Back to Journals » Journal of Asthma and Allergy » Volume 15
A Predictive Machine Learning Tool for Asthma Exacerbations: Results from a 12-Week, Open-Label Study Using an Electronic Multi-Dose Dry Powder Inhaler with Integrated Sensors
Authors Lugogo NL, DePietro M , Reich M, Merchant R, Chrystyn H, Pleasants R, Granovsky L, Li T, Hill T, Brown RW, Safioti G
Received 9 July 2022
Accepted for publication 2 November 2022
Published 11 November 2022 Volume 2022:15 Pages 1623—1637
DOI https://doi.org/10.2147/JAA.S377631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Njira L Lugogo,1 Michael DePietro,2 Michael Reich,3 Rajan Merchant,4 Henry Chrystyn,5 Roy Pleasants,6 Lena Granovsky,3 Thomas Li,2 Tanisha Hill,2 Randall W Brown,2 Guilherme Safioti7
1Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Michigan, Ann Arbor, MI, USA; 2Teva Branded Pharmaceutical Products R&D Inc, Parsippany, NJ, USA; 3Teva Pharmaceutical Industries Ltd, Tel Aviv, Israel; 4Woodland Clinic Medical Group, Allergy Department, Dignity Health, Woodland, CA, USA; 5Inhalation Consultancy Ltd, Leeds, UK; 6Population Health, University of Michigan, Ann Arbor, MI and Division of Pulmonary Disease and Critical Care Medicine, University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, NC, USA; 7Teva Pharmaceuticals Europe B.V, Amsterdam, the Netherlands
Correspondence: Njira L Lugogo, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Michigan, 300 North Ingalls St, Suite 2C40, Ann Arbor, MI, 48109, USA, Tel +1 734 647 6477, Email [email protected]
Purpose: Machine learning models informed by sensor data inputs have the potential to provide individualized predictions of asthma deterioration. This study aimed to determine if data from an integrated digital inhaler could be used to develop a machine learning model capable of predicting impending exacerbations.
Patients and Methods: Adult patients with poorly controlled asthma were enrolled in a 12-week, open-label study using ProAir® Digihaler®, an electronic multi-dose dry powder inhaler (eMDPI) with integrated sensors, as reliever medication (albuterol, 90 μg/dose; 1– 2 inhalations every 4 hours, as needed). Throughout the study, the eMDPI recorded inhaler use, peak inspiratory flow (PIF), inhalation volume, inhalation duration, and time to PIF. A model predictive of impending exacerbations was generated by applying machine learning techniques to data downloaded from the inhalers, together with clinical and demographic information. The generated model was evaluated by receiver operating characteristic area under curve (ROC AUC) analysis.
Results: Of 360 patients included in the predictive analysis, 64 experienced a total of 78 exacerbations. Increased albuterol use preceded exacerbations; the mean number of inhalations in the 24-hours preceding an exacerbation was 7.3 (standard deviation 17.3). The machine learning model, using gradient-boosting trees with data from the eMDPI and baseline patient characteristics, predicted an impending exacerbation over the following 5 days with an ROC AUC of 0.83 (95% confidence interval: 0.77– 0.90). The feature of the model with the highest weight was the mean number of daily inhalations during the 4 days prior to the day the prediction was made.
Conclusion: A machine learning model to predict impending asthma exacerbations using data from the eMDPI was successfully developed. This approach may support a shift from reactive care to proactive, preventative, and personalized management of chronic respiratory diseases.
Keywords: digital inhalers, machine learning, personalized medicine, predictive modeling
Plain Language Summary
Why was the study done?
An asthma attack is when a person’s asthma symptoms get worse over a short space of time. This study was done to see if we can use information from a digital inhaler to predict when someone may have an asthma attack before it happens. If we can use technology to predict an asthma attack by using inhaler data, then doctors and patients may be able to help to stop the attack from being too bad or prevent it from happening at all.
What did the researchers do and find?
The Digihaler® is an inhaler that has an electronic sensor built into it. The inhaler can record when a person uses it. It can also record how much air a person can breathe in, and how long and how fast they can breathe in for. These inhalers were used by the patients in this study. Using a computer programme and information from the inhalers, we developed a way of calculating how well we could predict if a patient would have an asthma attack within the next 5 days. Our results showed that our calculation was very good at predicting if a patient would have an asthma attack within the next 5 days. We also found that the number of times a patient used their inhaler in the few days before an attack was important for making a prediction.
What do these results mean?
An inhaler that can record how well a person breathes in can provide important information about that person’s asthma. This information can also help to predict if a person may have an asthma attack. Being able to do this may help doctors and patients to stop asthma attacks from happening.
