Back to Journals » Journal of Pain Research » Volume 16
A Silver Lining of Neuropathic Pain: Predicting Favorable Functional Outcome in Spinal Cord Injury
Authors Xu ML, Wu XB, Liang Y, Li N, Hu X, Lin XD, Sun MQ , Dai CQ, Niu D, Zhang YR, Cao H, Zhao CG, Sun XL , Yuan H
Received 28 March 2023
Accepted for publication 17 July 2023
Published 27 July 2023 Volume 2023:16 Pages 2619—2632
DOI https://doi.org/10.2147/JPR.S414638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Mu-Lan Xu,1,2,* Xiang-Bo Wu,1,* Ying Liang,3,* Ning Li,1 Xu Hu,1 Xiao-Dong Lin,1 Miao-Qiao Sun,1 Chun-Qiu Dai,1 Dan Niu,1 Yan-Rong Zhang,1 Hui Cao,1 Chen-Guang Zhao,1 Xiao-Long Sun,1 Hua Yuan1
1Department of Rehabilitation Medicine, Xi-Jing Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, Shaanxi, People’s Republic of China; 2Department of Rehabilitation Medicine, Shenshan Medical Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Shanwei, Guangdong, People’s Republic of China; 3Department of Health Statistics, Air Force Medical University (Fourth Military Medical University), Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Yuan; Xiao-Long Sun, Department of Rehabilitation Medicine, Xi-Jing Hospital, Air Force Medical University (Fourth Military Medical University), No. 15, Changle West Road, Xi’an, Shaanxi, 710032, People’s Republic of China, Tel/Fax +86-29-84775437 ; +86-29-84775439, Email [email protected]; [email protected]
Background: Neuropathic pain (NP) is a common and severe problem following spinal cord injury (SCI). However, its relationship with functional outcome remains unclear.
Methods: A retrospective explorative analysis was performed on SCI patients admitted to a tertiary academic medical center between January 2018 and June 2022. The candidate predictor variables, including demographics, clinical characteristics and complications, were analyzed with logistic and linear regression. Spinal Cord Independence Measure (SCIM) scores at discharge and mean relative functional gain (mRFG) of SCIM were as outcome parameters.
Results: A total of 140 SCI patients included for the final analysis. Among them, 44 (31.43%) patients were tetraplegics, and 96 (68.57%) patients were paraplegics; 68 (48.57%) patients developed NP, and 72 (51.43%) patients did not. Logistic and linear regression analyses of SCIM at discharge both showed that NP [OR=3.10, 95% CI (1.29,7.45), P=0.01; unstandardized β=11.47, 95% CI (4.95,17.99), P< 0.01; respectively] was significantly independent predictors for a favorable outcome (SCIM at discharge ≥ 50, logistic regression results) and higher SCIM total score at discharge (linear regression results). Besides, NP [unstandardized β=15.67, 95% CI (8.94,22.41), P< 0.01] was also independently associated with higher mRFG of SCIM scores. Furthermore, the NP group had significantly higher mRFG, SCIM total scores and subscales (self-care, respiration and sphincter management, and mobility) at discharge compared to the non-NP group. However, there were no significant differences in mRFG, SCIM total score or subscales at discharge among the NP subgroups in terms of locations (at level pain, below level pain, and both) or timing of occurrence (within and after one month after SCI). This study also showed that incomplete injury, lumbar-sacral injury level and non-anemia were significantly independent predictors for a favorable outcome, and higher mRFG of SCIM scores (except for non-anemia).
Conclusion: NP appears independently associated with better functional recovery in SCI patients, suggesting the bright side of this undesirable complication. These findings may help to alleviate the psychological burden of NP patients and ultimately restore their confidence in rehabilitation.
