Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
A Review: Cytochrome P450 in Alcoholic and Non-Alcoholic Fatty Liver Disease
Authors Jiang YJ , Cao YM, Cao YB, Yan TH, Jia CL, He P
Received 12 November 2023
Accepted for publication 16 March 2024
Published 1 April 2024 Volume 2024:17 Pages 1511—1521
DOI https://doi.org/10.2147/DMSO.S449494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Yu-Jie Jiang,1,2 Ye-Ming Cao,1 Yong-Bing Cao,1 Tian-Hua Yan,2 Cheng-Lin Jia,1 Ping He1
1Institute of Vascular Anomalies, Shanghai Academy of Traditional Chinese Medicine, Shanghai, 200082, People’s Republic of China; 2Department of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, 211100, People’s Republic of China
Correspondence: Cheng-Lin Jia; Ping He, Institute of Vascular Anomalies, Shanghai Academy of Traditional Chinese Medicine, Shanghai, 200082, People’s Republic of China, Tel/Fax +1 522 136 9352 ; +1 301 693 1191, Email [email protected]; [email protected]
Abstract: Alcoholic fatty liver disease (FALD) and non-alcoholic fatty liver disease (NAFLD) have similar pathological spectra, both of which are associated with a series of symptoms, including steatosis, inflammation, and fibrosis. These clinical manifestations are caused by hepatic lipid synthesis and metabolism dysregulation and affect human health. Despite having been studied extensively, targeted therapies remain elusive. The Cytochrome P450 (CYP450) family is the most important drug-metabolising enzyme in the body, primarily in the liver. It is responsible for the metabolism of endogenous and exogenous compounds, completing biological transformation. This process is relevant to the occurrence and development of AFLD and NAFLD. In this review, the correlation between CYP450 and liver lipid metabolic diseases is summarised, providing new insights for the treatment of AFLD and NAFLD.
Keywords: CYP450, liver metabolism, lipid accumulation, monooxygenases, alcoholic fatty liver disease, non-alcoholic fatty liver disease
Introduction
Liver metabolism disorders are primarily caused by an imbalance in lipid metabolism, which leads to the excessive accumulation of triglycerides (TG) and cholesterol (CHOL) in the liver. In addition, fatty metamorphosis induces liver inflammation and oxidative stress (OS), leading to cellular dysfunction, necrosis, and liver fibrosis. If untreated, cirrhosis develops and/or liver failure may occur.1 The global prevalence of hepatic lipid metabolism diseases is 32.4%, posing a huge burden on global health.2,3 Cytochrome P450 (CYP450) monooxygenases are abundant in the liver and essential for the metabolism of endogenous and exogenous compounds, including fatty acids. Thus, CYP450 serves as a novel target for the prevention and treatment of hepatic lipid metabolic diseases.4 Here, we summarised what is known about the relationship between hepatic CYP450 and lipid metabolism disorders.
Overview of CYP450
Naming and Distribution of CYP450
CYP450 belongs to a multifunctional redox superfamily.5 The discovery of it in rat liver microsomes dates back to 1958.6 In its reduced state, CYP450 maximum absorption peaks at 450 nm, which is the reason for its name.7 The CYP cDNA encodes 420–560 amino acids with a molecular weight of approximately 50 kDa, and has a conserved structural fold.8 CYP nomenclature is based on their sequence similarity, with a cut-off of 40% defining a family member and 55% or more defining a subfamily member.9 There are 18 known families and 43 known subfamilies of CYP450.10 Among them, the CYP1, 2, and 3 families are the most abundant in the human liver, accounting for approximately 70% of the total hepatic CYP450 content.11 CYP450 is widely distributed in the human body, and differential spectroscopy can detect its expression in various organs,12 including the liver, intestine, pancreas, brain, lungs, adrenal glands, kidneys, bone marrow, mast cells, skin, ovary and testis; however, experimental tests revealed that it is mainly expressed in the liver.13 In addition, the distribution of CYP450 in the liver is also different. For example, CYP1A is mainly expressed in the hepatocytes surrounding the small hepatic veins,14 while CYP3A is preferentially expressed in hepatocytes in the central area of the hepatic lobular.15
Structure and Function of CYP450
CYP450 is mainly distributed in the endoplasmic reticulum and mitochondrial inner membrane.16 It has a unique structure and is one of the most versatile biocatalysts in nature. CYP450 metabolises exogenous compounds, including drugs, ensuring their efficacy and controlling toxicity.11 Additionally, it catalyses endogenous substrates, including arachidonic acids, estradiol, cholesterol, vitamin D, and neurotransmitters.17 Its structure consists of 12 α helices and antiparallel β-folds,18 and is divided into cytosolic domain (CD), transmembrane domain (TD) and coheme, which is the source of CYP450 catalytic activity.19 Heme achieves electron transfer through the protoporphyrin structure20 (Fe3+ is located in the center of the site and binds to four nitrogen atoms in the same plane while binding to the sulfhydryl group of the bottom cysteine): (1) the substrate binds to the active center of the enzyme to form a complex of high iron ions.21 (2) CYP450 reductase (POR) slowly transfers an electron from Reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) to reduce iron to bivalent.22 (3) oxygen atoms are introduced into the substrate structure, oxidising ferrous to trivalent iron and producing peroxy ions.23 (4) cytochrome B5 achieves a second electron transfer to generate a molecule of water, and the CYP450 returns to the original position to continue to prepare for the next round of catalytic reaction, achieving cyclic catalysis.24 In the catalytic process, CYP450 interacts not only with POR and cytochrome B5, as mentioned above, but also with other CYP450 subtypes.12 For instance, during alcohol metabolism, CYP2E1 not only up-regulates its expression but also indirectly up-regulates the expression of CYP2A5.25
Correlation of CYP450 with Alcoholic and Non-Alcoholic Fatty Liver Disease
Recent meta-analyses indicate that the prevalence of fatty liver disease in the global adult population is increasing annually, with a worldwide prevalence of 30% and is one of the leading causes of death worldwide.26,27 Fatty liver diseases can be categorised into alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD) based on their pathogenesis.18 The root cause of it is an imbalance in the metabolism of triglycerides, cholesterol, and other lipids, which leads to their excessive accumulation in the liver.28 As a class of metabolic enzymes mainly expressed in the liver, CYP450 has a wide spectrum of substrates in the liver, almost covering all metabolic reactions in the liver, and maintaining the balance of lipid metabolism in the liver. Therefore, there is a dynamic balance between CYP450 and hepatic lipid metabolism. Disruption in CYP450 expression drives hepatic mitochondrial dysfunction and β-oxidation impairment, stimulating oxidative stress, endoplasmic reticulum stress, and inflammatory responses that exacerbate lipid metabolism disorders (Table 1).29,30 By contrast, correcting CYP450 expression delays disease progression.
