Trajectory analyses of adherence patterns in a real-life moderate to severe asthma population. J Allergy Clin Immunol Pract. 2020;8(6):1961–1969.e6. doi:10.1016/j.jaip.2019.12.002
62. Bingham Y, Sanghani N, Cook J, et al. Electronic adherence monitoring identifies severe preschool wheezers who are steroid responsive. Pediatr Pulmonol. 2020;55(9):2254–2260. doi:10.1002/ppul.24943
63. Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51(4):1701836. doi:10.1183/13993003.01836-2017
64. Boddy CE, Naveed S, Craner M, Murphy AC, Siddiqui S, Bradding P. Clinical outcomes in people with difficult-to-control asthma using electronic monitoring to support medication adherence. J Allergy Clin Immunol Pract. 2021;9(4):1529–1538.e1522. doi:10.1016/j.jaip.2020.10.059
65. Broeders ME, Molema J, Hop WC, Vermue NA, Folgering HT. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98(12):1173–1179. doi:10.1016/j.rmed.2004.04.010
66. Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Crit Care. 2002;6(1):30–44. doi:10.1186/cc1451
67. Lugogo NL, DePietro M, Reich M, et al. A Predictive Machine Learning Tool for Asthma Exacerbations: Results from a 12-week, open-label study using an electronic multi-dose dry powder inhaler with integrated Sensors. J Asthma Allergy. 2022;15:1623–1637.
68. Snyder LD, Safioti G, Reich M, et al. A predictive model for COPD exacerbations using ProAir Digihaler: a 12-week, open-label study. In:
69. Guerra B, Gaveikaite V, Bianchi C, Puhan MA. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26:143. doi:10.1183/16000617.0061-2016
70. Lee J, Huvanandana J, Foster JM, et al. Dynamics of inhaled corticosteroid use are associated with asthma attacks. Sci Rep. 2021;11(1):14715. doi:10.1038/s41598-021-94219-z
71. Killane I, Sulaiman I, MacHale E, et al. Predicting asthma exacerbations employing remotely monitored adherence. Healthcare Technol Lett. 2016;3(1):51–55. doi:10.1049/htl.2015.0058
72. Bukstein DA, Guerra Jr. DG, Huwe T, Davis RA. A review of shared decision-making: a call to arms for health care professionals. Ann Allergy Asthma Immunol. 2020;125(3):273–279. doi:10.1016/j.anai.2020.06.030
73. Alshabani K, Attaway AA, Smith MJ, et al. Electronic inhaler monitoring and healthcare utilization in chronic obstructive pulmonary disease. J Telemed Telecare. 2020;26(7–8):495–503. doi:10.1177/1357633X19850404
74. Chan AHY, Stewart AW, Harrison J, Black PN, Mitchell EA, Foster JM. Electronic adherence monitoring device performance and patient acceptability: a randomized control trial. Expert Rev Med Devices. 2017;14(5):401–411. doi:10.1080/17434440.2017.1322505
75. Kuipers E, Poot CC, Wensing M, Chavannes NH, de Smet PA, Teichert M. Self-Management Maintenance Inhalation Therapy With eHealth (SELFIE): observational study on the use of an electronic monitoring device in respiratory patient care and research. J Med Internet Res. 2019;21(5):e13551. doi:10.2196/13551
76. Foster JM, Reddel HK, Usherwood T, Sawyer SM, Smith L. Patient-perceived acceptability and behaviour change benefits of inhaler reminders and adherence feedback: a qualitative study. Respir Med. 2017;129:39–45. doi:10.1016/j.rmed.2017.05.013
77. Johnston DS. Digital maturity: are we ready to use technology in the NHS? Future Healthc J. 2017;4(3):189–192. doi:10.7861/futurehosp.4-3-189
78. May SG, Huber C, Roach M, et al. Adoption of digital health technologies in the practice of behavioral health: qualitative case study of glucose monitoring technology. J Med Internet Res. 2021;23(2):e18119. doi:10.2196/18119
79. Kan K, Shaunfield S, Kanaley M, et al. Health provider perspectives of electronic medication monitoring in outpatient asthma care: a qualitative investigation using the consolidated framework for implementation research. J Asthma. 2022;59(2):342–351. doi:10.1080/02770903.2020.1846745
80. Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178–192. doi:10.1016/j.diabres.2017.08.005
81. Klonoff DC, Kleidermacher DN. Now Is the time for a cybersecurity standard for connected diabetes devices. J Diabetes Sci Technol. 2016;10(3):623–626. doi:10.1177/1932296816647516
82. Ekhlaspour L, Tabatabai I, Buckingham B. A Review of continuous glucose monitoring data interpretation in the age of automated insulin delivery. J Diabetes Sci Technol. 2019;13(4):645–663. doi:10.1177/1932296819851790
83. Price D, Jones R, Pfister P, et al. Maximizing Adherence and Gaining New Information For Your Chronic Obstructive Pulmonary Disease (MAGNIFY COPD): study protocol for the pragmatic, cluster randomized trial evaluating the impact of dual bronchodilator with add-on sensor and electronic monitoring on clinical outcomes. Pragmat Obs Res. 2021;12:25–35. doi:10.2147/POR.S302809
84. Sportel ET, Oude Wolcherink MJ, van der Palen J, et al. Does immediate smart feedback on therapy adherence and inhalation technique improve asthma control in children with uncontrolled asthma? A study protocol of the IMAGINE I study. Trials. 2020;21(1):801. doi:10.1186/s13063-020-04694-4
85. Hollenbach J, Simoneau T, Sun Y, et al. Design, methods, and baseline characteristics of a pilot, randomized, controlled trial of the effects of an electronic monitoring device on medication adherence in children with asthma. Contemp Clin Trials Commun. 2021;21:100706.
86. van Boven JFM, Cushen B, Sulaiman I, et al. Personalising adherence-enhancing interventions using a smart inhaler in patients with COPD: an exploratory cost-effectiveness analysis. NPJ Prim Care Respir Med. 2018;28(1):24. doi:10.1038/s41533-018-0092-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