Keywords: spinal cord injury, neuropathic pain, functional outcome, SCIM, mRFG, retrospective study
Introduction
Spinal cord injury (SCI) is a debilitating condition which often leads to long-term or permanent mobility disability, chronic risk of various complications, and requires extensive medical resources.1,2 An epidemiology study showed that the overall global incidence of SCI was 10.5 cases per 100,000 persons.3 Although mobility is the most critical factor determining functional outcome,4 additional sequelae such as neuropathic pain (NP), bladder and rectum dysfunctions also decrease quality of life considerably.5,6
NP is one of the most common complications following SCI, with an incidence rate of 30–59%.7,8 The mechanisms of NP remain obscure. Residual structures and the neuroplasticity in spinal cord and cortices may contribute to the cause of NP.9 Persisting nociceptive stimuli in residual afferents increase spontaneous ectopic activity in injured myelinated afferents and the corresponding dorsal root ganglion (DRG) neuronal bodies, inducing alterations in discharge and excitability of the thalamus, which may contribute to the genesis of NP.9–11
The existing treatments for NP have limited success. Although drugs such as calcium channel modulator, opioids and tricyclic anti-depressants, have been recommended as the main treatment of NP, whereas only about 1/3 patients receive pain relief, and some patients have no choice but to stop the drugs because of the severe side effects.7 This is mainly because the targets of most conventional drugs exist both inside and outside the pain signal pathways. An animal study suggested that the Human Mas-related G protein-coupled receptor X1 (MRGPRX1) in trigeminal ganglia and DRG may be a potential target for pain relief because of its restricted expression in primary nociceptive neurons. Activation of MRGPRX1 attenuates inflammatory and NP-related behavior in rodent models.12 However, there is a long way to go before it can be translated into clinical application. In addition to drug treatments, noninvasive brain stimulation (NIBS), such as repetitive transcranial magnetic stimulation (rTMS), is widely used in clinic at present. Accumulated researches have demonstrated that rTMS can relieve pain in SCI patients with NP. However, rTMS is not effective for NP in all patients.
NP may influence sleep, emotional regulation, employment, and overall quality of life significantly.7,8 Besides, it is reported that remarkably higher health care resource utilization and total costs are incurred by SCI patients with NP compared with those without.13 NP therefore places heavy mental and economic burdens on SCI patients and their family, and considered as an unfavorable factor for the rehabilitation process. However, the presence of NP may also imply functional spinothalamic tracts below the level of injured spinal cord, as these sensory tracts are responsible for transmitting pain.14 This would suggest that the injury may be incomplete, especially in patients diagnosed with complete injury by clinical examination. Besides, several studies have reported that spinal cord lesion induces neuroplasticity. For example, reorganization within the central and peripheral nervous systems, which may contribute to motor recovery following SCI,15,16 while neuroplasticity might also be responsible for the development of NP.17 Hyperactivity and reorganization of the motor-related cortices and spinal cord have been observed in SCI patients with NP.18,19 Our recent studies revealed that high-frequency repetitive transcranial magnetic stimulation (rTMS) over the motor cortex could alleviate SCI-related NP, enhance motor-evoked potential (MEP) amplitude, and ameliorate motor cortex hypersensitivity.6,19 All the above suggest potential interaction between NP and favorable functional outcome.
Therefore, we speculated that SCI patients with NP might have better functional recovery than those without. The aim of this study was to investigate the relationship between NP and the degree of functional recovery of SCI patients, and to further analyze the differences in functional outcome of NP and non-NP groups as well as NP subgroups, in terms of the location and occurrence time of NP.
Methods
Study Settings and Participants
The study was conducted in the inpatient general rehabilitation unit at Xi-Jing Hospital, a tertiary academic medical care institution in Xi’an, People’s Republic of China. Participants 18 years and older hospitalized with SCI between January 2018 and June 2022 were recruited for this study. Exclusion criteria were: (1) severe coexisting systemic disease, such as cancer, neurologic or mental disorders, etc.; (2) American Spinal Injury Association (ASIA) impairment scale (AIS) graded E; (3) length of stay (LOS) < 10 days; (4) incomplete assessment data. This study complies with the Declaration of Helsinki and was approved by the Institutional Review Board of the medical center (No. KY20222096-C-1). Additional informed consent was not required due to the retrospective observational study design.
Data Collection
Predictor variables with a possible association with rehabilitation were retrospectively collected in a deidentified manner. Three variables concerned demographics: (1) gender; (2) age; (3) education level, categorized as low (junior high school education or below), medium (senior or vocational high school education), or high (university education or above);20 (4) career, categorized as manual, intellectual, or other.21
LOS and duration of SCI were also collected since they were important factors for functional outcomes.