 |
Table 1 CYP450 Regulation in Liver Lipid Metabolism-Related Diseases |
Correlation Between CYP450 and AFLD
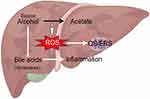
AFLD progression is closely related to the amount and duration of alcohol consumption.61 Excessive alcohol consumption leads to elevated CYP450 activity in hepatic microsomes. This results in reactive oxygen species (ROS) accumulation during alcohol metabolism, which contributes to the onset of mitochondrial oxidative or endoplasmic reticulum stress (ERS). ERS impedes the tricarboxylic acid cycle (TAC) and negatively affects cellular fatty acid β-oxidation function.40 Consequently, ethanol and its metabolites become more toxic to the liver. In addition, the cholestasis-induced inflammatory response contributes to the development of AFLD.41 Furthermore, excessive alcohol intake induces pore size enlargement within liver endothelial cells. Consequently, triglyceride-rich chylomicron remnants and their associated particles enter the hepatocytes, resulting in the accumulation of fat.42 (Figure 1).
CYP1 and AFLD
Changes in CYP1 expression play a regulatory role in the development of AFLD among the CYP1, 2, and 3 families, except for the CYP3 family, which is dominated by drug metabolism. Zhang et al found that CYP1A1 upregulation increases aromatic hydrocarbon receptor (AhR) expression and activates the transformation of hepatic stellate cells to proliferative, fibrotic, and contractile myofibroblasts to further promote the evolution of steatohepatitis to hepatic fibrosis.62 The CYP family, located mainly in the smooth endoplasmic reticulum, is a major component of the mitochondrial ethanol oxidation system.35 Chronic alcohol consumption leads to enhanced CYP1A2 activity in the smooth endoplasmic reticulum, which significantly promotes microsomal ethanol oxidation, metabolises ethanol to highly reactive acetaldehyde, stimulates the production of ROS, leads to the failure of deoxyribonucleic acid (DNA) repair in hepatocytes, lipid peroxidation, and mitochondrial damage, as well as promotes AFLD and hepatocellular carcinoma (HCC).36,63
CYP2 and AFLD
Similar to the CYP1 family, the CYP2 family plays a significant role in the development of AFLD. Chen et al verified, in a mouse model of alcoholic fatty liver, that high CYP2A5 expression acts on the peroxisome proliferators activated receptors α- fibroblast growth factor 21 (PPARα-FGF21) signalling pathway, which further enhances insulin sensitivity and blunted the development of AFLD.52 CYP2E1 is highly catalytic in alcohol consumption and is strongly correlated with AFLD.64 Attal et al suggested that CYP2E1 is dependent on the sirtuin-1-foxo1 pathway to increase the expression and secretion of fatty acid-binding protein-4 (FABP4) mRNA in adipocytes and macrophages. CYP2E1 inhibition reduces fatty acid synthesis, effectively alleviating the formation of simple fatty liver in early AFLD.58 In addition, Yuan et al discovered that CYP2E1 suppression impedes ethanol oxidation, decreases ROS and free radical production, hinders lipid peroxidation in hepatocytes, and enhances the stability and function of cell membranes. Consequently, it prevents ethanol-induced liver injury.65 Nagappan et al found that CYP2E1 inhibition significantly increases the expression of antioxidant enzymes, including super oxide dismutase (SOD) and catalase (CAT), as well as hepatic glutathione levels. This inhibition decreased oxidative stress and inflammatory responses, significantly improving alcohol-induced liver fibrosis.66 Li et al found that CYP2E1 inhibition enhances alcohol metabolism, improves oxidative stress, and reduces cytotoxic malondialdehyde (MDA) produced during lipid peroxidation.67 Gao et al demonstrated that the use of CYP2E1 inhibitors reduced NLRP3 signal transduction, caspase-1 expression and ROS production in the liver of mice.68 The above studies suggest that inhibition of the stress response and activation of inflammatory vesicles may be an attractive pharmacological entry point for the treatment of AFLD. Additionally, numerous researchers have observed the therapeutic potential of CYP2E1 inhibitors and have endeavoured to develop them as a rational drug for the extended treatment of alcoholic steatohepatitis.69 However, it is not invariable. In contrast to the above findings, a clinical study indicated that CYP2E1 was upregulated in the advanced stages of hepatocellular carcinoma.70 Leung et al also found that in a mouse model of alcoholic fatty liver disease, CYP2E1 is upregulated, resulting in excess ROS production, activation of the Nrf2 signalling pathway, CYP2A5 upregulation, and inhibition of disease progression.25 Furthermore, Ronis et al discovered that tumors in mice fed with alcohol and in patients with alcohol-related HCC exhibited elevated levels of CYP2W1.33
Other CYP Families and AFLD
Apart from the CYP1 and CYP2 families, the CYP4, CYP7, and CYP26 families are also involved in AFLD. Tête et al found that CYP4A inhibition suppresses the toxic effects of hepatocyte death on the pathological progression of steatosis under ethanol exposure and effectively alleviates AFLD progression.31 Alcohol disrupts bile acid (BA) synthesis as well as hepatic and intestinal circulation. Additionally, it damages liver cells, leading to hepatotoxicity.32 Hartman et al found that in mice, the CYP7A1 (a rate-limiting enzyme in the classical metabolic pathway of BAs) inhibition targets the bile acids-farnesoid X receptor- fibroblast growth factor 15 (Bas-FXR-FGF15) signalling pathway to regulate liver glucose metabolism and stimulate protein synthesis and promote liver regeneration and repair.34,71 Furthermore, alcohol consumption reduces the supply of vitamin A to the hepatic stellate cells, promoting fibrosis progression.72 Erdouse et al demonstrated that chronic alcohol consumption reduces hepatic vitamin A levels, which correlates with the expression of the retinoic acid-specific hydroxylases CYP26A1 and CYP26B1. Chronic alcohol consumption upregulates CYP26 expression, depletes vitamin A content in hepatic stellate cells, and stimulates the development of hepatic fibrosis.47