Two variables concerned medical history: (1) diabetes, defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L; 2-h plasma glucose (PG) ≥ 11.1 mmol/L; classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 11.1 mmol/L; HbA1c ≥ 6.5%; self-reported physician-confirmed diagnosis;22 (2) hypertension, defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg by repeated examination or self-reported hypertension treatment with antihypertensive medications;23 both were coded as present or absent.
Three variables were related to injury condition: (1) cause of injury, coded as heavy pound, traffic accidents, fall, or other;24 (2) injury level, based on the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)25 and coded as cervical, thoracic, or lumbar-sacral;26 (3) injury severity, classified according to the AIS and coded as complete (AIS A) or incomplete (AIS B, C and D).27
Six variables involved complications after SCI: (1) anemia, defined as hemoglobin concentration less than 13.0 g/dL for males or 12.0 g/dL for females;28 (2) venous thrombosis (VT), confirmed by ultrasound;29 (3) pressure ulcers, diagnosed by physician examination;30 (4) urinary tract infection (UTI), diagnosed according to reported signs and symptoms, and urine culture results;23 (5) hypoproteinemia, defined as serum albumin concentration < 30 g/L;31 (6) NP. The diagnosis and classification (at level pain, below level pain, or both) of NP were based on the Spinal Cord Injury Pain Instrument (SCIPI)32 and the International Spinal Cord Injury Pain Classification (ISCIP),33 both were specially designed for NP in patients with SCI.7,34 All the above factors were coded as present or absent.
Definition of Outcomes
The third version of Spinal Cord Independence Measure (SCIM III, simplified as SCIM in the following) total score at discharge was used to measure functional outcome. SCIM is recognized as the best comprehensive, SCI-specific functional status instrument,35 and it is extensively applied in SCI.25 The SCIM scale consists of 19 items which are organized into three subscales: self-care, respiration and sphincter management, mobility. To distinguish between good and poor outcome, an SCIM total score at discharge cutoff was applied: scores < 50 were defined as poor and scores ≥ 50 were defined as good outcome.
Since the final functional status does not take injury severity and level and thus rehabilitation potential into account, certain rehabilitation impact indices have been devised to give consideration to the baseline functional condition, including mean relative functional gain (mRFG) and absolute functional gain (AFG).36 Among them, mRFG, which has been widely used in related researches, is considered superior to AFG because the latter does not consider the potential maximal functional improvement.37
Therefore, the outcomes included the following: (1) mRFG, which quantifies the amount of functional gain achieved as a percentage of the total functional gain possible using the formula (Discharge Total SCIM – Admission Total SCIM) / (Maximum Total SCIM Score – Admission Total SCIM) ×100%; (2) the SCIM a) total score (max total score of 100), b) self-care sub-scale, reflecting the ability to wash and dress (max total score of 20), c) respiration and sphincter management sub-scale, reflecting respiration, bladder and rectum function (max total score of 40), and d) mobility sub-scale (max score of 40). These sub-scales represent some of the most significant problems among patients with SCI.38
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics version 16.0. Continuous variables were presented as mean ± SD (normally distributed) or median and quartile range (QR, not normally distributed). Categorical variables were presented as counts and percentages. Chi-square tests were performed for categorical variables, and Mann–Whitney U-tests (not normally distributed) or Student’s t-tests (normally distributed) for continuous variables were conducted in univariate comparisons of clinical and demographic variables. A forward stepwise multivariate logistic regression was then performed in order to identify independent predictors of SCIM at discharge dichotomized. Results were expressed by odds ratio (OR) and 95% confidence interval (CI). To evaluate the effect of NP on functional gain, and to control for confounding effects of relevant demographic and clinical factors, we conducted linear regression analyses for each outcome variable separately (SCIM at discharge and mRFG). For all the tests, values of P<0.05 were considered statistically significant.
Results
Study Population
Between January 2018 and June 2022, 201 patients with SCI were admitted to the study hospital. Nine patients <18 years old were excluded. Of the 192 adult patients with SCI in the study population, 6 with serious disease, 2 with AIS graded E, 5 with LOS < 10 days, and 39 with incomplete assessments were excluded. A total of 140 SCI patients with complete assessments were included for the final analysis (Figure 1). The demographic and clinical characteristics of the individuals with or without complete assessments were comparable (Supplementary Table 1).
Among 140 SCI patients included, 44 (31.43%) patients were tetraplegics, and 96 (68.57%) patients were paraplegics; 68 (48.57%) patients developed NP, and 72 (51.43%) patients did not by the end of data collection. According to the time of pain occurrence, 33 (48.53%) patients were within one month and 35 (51.47%) were after one month since injury. In terms of pain location, 15 (22.06%) cases were at-level pain, 23 (33.82%) cases were below-level pain, and 15 (22.06%) cases were both.
Univariate Analyses and Multivariate Logistic Regression of SCIM at Discharge Dichotomized
The 140 included SCI patients were then divided into either the poor prognostic group (n=77, SCIM≥50) or the good prognostic group (n=63, SCIM<50). Univariate analysis indicated no differences between the two groups with respect to most demographic characteristics except for gender (Table 1). Regarding clinical characteristics, SCIM outcomes were significantly different among injury level (P<0.01), injury severity (P<0.01), anemia (P=0.01), and NP (P=0.01). However, there were no differences between the two groups in other clinical factors, including LOS and duration of SCI (Table 1).
 |
Table 1 Univariate Analysis of Functional Outcome |
Therefore, gender, injury degree, injury level, anemia, and NP were entered into the multivariate logistic regression analysis. The results showed that NP [OR=3.10, 95% CI (1.29,7.45), P=0.01], non-anemia [OR=2.99, 95% CI (1.22,7.30), P=0.02], incomplete injury [OR=13.52, 95% CI (5.16,35.42), P<0.01], and lumbar-sacral injury level [OR=6.22, 95% CI (1.74,22.18), P=0.01] were significant independent predictive factors for good prognostic outcome (Figure 2).
Univariate and Multivariate Linear Regression of SCIM at Discharge
The univariate and multivariate linear regression analyses were further performed to validate the relationship between predictive factors and SCIM at discharge. Multivariate linear regression demonstrated that the overall model was significant (F=22.04, P<0.01, adjusted R2=0.43). The results indicated that NP [unstandardized β=11.47, 95% CI (4.95,17.99), P<0.01], non-anemia [unstandardized β=10.68, 95% CI (4.16,17.19), P<0.01], incomplete injury [unstandardized β=24.82, 95% CI (17.99,31.64), P<0.01], and lumbar-sacral injury level [unstandardized β=16.02, 95% CI (6.90,25.13), P<0.01] were independent predictors of SCIM total score at discharge, while the thoracic injury level was marginally significant [unstandardized β=7.05, 95% CI (−0.54, 14.64), P=0.07] (Table 2).
 |
Table 2 Linear Regressions Predicting Spinal Cord Independence Measure Total Score at Discharge |
Univariate and Multivariate Linear Regression of SCIM mRFG on SCIM and Its Sub-Scales
We then performed a linear regression of mRFG of SCIM score to investigate the predictive value of demographic and clinical factors for potential maximal functional improvement. Univariate and multivariate linear regression analyses are shown in Table 3. Multivariate linear regression showed the overall model was significant (F=17.23, P<0.01, adjusted R2=0.32). NP [unstandardized β=15.67, 95% CI (8.94,22.41), P<0.01], incomplete injury [unstandardized β=19.06, 95% CI (11.93,26.19), P<0.01], and lumbar-sacral injury level [Unstandardized β=11.43, 95% CI (1.90,20.96), P<0.01] were significant predictors of mRFG on SCIM total score, while the thoracic injury level was not [unstandardized β=2.48, 95% CI (−5.45,10.40), P=0.54]. Moreover, multivariate linear regressions of mRFG on SCIM sub-scales also revealed that NP and incomplete injury were significant predictors (Supplementary Tables 2-4).
 |
Table 3 Linear Regressions Predicting Mean Relative Function Gain of Spinal Cord Independence Measure Total Score |
Comparison of mRFG on SCIM and Its Sub-Scales
We further analyzed differences in mRFG between NP and non-NP groups, as well as among NP subgroups. According to the Mann–Whitney U analysis, the NP group had a significantly higher mRFG of SCIM total score as well as its sub-scales relative to the non-NP group (P<0.05, Figure 3). However, the Kruskal–Wallis H-test showed no statistical difference of mRFG among the three NP subgroups (at level pain, below level pain, or both). See Supplementary Table 5 for details.