Correlation Between CYP450 and NAFLD
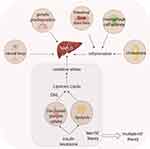
A fundamental theory formulated by Day and James in 1998 states that the progression of NAFLD can be characterised by a “two-hit” model.45 The initial impact results in the accumulation of hepatic triglycerides in the fatty liver, which may be benign and reversible. If oxidative stress occurs again, it may lead to non-alcoholic steatohepatitis (NASH). This is because hepatocytes are exposed to ROS and inflammatory cytokines, leading to death and irreversible remodelling of the liver tissue. However, this view seems too simplistic in its attempt to capture the multifaceted nature of NAFLD in humans. “Multiple-hit” theory was born, which involves insulin resistance (IR), oxidative stress, inflammation, genetics, intestinal flora, and immunity.46,50 Changes in CYP450 activity are closely linked to the pathogenesis of NAFLD.73 Several CYP450 isoforms mediate insulin action (eg CYP2J2 and CYP2E1), affect lipid synthesis and accumulation in the liver, and enhance liver susceptibility.43 ROS produced during CYP450 metabolism can strike hepatocytes with pre-existing lipid deposits, induce hepatic inflammatory responses, and promote diffuse hepatic fibrosis, regenerative nodule formation, and liver failure.44 (Figure 2).
CYP1 and NAFLD
CYP1 is an enzyme involved in the biosynthesis and metabolism of lipid mediators.49 Lu et al discovered that the absence of CYP1A1 and CYP1A2, in mice, obstructs the cholesterol metabolism pathway, resulting in the accumulation of cholesterol and cholesteryl esters. Moreover, CYP1A-inducing substances can counteract this lipid deposition phenotype.48 He and Huang et al demonstrated that inhibition of CYP1A1 was effective in suppressing OS levels and alleviating the symptoms of steatohepatitis in terms of decreasing ROS production and elevating the level of the antioxidant Superoxide Dismutase (SOD), respectively.38,74 Zhang et al showed that inhibiting the enzymatic activity of CYP1A1 enhanced glucose metabolism in obese mice induced by a high-fat diet. Furthermore, it decreased the quantity of triglycerides in the liver.57 Zhu et al found that overexpression of CYP1A1, an oestrogen-metabolising enzyme, affects the oestrogen signalling pathway, inhibits fatty acid oxidation and enhances fat mobilization.60 Li et al discovered that fatty acid synthase (FAS) and acetyl-CoA carboxylase β (ACCβ) expression was downregulated in the liver of CYP1B1 null mice. This effectively reduces the synthesis of intrahepatic fat, thereby slowing the formation of simple fatty liver.56 CYP1A2, unlike CYP1A1 and CYP1B1, reduces metabolic activity in patients with cirrhosis.55
CYP2 and NAFLD
The CYP2 family is one of the largest CYP450 families, which profoundly affects the metabolism and synthesis of endogenous lipids in the liver.75 Morgan et al found that the CYP2A5 upregulation suppresses X-box binding protein1 (XBP1) splicing and caspase-3 cleavage, resulting in the inhibition of hepatic injury caused by endoplasmic reticulum stress. Consequently, the progression from steatosis to fibrosis and cirrhosis is effectively prevented.53 Li et al discovered that overexpression of CYP2B6 hinders the phosphorylation of insulin receptor substrates and the membrane translocation of glucose transporter 2 (GLUT2), exacerbating IR and promoting lipid accumulation in the liver.37 Furthermore, Ghoshal et al observed that CYP2C44 suppresses the expression of genes involved in gluconeogenesis (glycogen synthase 2, glucose 6-phosphatase, phosphoenolpyruvate carboxykinase, and fructose 1,6-bisphosphatase); stimulates insulin signalling; and inhibits hepatic lipid synthesis.54 Muller et al observed that overexpression of CYP2D6 increased the risk of autoimmune liver diseases (such as autoimmune hepatitis) in the presence of pre-existing metabolic liver injury.39 Smith et al discovered that CYP2E1 upregulation promotes ROS production, triggering the activation of the JNK pathway to phosphorylate insulin receptor substrates. This action reduces the sensitivity to insulin signalling and prompts hormone-sensitive lipase activation. It also catalyses adipose tissue hydrolysis, which releases significant amounts of fatty acids into the bloodstream, eventually affecting the liver. This process enhances hepatic triglyceride synthesis and storage, promotes inflammation, and fibrosis stimulation.51 Leclercq et al identified an alternative pathway for FA hydroxylation in CYP2E1 null mice, in which CYP4A is activated, causing an increase in OS levels. This response promotes inflammatory hepatic injury and fibrosis.76 In addition, Song and Dang found that decreased CYP2E1 activity activates hepatic PPARα and increases the expression and secretion of its target gene, FGF21, to induce white steatosis and effectively metabolise accumulated adipose tissue. FGF21 induces white fat browning and effectively metabolises accumulated adipose tissue, thereby facilitating disease treatment.77,78