Comparison of SCIM and Its Sub-Scales on Admission and Discharge
To determine whether there was any functional status difference at admission and discharge between NP and non-NP groups, we compared the SCIM total score and sub-scales. Results showed no significant differences between the two groups on the total score of SCIM or its sub-scales at admission. However, the rehabilitation assessments of the NP group were significantly superior to those of the non-NP group at discharge (P<0.05, Figure 4). We also investigated the rehabilitation assessments across the NP subgroups (at level pain, below level pain, and both), but no statistical differences were found, as shown in Supplementary Table 6.
Comparison of mRFG on SCIM Between NP Developed Within and Past One Month After Injury
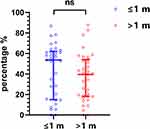
To explore the predictive effect of different timing of NP emergence on functional recovery, we compared the mRFG of patients who developed NP within one month [medium (QR), 53.68 (15.04,62.21)] after injury and those over one month [medium (QR), 39.53 (18.07,54.02)]. However, the result showed that there was no statistically significant difference (P=0.19, Figure 5).
Discussion
In this study, logistic and linear regression analyses were conducted using SCIM at discharge dichotomized or not, respectively, as outcome. Linear regression analyses using mRFG as outcome were also performed. Multivariate logistic and linear regression of SCIM at discharge showed that NP, non-anemia, incomplete injury, and lumbar-sacral injury level were significant independent predictive factors. However, when taking baseline functional condition and rehabilitation potential into account, non-anemia was excluded from the mRFG linear regression model. The possible reason is that the link between anemia and functional recovery may not be as strong as other three factors mentioned above. mRFG analysis indicated that the above factors except for non-anemia were related to favorable functional outcomes of SCI patients. In short, these analyses revealed that NP, as well as injury severity and level, were significantly related to the functional outcome of SCI patients. Sufficient evidence has verified that injury severity and level are important determinant factors of SCI functional outcome.4 However, few studies investigate the prognostic value of NP. Further analyses revealed that patients with NP had superior outcome to those without NP, with respect to mRFG as well as SCIM total score and its subscales. However, there were no statistical differences among the NP subgroups (at level pain, below level pain and both), or subgroups that developed NP within and over one month after injury in the predictive effects on functional recovery.
SCI often results in severe neurological impairments, including motor, self-care, respiration and sphincter dysfunctions.39 Fortunately, a portion of patients experience some degree of functional recovery, and previous researches have revealed several possible underlying mechanisms.14,15,40 First, residual neural pathways allow for spontaneous recovery. The primary reason for better outcome in patients with incomplete injury compared to complete is more residual neural pathways. An electrophysiological study has confirmed the presence of preserved descending motor pathways even in individuals with motor-complete SCI.41 These residual descending fibers may sprout into intact pathways below the injury level to contact with distal targets.42 MRI studies have indicated that the width of residual tissue bridges spanning the lesion may predict functional recovery after SCI.43 Second, molecular and structural alterations of synapses and sprouting of axons or dendrites can occur spontaneously after SCI, resulting in anatomical reorganization of the central and peripheral nervous systems, such as neuroplasticity.15 Reorganization of the corticospinal tract to the spinal and brainstem pathways has been demonstrated in motor recovery.18 Moreover, a study in animals also showed that the reorganization of descending and propriospinal connections may contribute to pronounced functional recovery after SCI.40
In this study, the functional prognostic value of NP was verified using both logistic and linear multivariate analyses by adjusting the potential confounding factors. Along with the above study that tissue bridges predict better functional recovery, an additional study has indicated that these tissue bridges are involved in the development and maintenance of NP.44 It has also been suggested that there is preserved function of the spinothalamic tract in most patients with NP following clinical diagnosis of complete SCI.14 Spinothalamic tract afferents projecting through a damaged spinal cord region may contribute to the development of NP.45 In addition to these structural factors, the neuroplasticity following SCI, which is necessary for neurological function recovery, is also an important cause of NP.9 Persisting nociceptive stimuli in residual afferents induce alterations in discharge and excitability of the thalamus, and these inputs might cause cortical reorganization, which may in turn contribute to the onset of NP.14 A study has exhibited a pronounced correlation between phantom-limb pain and the degree of reorganization in the primary somatosensory cortex (S1) and thalamus.46 Therefore, the emergence of NP may probably indicate favorable outcome from the perspectives of residual structures and neuroplasticity.
This study also found that the NP group was superior to the non-NP group in multiple functionality dimensions, including self-care, sphincter function and mobility, according to SCIM assessments. A previous animal study has indicated that bladder function after SCI is related to the plastic changes of bladder afferent pathways and synaptic connections in reorganization of the spinal cord.47 The reorganization of synaptic connections in the spinal cord and alterations of afferent neurons can induce the formation of a new micturition reflex.48 Changes in the function of peripheral regions might increase the expression of neurotrophic factors and subsequently induce neuroplasticity in peripheral and spinal neural pathways.49 Regarding mobility, although primary motor cortex (M1) and premotor cortex (PMC) are usually considered to be motor-related areas, studies have revealed that these regions play critical roles in pain regulation as well.50 A recently published animal study has revealed layer-specific pain relief pathways originating from M1.51 Our functional near-infrared spectroscopy (fNIRS) study also found hyperactivity of M1 and PMC in SCI patients with NP. Interestingly, after four weeks of high-frequency rTMS on M1, the pain intensity significantly decreased, meanwhile the overactivation of M1 and PMC was remarkably suppressed.19 The present study further demonstrated the close interaction between NP and motor function recovery.
It is interesting to find that there were no significant differences in functional recovery among the at-, below-, and both-level NP phenotypes, or between the different timings of NP occurrence. Typically, at-level pain precedes the onset of below-level, since the at-level pain may primarily arise from the perturbation of spinal nerves adjacent to the lesion level, while the maladaptive changes in spinal and supraspinal structures may be involved in the development of below-level pain.52 Therefore, there is a close association between the site and time of pain occurrence. Our study suggests that NP occurrence predicts favorable outcome for SCI in a location- and time-independent manner.
Of note, NP following SCI has been reported as a major cause of distress in patients.53 Nevertheless, the predictive value of NP on functional recovery may encourage these patients to look on the bright side of this undesirable symptom. Thus, our findings may help alleviate the psychological burdens of SCI patients and ultimately restore their confidence in rehabilitation. In this way, the predictive value of NP may function as a key to start the virtuous cycle of functional recovery for patients suffering from SCI.
There are several limitations in the present study to be noted. First, because of the retrospective study design, we could only extract available routine data. As a result, not all relevant confounding factors were well recorded. For example, pain intensity and psychological symptoms such as depression and anxiety were missing, therefore we did not conduct analyses concerning the correlation between the intensity of pain and the degree of functional recovery. However, these data are imperative for further research. Our study analyses were also based on data collected from a relatively small sample size from a single medical center, and the assessment data were not well documented. Therefore, we compared the demographic characteristics of the patients with complete assessment data and those without, and found that there was no significant selection bias in this study, reiterating its reliability. Despite this, large sample studies from multiple centers are still needed to further validate our conclusions. In spite of these limitations, we contend that the present study provides important implications to help alter the perspective of NP following SCI among both patients and medical practitioners.
Conclusion
Previous studies revealed that the greater degrees of NP relief, the more functional improvements were achieved in SCI patients.54 The present study showed the first time that the occurrence of NP was independently related to the functional recovery of patients with SCI. Specifically, SCI patients with NP had better prognoses in the aspects of SCIM subscales including self-care, respiration and sphincter management, and mobility, compared to those without NP. The silver lining of NP should be paid enough attention during SCI rehabilitation, which may help alleviate the psychological burden of NP patients and ultimately restore their confidence in functional recovery.
Acknowledgments
The authors thank the team of rehabilitation medicine at Xi-Jing Hospital, Air Force Medical University (Fourth Military Medical University).
Data Statement
Dr. Hua Yuan has full control of all primary data. All data of the study are available in Rehabilitation Medicine, Xi-Jing Hospital, Air Force Medical University (Fourth Military Medical University), and may be available upon reasonable request, after the permission from Ministry of Science and Technology of the People’s Republic of China.
Funding
This work was supported by National Natural Science Foundation of China [grant numbers 82072534, 82272591], Shanxi Provincial Key R&D Program-International Science and Technology Cooperation Program [grant number 2020KW-050], and Young Elite Scientists Sponsorship Program by CAST [grant number 2018QNRC001].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olusanya A, Yearsley A, Brown N, et al. Capsaicin 8% patch for spinal cord injury focal neuropathic pain, a randomized controlled trial. Pain Med. 2023;24(1):71–78.