Chen et al discovered that CYP2J2 upregulation, in mice induced with high-fat diets, decreased the activation of the nuclear factor kappa B/c-Jun N-terminal kinase (NF-Κb/JNK) signalling pathway, thereby improving the antioxidant defence system and reducing oxidative stress and inflammatory responses. These findings have ultimately led to the successful prevention and treatment of NAFLD.79 Zhang et al demonstrated that CYP2J2 upregulation increases insulin sensitivity through fatty acid oxidation mediated by the AMP-activated protein kinase (AMPK) pathway. This process reduces circulating fatty acid production and intrahepatic lipid accumulation.80 Razdan et al discovered that when CYP2J5 downregulation significantly reduces hepatic and circulating epoxyeicosatrienoic acids (EETs) levels. This loss also reduces adipose tissue expansion, promotes adipogenic gene (for example, FABP4) expression, leads to glucose intolerance, and enhances intrahepatic lipid synthesis.81 Maayah found that CYP2J6 catalyses the formation of EETs from arachidonic acid, enhancing insulin sensitivity and regulating lipid metabolism.82
Other CYP Families and NAFLD
Families other than CYP 1, and 2 are also of interest in the study of non-alcoholic fatty liver disease. Cholesterol-synthesising BAs have been identified as signalling molecules that progressively drive HCC development in patients with non-alcoholic steatohepatitis.83 Ji et al found that CYP7A1 downregulation significantly lowers the risk of liver tumour formation due to limited bile acid synthesis.84 This highlights the use of CYP7A1 as a therapeutic target; downregulating CYP7A1 serves as a preventive measure against liver tumourigenesis development. Qin et al noted that CYP3A inhibition inhibits intrahepatic bile acid metabolism and the release of inflammatory cytokines, which increases hepatic susceptibility and promotes fibrosis and cirrhosis development.85 Dong et al found that CYP7A1 activation enhances hepatic bile acid biosynthesis to counteract high-fat diet-induced steatosis.86 Pathak et al found that CYP8B1 overexpression increases 12α-hydroxybile acid production and activates lipid synthesis genes, including sterol regulatory element binding protein 1c (SREBP-1c) and FAS, thereby increasing hepatic lipid accumulation.87 Hendrikx et al showed, in liver Kupffer cells, that CYP27A1 overexpression downregulates inflammatory gene expression and upregulates cholesterol transporter protein expression, effectively alleviating liver lipid accumulation and inflammation.88
Filipović et al revealed that CYP24A1 enhances the hydroxylation response of vitamin D. CYP24A1 upregulation curbs insulin secretion in pancreatic β-cells of rats, reduces intracellular Ca2+ concentration, and enhances glucose transporter protein activity. In addition, it restrains peripheral IR and delays hepatic lipid accumulation.89 Barchetta et al found that CYP27A1 inhibition hindered vitamin D metabolism and insulin signalling. This leads to reduced hepatic angiopoietin-like protein 3 (Angptl33) expression and enhanced hormone-sensitive triglyceride lipase (HSL) activity, which promotes hepatic lipid synthesis.90 Yang et al found that CYP19A1 upregulation promotes oestrogen production, attenuates IR via the transcription factor forkhead box o 1 (Foxo1), and inhibits lipid accumulation in the liver.91 Evangelos et al reported that in an insulin resistant state, CYP7B1 stimulation leads to the advancement of steatohepatitis from simple steatohepatitis.92
CYP4A and CYP4F play important roles in hepatic fatty acid metabolism. Hardwick found that a high-fat diet induces the expression of CYP4A and CYP4F, which promotes the synthesis of stearoyl-coenzyme A desaturase 1 (SCD-1) and inhibits the assembly of TG into very low density lipoprotein (VLDL), consequently favouring steatosis and steatohepatitis.93 Similar to CYP2E1, Osborne found that in human tissue samples, CYP4V2 was elevated in steatosis, but decreased in patients with cirrhosis and HCC.94
Conclusion
In summary, CYP450 is a crucial member of the hepatic enzyme system. It regulates systemic metabolic homeostasis by altering the transcription and expression of its upstream factors. It also participates in ROS generation, steroid metabolism, immune inflammatory response, and other pathways. Relevant studies on diseases related to liver lipid metabolism have attracted wide attention. It has been suggested that targeting CYP450 could be a potential approach for treating emerging diseases that regulate lipid metabolism in the liver. This may provide ideas for treating clinical diseases related to lipid metabolism disorders. However, CYP450 polymorphism exhibits variation across different species, and even among individuals of the same species. When introducing CYP450-targeted drugs to the clinic, it is important to verify extrapolation and consider individual differences among patients. At the same time, CYP450 exists widely in the human body, and achieving precise treatment requires further in-depth research. Consequently, research on CYP450 targeted drugs has mostly stalled in the laboratory. Few drugs have progressed to the early clinical trial stage, and there are still no mature clinical preparations. Further studies are required to confirm the presence of unknown but potentially serious adverse reactions during targeted CYP450 therapy. Therefore, CYP450 could be used as a clinical treatment strategy by targeting its upstream gene/protein as a therapeutic target for clinical drug development, focusing on its indirect regulatory role.