2. Chikuda H, Koyama Y, Matsubayashi Y, et al. Effect of Early vs Delayed Surgical Treatment on Motor Recovery in Incomplete Cervical Spinal Cord Injury With Preexisting Cervical Stenosis: a Randomized Clinical Trial. JAMA Netw Open. 2021;4(11):e2133604.
3. Margot-Duclot A, Tournebise H, Ventura M, Fattal C. What are the risk factors of occurence and chronicity of neuropathic pain in spinal cord injury patients? Ann Phys Rehabil Med. 2009;52(2):111–123.
4. Burns AS, Marino RJ, Flanders AE, Flett H. Clinical diagnosis and prognosis following spinal cord injury. Handb Clin Neurol. 2012;109:47–62.
5. Tibbett JA, Field-Fote EC, Thomas CK, Widerstrom-Noga EG. Spasticity and Pain after Spinal Cord Injury: impact on Daily Life and the Influence of Psychological Factors. PM R. 2020;12(2):119–129.
6. Zhao CG, Sun W, Ju F, et al. Analgesic Effects of Directed Repetitive Transcranial Magnetic Stimulation in Acute Neuropathic Pain After Spinal Cord Injury. Pain Med. 2020;21(6):1216–1223.
7. Hatch MN, Cushing TR, Carlson GD, Chang EY. Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 2018;384:75–83.
8. Finnerup NB, Norrbrink C, Trok K, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain. 2014;15(1):40–48.
9. Finnerup NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord. 2017;55(12):1046–1050.
10. Fernandes V, Sharma D, Vaidya S, et al. Cellular and molecular mechanisms driving neuropathic pain: recent advancements and challenges. Expert Opin Ther Targets. 2018;22(2):131–142.
11. Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16(8):1024–1031.
12. Li Z, Tseng PY, Tiwari V, et al. Targeting human Mas-related G protein-coupled receptor X1 to inhibit persistent pain. Proc Natl Acad Sci U S A. 2017;114(10):E1996–E2005.
13. Margolis JM, Juneau P, Sadosky A, et al. Health care utilization and expenditures among Medicaid beneficiaries with neuropathic pain following spinal cord injury. J Pain Res. 2014;7:379–387.
14. Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131(Pt 9):2387–2400.
15. Filli L, Schwab ME. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res. 2015;10(4):509–513.
16. Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol. 2021;17(1):53–62.
17. Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111(4):761–773.
18. Asboth L, Friedli L, Beauparlant J, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21(4):576–588.
19. Sun X, Long H, Zhao C, et al. Analgesia-enhancing effects of repetitive transcranial magnetic stimulation on neuropathic pain after spinal cord injury:An fNIRS study. Restor Neurol Neurosci. 2019;37(5):497–507.
20. Schrempft S, van Jaarsveld C, Fisher A, et al. Variation in the Heritability of Child Body Mass Index by Obesogenic Home Environment. JAMA Pediatr. 2018;172(12):1153–1160.
21. Svensson T, Kitlinski M, Engstrom G, Melander O. Psychological stress and risk of incident atrial fibrillation in men and women with known atrial fibrillation genetic risk scores. Sci Rep. 2017;7:42613.
22. Association AD. 2. Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement_1):S15–S33.
23. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004.
24. Cao BH, Wu ZM, Liang JW. Risk Factors for Poor Prognosis of Cervical Spinal Cord Injury with Subaxial Cervical Spine Fracture-Dislocation After Surgical Treatment: a CONSORT Study. Med Sci Monit. 2019;25:1970–1975.
25. van Zyl N, Hill B, Cooper C, Hahn J, Galea MP. Expanding traditional tendon-based techniques with nerve transfers for the restoration of upper limb function in tetraplegia: a prospective case series. Lancet. 2019;394(10198):565–575.
26. Scivoletto G, Farchi S, Laurenza L, Molinari M. Traumatic and non-traumatic spinal cord lesions: an Italian comparison of neurological and functional outcomes. Spinal Cord. 2011;49(3):391–396.
27. Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535–546.