Abbreviations
CYP450, cytochrome P450; TG, triglycerides; CHOL, cholesterol; NADPH, nicotinamide adenine dinucleotide phosphate; NADH, nicotinamide adenine dinucleotide; POR, cytochrome P450 reductase; AFLD, alcoholic fatty liver disease; NAFLD, non-alcoholic fatty liver disease; ROS, reactive oxygen species; ERS, endoplasmic reticulum stress; TAC, tricarboxylic acid cycle; AhR, aromatic hydrocarbon receptor; DNA, deoxyribonucleic acid; FABP4, fatty acid-binding protein-4; SOD, super oxide dismutase; CAT, catalase; MDA, malondialdehyde; PPARα-FGF21, proliferators activated receptors α- fibroblast growth factor 21; BAs-FXR-FGF15, bile acids-farnesoid X receptor- fibroblast growth factor 15; NASH, non-alcoholic steatohepatitis; FAS, fatty acid synthase; ACCβ, acetyl-CoA carboxylase β; NF-κB/JNK, nuclear factor kappa B/c-Jun N-terminal kinase; EETs, epoxyeicosatrienoic acids; AMPK, AMP-activated protein kinase; XBP1 X-box binding protein1; Angptl33, angiopoietin-like protein 3; HSL, hormone-sensitive triglyceride lipase; Foxo1, factor forkhead box o 1; SREBP 1c, sterol regulatory element binding protein 1c; SCD-1. stearoyl-coenzyme A desaturase 1; hepatocellular carcinoma, HCC; IR, insulin resistance.
Acknowledgments
This article was financially supported by National Natural Science Foundation of China (81874378), Project within the budget of Shanghai University of Traditional Chinese Medicine (2021LK062), and the Special project for clinical research in health industry of Shanghai Health Commission (202140334).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gopal T, Ai W, Casey CA, et al. A review of the role of ethanol-induced adipose tissue dysfunction in alcohol-associated liver disease. Alcohol Clin Exp Res. 2021;45(10):1927–1939. doi:10.1111/acer.14698
2. Devarbhavi H, Asrani SK, Arab JP, et al. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516–537. doi:10.1016/j.jhep.2023.03.017
3. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. doi:10.1016/S2468-1253(22)00165-0
4. Gómez-Lechón MJ, Jover R, Donato MT. Cytochrome p450 and steatosis. Curr Drug Metab. 2009;10(7):692–699. doi:10.2174/138920009789895543
5. Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes: II. Solubilization, purification, and properties. J Biol Chem. 1964;239(7):2379–2385. doi:10.1016/S0021-9258(20)82245-5
6. Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–1162. doi:10.1016/S0140-6736(02)11203-7
7. Omura T. Forty years of cytochrome P450. Biochem Biophys Res Commun. 1999;266(3):690–698. doi:10.1006/bbrc.1999.1887
8. Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi:10.1016/j.redox.2014.11.008
9. de Montellano PR O. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem Rev. 2010;110(2):932–948. doi:10.1021/cr9002193
10. Pankov KV, McArthur AG, Gold DA, et al. The cytochrome P450 (CYP) superfamily in cnidarians. Sci Rep. 2021;11(1):9834. doi:10.1038/s41598-021-88700-y
11. Zhao M, Ma J, Li M, et al. Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci. 2021;22(23):12808. doi:10.3390/ijms222312808
12. lewka A, Kamiński M, Plewka D. Ontogenesis of hepatocyte respiration processes in relation to rat liver cytochrome P450-dependent monooxygenase system. Mech Ageing Dev. 1998;105(3):197–207. doi:10.1016/S0047-6374(98)00086-4
13. Song Y, Li C, Liu G, et al. Drug-metabolizing cytochrome P450 enzymes have multifarious influences on treatment outcomes. Clin Pharmacokinet. 2021;60(5):585–601. doi:10.1007/s40262-021-01001-5
14. McKinnon RA, Hall PD, Quattrochi LC, et al. Localization of CYP1A1 and CYP1A2 messenger RNA in normal human liver and in hepatocellular carcinoma by in situ hybridization. Hepatology. 1991;14(5):848–856. doi:10.1002/hep.1840140517
15. Fanni D, Pinna F, Gerosa C, et al. Anatomical distribution and expression of CYP in humans: neuropharmacological implications. Drug Dev Res. 2021;82(5):628–667. doi:10.1002/ddr.21778
16. Matsuura S, Fujii-Kuriyama Y, Tashiro Y. Immunoelectron microscope localization of cytochrome P-450 on microsomes and other membrane structures of rat hepatocytes. J Cell Biol. 1978;78(2):503–519. doi:10.1083/jcb.78.2.503
17. Omura T. Mitochondrial P450s. Chem Biol Interact. 2006;163(1–2):86–93. doi:10.1016/j.cbi.2006.06.008
18. Šrejber M, Navrátilová V, Paloncýová M, et al. Membrane-attached mammalian cytochromes P450: an overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J Inorg Biochem. 2018;183:117–136. doi:10.1016/j.jinorgbio.2018.03.002
19. Barnaba C, Sahoo BR, Ravula T, et al. Cytochrome-P450-induced ordering of microsomal membranes modulates affinity for drugs. Angew Chem Int Ed Engl. 2018;57(13):3391–3395. doi:10.1002/anie.201713167
20. Kappas A, Drummond GS. Control of heme metabolism with synthetic metalloporphyrins. J Clin Invest. 1986;77(2):335–339. doi:10.1172/JCI112309