28. Butt AA, Yan P, Lo RVR, et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med. 2015;175(2):178–185.
29. Olaf M, Cooney R. Deep Venous Thrombosis. Emerg Med Clin North Am. 2017;35(4):743–770.
30. Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol. 2015;173(2):379–390.
31. Conner BJ. Treating Hypoalbuminemia. Vet Clin North Am Small Anim Pract. 2017;47(2):451–459.
32. Franz S, Schuld C, Wilder-Smith EP, et al. Spinal Cord Injury Pain Instrument and painDETECT questionnaire: convergent construct validity in individuals with Spinal Cord Injury. Eur J Pain. 2017;21(10):1642–1656.
33. Bryce TN, Ivan E, Dijkers M. Proposed International Spinal Cord Injury Pain (ISCIP) Classification:: preliminary Validation Data. Top Spinal Cord Inj Rehabil. 2012;18(2):143–145.
34. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532–545.
35. Catz A, Itzkovich M, Tesio L, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: rasch psychometric validation. Spinal Cord. 2007;45(4):275–291.
36. Koh GC, Chen CH, Petrella R, Thind A. Rehabilitation impact indices and their independent predictors: a systematic review. BMJ Open. 2013;3(9):e003483.
37. Jaywant A, Toglia J, Gunning FM, O’Dell MW. Subgroups Defined by the Montreal Cognitive Assessment Differ in Functional Gain During Acute Inpatient Stroke Rehabilitation. Arch Phys Med Rehabil. 2020;101(2):220–226.
38. Sharif S, Jazaib AM. Outcome Prediction in Spinal Cord Injury: myth or Reality. World Neurosurg. 2020;140:574–590.
39. Pfyffer D, Huber E, Sutter R, Curt A, Freund P. Tissue bridges predict recovery after traumatic and ischemic thoracic spinal cord injury. Neurology. 2019;93(16):e1550–e1560.
40. Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69–74.
41. Squair JW, Bjerkefors A, Inglis JT, Lam T, Carpenter MG. Cortical and vestibular stimulation reveal preserved descending motor pathways in individuals with motor-complete spinal cord injury. J Rehabil Med. 2016;48(7):589–596.
42. Rejc E, Smith AC, Weber KN, et al. Spinal Cord Imaging Markers and Recovery of Volitional Leg Movement With Spinal Cord Epidural Stimulation in Individuals With Clinically Motor Complete Spinal Cord Injury. Front Syst Neurosci. 2020;14:559313.
43. Huber E, Lachappelle P, Sutter R, Curt A, Freund P. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol. 2017;81(5):740–748.
44. Pfyffer D, Vallotton K, Curt A, Freund P. Tissue bridges predict neuropathic pain emergence after spinal cord injury. J Neurol Neurosurg Psychiatry. 2020;91(10):1111–1117.
45. Soler MD, Kumru H, Vidal J, et al. Referred sensations and neuropathic pain following spinal cord injury. Pain. 2010;150(1):192–198.
46. Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124(Pt 11):2268–2277.
47. de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235(1):123–132.
48. Goetz LL, Cardenas DD, Kennelly M, et al. International Spinal Cord Injury Urinary Tract Infection Basic Data Set. Spinal Cord. 2013;51(9):700–704.
49. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84.
50. Park E, Cha H, Kim E, et al. Alterations in power spectral density in motor- and pain-related networks on neuropathic pain after spinal cord injury. Neuroimage Clin. 2020;28:102342.
51. Gan Z, Gangadharan V, Liu S, et al. Layer-specific pain relief pathways originating from primary motor cortex. Science. 2022;378(6626):1336–1343.
52. Rosner J, Negraeff M, Belanger LM, et al. Characterization of Hyperacute Neuropathic Pain after Spinal Cord Injury: a Prospective Study. J Pain. 2022;23(1):89–97.
53. Kim HY, Lee HJ, Kim TL, et al. Prevalence and Characteristics of Neuropathic Pain in Patients With Spinal Cord Injury Referred to a Rehabilitation Center. Ann Rehabil Med. 2020;44(6):438–449.
54. Sadosky A, Parsons B, Emir B, Nieshoff EC. Pain relief and functional improvement in patients with neuropathic pain associated with spinal cord injury: an exploratory analysis of pregabalin clinical trials. J Pain Res. 2016;9:405–416.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.