21. Hedison TM, Scrutton NS. Tripping the light fantastic in membrane redox biology: linking dynamic structures to function in ER electron transfer chains. FEBS J. 2019;286(11):2004–2017. doi:10.1111/febs.14757
22. Yan B, Ai Y, Sun Q, et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 2021;81(2):355–369.e10. doi:10.1016/j.molcel.2020.11.024
23. Kadkhodayan S, Coulter ED, Maryniak DM, et al. Uncoupling oxygen transfer and electron transfer in the oxygenation of camphor analogues by cytochrome P450-CAM. Direct observation of an intermolecular isotope effect for substrate C-H activation. J Biol Chem. 1995;270(47):28042–28048. doi:10.1074/jbc.270.47.28042
24. Duggal R, Denisov IG, Sligar SG. Cytochrome b enhances androgen synthesis by rapidly reducing the CYP17A1 oxy-complex in the lyase step. FEBS Lett. 2018;592(13):2282–2288. doi:10.1002/1873-3468.13153
25. Leung TM, Lu Y. Alcoholic liver disease: from CYP2E1 to CYP2A5. Curr Mol Pharmacol. 2017;10(3):172–178. doi:10.2174/1874467208666150817111846
26. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150(8):1778–1785. doi:10.1053/j.gastro.2016.03.005
27. Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. doi:10.21037/tgh.2019.09.08
28. Zhang S, Hong F, Ma C, et al. Hepatic lipid metabolism disorder and atherosclerosis. Endocr Metab Immune Disord Drug Targets. 2022;22(6):590–600. doi:10.2174/1871530322666211220110810
29. Svegliati-Baroni G, Pierantonelli I, Torquato P, et al. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2019;144:293–309. doi:10.1016/j.freeradbiomed.2019.05.029
30. Rey-Bedon C, Banik P, Gokaltun A, et al. CYP450 drug inducibility in NAFLD via an in vitro hepatic model: understanding drug-drug interactions in the fatty liver. Biomed Pharmacother. 2022;146:112377. doi:10.1016/j.biopha.2021.112377
31. Tête A, Gallais I, Imran M, et al. MEHP/ethanol co-exposure favors the death of steatotic hepatocytes, possibly through CYP4A and ADH involvement. Food Chem Toxicol. 2020;146:111798. doi:10.1016/j.fct.2020.111798
32. Spatz M, Ciocan D, Merlen G, et al. Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis. JHEP Rep. 2021;3(2):100230. doi:10.1016/j.jhepr.2021.100230
33. Ronis MJ, Mercer KE, Shankar K, et al. Potential role of gut microbiota, the proto-oncogene PIKE (Agap2) and cytochrome P450 CYP2W1 in promotion of liver cancer by alcoholic and nonalcoholic fatty liver disease and protection by dietary soy protein. Chem Biol Interact. 2020;325:109131. doi:10.1016/j.cbi.2020.109131
34. Hartmann P, Hochrath K, Horvath A, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67(6):2150–2166. doi:10.1002/hep.29676
35. Teschke R. Microsomal ethanol-oxidizing system: success over 50 years and an encouraging future. Alcohol Clin Exp Res. 2019;43(3):386–400. doi:10.1111/acer.13961
36. Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS. Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res. 1998;22(9):2125–2132.
37. Li Y, Zhang D, Gao Y, et al. METTL3 exacerbates insulin resistance in hepatocytes by regulating m6A modification of cytochrome P450 2B6. Nutr Metab. 2023;20(1):40. doi:10.1186/s12986-023-00762-z
38. Huang B, Bao J, Cao Y-R, et al. Cytochrome P450 1A1 (CYP1A1) catalyzes lipid peroxidation of oleic acid-induced HepG2 cells. Biochemistry. 2018;83(5):595–602. doi:10.1134/S0006297918050127
39. Müller P, Messmer M, Bayer M, et al. Non-alcoholic fatty liver disease (NAFLD) potentiates autoimmune hepatitis in the CYP2D6 mouse model. J Autoimmun. 2016;69:51–58. doi:10.1016/j.jaut.2016.02.007
40. Osna NA, Rasineni K, Ganesan M, et al. Pathogenesis of Alcohol-Associated Liver Disease. J Clin Exp Hepatol. 2022;12(6):1492–1513. doi:10.1016/j.jceh.2022.05.004
41. Donepudi AC, Ferrell JM, Boehme S, et al. Deficiency of cholesterol 7α-hydroxylase in bile acid synthesis exacerbates alcohol-induced liver injury in mice. Hepatol Commun. 2017;2(1):99–112. doi:10.1002/hep4.1129
42. Veith A, Moorthy B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr Opin Toxicol. 2018;7:44–51. doi:10.1016/j.cotox.2017.10.003
43. Massart J, Begriche K, Fromenty B. Cytochrome P450 2E1 should not be neglected for Acetaminophen-induced liver injury in metabolic diseases with altered insulin levels or glucose homeostasis. Clin Res Hepatol Gastroenterol. 2021;45(1):101470. doi:10.1016/j.clinre.2020.05.018
44. Sukkasem N, Chatuphonprasert W, Jarukamjorn K. Cytochrome P450 expression-associated multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) in HepG2 cells. Trop J Pharm Res. 2020;19(4):707–714. doi:10.4314/tjpr.v19i4.5
45. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi:10.1016/S0016-5085(98)70599-2
46. Guo X, Yin X, Liu Z, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int J Mol Sci. 2022;23(24):15489. doi:10.3390/ijms232415489
47. Ferdouse A, Agrawal RR, Gao MA, et al. Alcohol induced hepatic retinoid depletion is associated with the induction of multiple retinoid catabolizing cytochrome P450 enzymes. PLoS One. 2022;17:e0261675.
48. Lu J, Shang X, Yao B. The role of CYP1A1/2 in cholesterol ester accumulation provides a new perspective for the treatment of hypercholesterolemia. Acta Pharm Sin B. 2023;13(2):648–661. doi:10.1016/j.apsb.2022.08.005
49. Divanovic S, Dalli J, Jorge-Nebert LF, et al. Contributions of the three CYP1 monooxygenases to pro-inflammatory and inflammation-resolution lipid mediator pathways. J Immunol. 2013;191(6):3347–3357. doi:10.4049/jimmunol.1300699
50. Deng A, Liu F, Tang X, et al. Water extract from artichoke ameliorates high-fat diet-induced non-alcoholic fatty liver disease in rats. BMC Complement Med Ther. 2022;22(1):308. doi:10.1186/s12906-022-03794-9
51. Gonzalez A, Huerta-Salgado C, Orozco-Aguilar J, et al. Role of oxidative stress in hepatic and extrahepatic dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid Med Cell Longev. 2020;2020:1617805. doi:10.1155/2020/1617805
52. Chen X, Ward SC, Cederbaum AI, et al. Alcoholic fatty liver is enhanced in CYP2A5 knockout mice: the role of the PPARα-FGF21 axis. Toxicology. 2017;379:12–21. doi:10.1016/j.tox.2017.01.016
53. Morgan L, Antenos M, Kirby GM. Nrf2-mediated induction of Cyp2a5 partially protects against reductive endoplasmic reticulum stress in mouse hepatocytes. Toxicology. 2022;471:153162. doi:10.1016/j.tox.2022.153162
54. Ghoshal K, Li X, Peng D, et al. EET analog treatment improves insulin signaling in a genetic mouse model of insulin resistance. Diabetes. 2021;71(1):db210298.
55. Chen H, Shen ZY, Xu W, et al. Expression of P450 and nuclear receptors in normal and end-stage Chinese livers. World J Gastroenterol. 2014;20(26):8681–8690. doi:10.3748/wjg.v20.i26.8681
56. Li F, Zhu W, Gonzalez FJ. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol Ther. 2017;178:18–30. doi:10.1016/j.pharmthera.2017.03.007
57. Zhang Y, Fu Q, Wu T, et al. 5-Methoxyflavone ameliorates non-alcoholic fatty liver disease through targeting the cytochrome P450 1A1. Free Radic Biol Med. 2023;195:178–191. doi:10.1016/j.freeradbiomed.2022.12.093
58. Attal N, Marrero E, Thompson KJ, et al. Cytochrome P450 2E1-dependent hepatic ethanol metabolism induces fatty acid-binding protein 4 and steatosis. Alcohol Clin Exp Res. 2022;46(6):928–940. doi:10.1111/acer.14828
59. Smith GI, Shankaran M, Yoshino M, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453–1460. doi:10.1172/JCI134165
60. Zhu X-Y, Xia H-G, Wang Z-H, et al. In vitro and in vivo approaches for identifying the role of aryl hydrocarbon receptor in the development of nonalcoholic fatty liver disease. Toxicol Lett. 2020;319:85–94. doi:10.1016/j.toxlet.2019.10.010
61. Paradies G, Paradies V, Ruggiero FM, et al. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(39):14205–14218. doi:10.3748/wjg.v20.i39.14205
62. Zhang HF, Lin XH, Yang H, et al. Regulation of the activity and expression of aryl hydrocarbon receptor by ethanol in mouse hepatic stellate cells. Alcohol Clin Exp Res. 2012;36(11):1873–1881. doi:10.1111/j.1530-0277.2012.01787.x
63. Taniai M. Alcohol and hepatocarcinogenesis. Clin Mol Hepatol. 2020;26(4):736–741. doi:10.3350/cmh.2020.0203
64. Teschke R, Moreno F, Petrides AS. Hepatic microsomal ethanol oxidizing system (MEOS): respective roles of ethanol and carbohydrates for the enhanced activity after chronic alcohol consumption. Biochem Pharmacol. 1981;30(13):1745–1751. doi:10.1016/0006-2952(81)90004-6
65. Yuan R, Tao X, Liang S, et al. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed Pharmacother. 2018;99:537–542. doi:10.1016/j.biopha.2018.01.079
66. Nagappan A, Kim J-H, Jung DY, et al. Cryptotanshinone from the Salvia miltiorrhiza Bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways. Int J Mol Sci. 2019;21(1):265. doi:10.3390/ijms21010265
67. Li BY, Mao QQ, Gan RY, et al. Protective effects of tea extracts against alcoholic fatty liver disease in mice via modulating cytochrome P450 2E1 expression and ameliorating oxidative damage. Food Sci Nutr. 2021;9(10):5626–5640. doi:10.1002/fsn3.2526
68. Gao N, Chen J, Li Y, et al. The CYP2E1 inhibitor Q11 ameliorates LPS-induced sepsis in mice by suppressing oxidative stress and NLRP3 activation. Biochem Pharmacol. 2023;214:115638. doi:10.1016/j.bcp.2023.115638
69. Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362–376. doi:10.1007/s00535-017-1415-1
70. Zhu L, Yang X, Feng J, et al. CYP2E1 plays a suppressive role in hepatocellular carcinoma by regulating Wnt/Dvl2/β-catenin signaling. J Transl Med. 2022;20(1):194. doi:10.1186/s12967-022-03396-6
71. Katafuchi T, Makishima M. Molecular basis of bile acid-FXR-FGF15/19 signaling axis. Int J Mol Sci. 2022;23(11):6046. doi:10.3390/ijms23116046
72. Chen G. The link between hepatic vitamin A metabolism and nonalcoholic fatty liver disease. Curr Drug Targets. 2015;16(12):1281–1292. doi:10.2174/1389450116666150325231015
73. Jamwal R, Barlock BJ. Nonalcoholic Fatty Liver Disease (NAFLD) and hepatic cytochrome P450 (CYP) enzymes. Pharmaceuticals. 2020;13(9):222. doi:10.3390/ph13090222
74. He Q, Li J-K, Li F, et al. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2015;21(7):2058–2066. doi:10.3748/wjg.v21.i7.2058
75. Kirischian N, McArthur AG, Jesuthasan C, et al. Phylogenetic and functional analysis of the vertebrate cytochrome p450 2 family. J Mol Evol. 2011;72(1):56–71. doi:10.1007/s00239-010-9402-7
76. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–1075. doi:10.1172/JCI8814
77. Song BJ, Abdelmegeed MA, Cho YE, et al. Contributing roles of CYP2E1 and other cytochrome P450 isoforms in alcohol-related tissue injury and carcinogenesis. Adv Exp Med Biol. 2019;1164:73–87.
78. Dang TTH, Yun JW. Cytochrome P450 2E1 (CYP2E1) positively regulates lipid catabolism and induces browning in 3T3-L1 white adipocytes. Life Sci. 2021;278:119648. doi:10.1016/j.lfs.2021.119648
79. Chen G, Xu R, Zhang S, et al. CYP2J2 overexpression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice. Am J Physiol Endocrinol Metab. 2015;308(2):E97–E110. doi:10.1152/ajpendo.00366.2014
80. Zhang S, Chen G, Li N, et al. CYP2J2 overexpression ameliorates hyperlipidemia via increased fatty acid oxidation mediated by the AMPK pathway. Obesity. 2015;23(7):1401–1413. doi:10.1002/oby.21115
81. Razdan A, Main NM, Chiu V, et al. Targeting the eicosanoid pathway in hepatocellular carcinoma. Am J Cancer Res. 2021;11(6):2456–2476.
82. Maayah ZH, McGinn E, Al Batran R, Gopal K, Ussher JR, El-Kadi AOS. Role of cytochrome p450 and soluble epoxide hydrolase enzymes and their associated metabolites in the pathogenesis of diabetic cardiomyopathy. J Cardiovasc Pharmacol. 2019;74(3):235–245. doi:10.1097/FJC.0000000000000707
83. Conde de la Rosa L, Garcia-Ruiz C, Vallejo C, et al. STARD1 promotes NASH-driven HCC by sustaining the generation of bile acids through the alternative mitochondrial pathway. J Hepatol. 2021;74(6):1429–1441. doi:10.1016/j.jhep.2021.01.028
84. Qin X, Zhang Y, Lu J, Huang S, Liu Z, Wang X. CYP3A deficiency alters bile acid homeostasis and leads to changes in hepatic susceptibility in rats. Toxicol Appl Pharmacol. 2021;429:115703. doi:10.1016/j.taap.2021.115703
85. Ji S, Liu Q, Zhang S, et al. FGF15 activates hippo signaling to suppress bile acid metabolism and liver tumorigenesis. Dev Cell. 2019;48(4):460–474. doi:10.1016/j.devcel.2018.12.021
86. Dong Z, He F, Yan X, et al. Hepatic reduction in cholesterol 25-hydroxylase aggravates diet-induced steatosis. Cell Mol Gastroenterol Hepatol. 2022;13(4):1161–1179. doi:10.1016/j.jcmgh.2021.12.018
87. Pathak P, Chiang JYL. Sterol 12α-hydroxylase aggravates dyslipidemia by activating the Ceramide/Mtorc1/SREBP-1C pathway via FGF21 and FGF15. Gene Expr. 2019;19(3):161–173. doi:10.3727/105221619X15529371970455
88. Hendrikx T, Jeurissen ML, Bieghs V, et al. Hematopoietic overexpression of Cyp27a1 reduces hepatic inflammation independently of 27-hydroxycholesterol levels in Ldlr(-/-) mice. J Hepatol. 2015;62(2):430–436. doi:10.1016/j.jhep.2014.09.027
89. Filipović N, Bočina I, Restović I, et al. Ultrastructural characterization of vitamin D receptors and metabolizing enzymes in the lipid droplets of the fatty liver in rat. Acta Histochemica. 2020;122(2):151502. doi:10.1016/j.acthis.2020.151502
90. Barchetta I, Cimini FA, Chiappetta C, et al. Relationship between hepatic and systemic angiopoietin-like 3, hepatic Vitamin D receptor expression and NAFLD in obesity. Liver Int. 2020;40(9):2139–2147. doi:10.1111/liv.14554
91. Yang Y, Wang P. Association of CYP19A1 and CYP1A2 genetic polymorphisms with type 2 diabetes mellitus risk in the Chinese Han population. Lipids Health Dis. 2020;19(1):187. doi:10.1186/s12944-020-01366-9
92. Evangelakos I, Schwinge D, Worthmann A, et al. Oxysterol 7-α hydroxylase (CYP7B1) attenuates metabolic-associated fatty liver disease in mice at thermoneutrality. Cells. 2021;10(10):2656. doi:10.3390/cells10102656
93. Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75(12):2263–2275. doi:10.1016/j.bcp.2008.03.004
94. Osborne N, Leahy C, Lee YK, et al. CYP4V2 fatty acid omega hydroxylase, a druggable target for the treatment of metabolic associated fatty liver disease (MAFLD). Biochem Pharmacol. 2022;195:114841. doi:10.1016/j.bcp.2021.114841
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.