Introduction
Correct use of asthma medications is important to minimize symptoms and avoid exacerbations.1,2 Inhalation therapies for asthma are reliant on correct inhaler technique for optimal deposition of medication.3–5 For dry powder inhalers (DPIs), appropriate technique involves performing a deep and forceful inhalation in order to adequately de-aggregate and disperse the inhaled particles for delivery to the lungs.6–8 A well-established measure of inhalation technique is peak inspiratory flow (PIF),8 which is an important driver of de-agglomeration of particles inside DPIs, and is shown to decline with hyperinflation and airway disease exacerbations.9
ProAir® Digihaler® (Teva Pharmaceuticals, Israel) is a US Food and Drug Administration approved electronic multi-dose DPI. The Digihaler incorporates integrated sensors that detect device actuation (opening of the cap, which prepares a dose) and inhalation parameters such as PIF, inhalation duration, inhalation volume, and time to PIF.10 In a study of 150 participants, the Digihaler was shown to provide accurate measurements of inhalation parameters when used by patients.11 The goal of measuring these parameters was to provide information about real-life inhaler technique and information about short-acting beta2-agonist (SABA) use frequency, which might be useful to healthcare providers (HCPs) and patients.
It was hypothesized that data from the Digihaler could be used to detect deterioration in a patient’s clinical status prior to an asthma exacerbation based on changes in inhalation parameters and albuterol use. The period of an incipient exacerbation (early loss of control) is considered the “yellow zone” in asthma action plans. Asthma action plans have been used for many years to empower patients to detect early declines in lung function coupled with increased asthma symptoms and are part of standard guideline-based asthma care.12 Identifying the “yellow zone” provides increased awareness of deterioration and, when combined with early treatment adjustments, can pre-empt more severe exacerbations.13,14 However, asthma action plans and “yellow zone” identification have not been integrated into practice as widely as possible and optimizing them would represent an advance in asthma treatment.15
Used for a variety of clinical applications,16–20 machine learning entails the use of computer algorithms to develop a model capable of making accurate predictions based on data inputs.21 One machine learning technique is supervised learning, in which the model is “trained” to classify data using input data with corresponding labels (or outcomes), determining if any relationships exist between the input data and the label. After the model has been trained, it will attempt to classify new data based on the previous training data.22 Decision trees, which hierarchically classify data inputs according to pre-specified characteristics, are a well-established approach in computational biology and have proven amenable to adaptation into machine learning algorithms.23 Gradient-boosting is a supervised machine learning technique that utilizes decision trees to combine and optimize diverse data inputs in an iterative process that seeks to maximize the accuracy of a probabilistic model.24
A number of previous studies have attempted to develop models to predict a patient’s future risk of exacerbations.25–27 Most existing models currently predict the overall risk of developing exacerbations within a certain time frame (usually 1 month or 1 year) based on various combinations of clinical and billing data but are unable to specifically predict imminent events.26,27 A model taking into account day-to-day variations in disease activity based on baseline SABA use, inhalation parameters, and symptomatology may allow for more timely identification of potential deteriorations in asthma control and enable pre-emptive intervention in time to prevent a severe exacerbation.
Our aim was to determine if it was feasible to use data from ProAir Digihaler, along with baseline demographic information and clinical data, to develop a machine learning model capable of predicting impending asthma exacerbations as an initial step in developing a more robust system.
Materials and Methods
Study Design and Participants
This 12-week, open-label study was conducted across 45 study centers in the USA between February 2017 and February 2018. The study consisted of a 2-week screening period and a 12-week intervention period (NCT02969408). During the baseline visit (study Day 1), patients were trained on correct inhaler technique. A schematic design of the study is described in Figure 1. Details of sample size selection can be found in the Supplementary Methods.
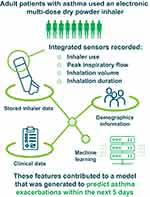 |
Figure 1 A schematic overview of the study. |
The study population comprised adult patients with a physician diagnosis of asthma, at least one moderate or severe asthma exacerbation over the 12 months prior to screening, and poorly controlled asthma as defined by an Asthma Control Questionnaire-5 (ACQ-5) score of ≥1.5.28 For this study, exacerbations were defined according to the 2009 American Thoracic Society/European Respiratory Society recommendation.29 Moderate exacerbations were those involving worsening asthma and requiring administration of systemic corticosteroids (SCS) above baseline for at least 3 days, or an unscheduled HCP visit (eg, doctor’s office or emergency care) associated with an increase in asthma therapy. Severe exacerbations were those requiring both administration of SCS as above and an unscheduled HCP visit. All patients were required to be on moderate or high doses of inhaled corticosteroids, equivalent to at least 440 µg daily of fluticasone propionate, with or without other asthma maintenance medications (long-acting beta2-agonist, leukotriene antagonist, long-acting antimuscarinic agent, biologic, or maintenance oral corticosteroids). Patients were excluded if they had any confounding underlying lung disorder other than asthma or had used any investigational drugs within five half-lives of discontinuation.
Patients were required to discontinue all other reliever medication containing SABA or short-acting antimuscarinic agents and replace them with the Digihaler (albuterol, 90 µg/dose) as their reliever medication (1–2 inhalations every 4 hours, as needed) for the duration of the study, alongside their usual maintenance therapy. Patients were supplied with seven Digihaler devices: three were provided on Day 1 and a subsequent four additional devices were provided by courier on Day 21. The Digihaler devices contain sensors within an integrated electronic module that recorded a timestamp with each use, along with PIF, inhalation volume, time to PIF, and inhalation duration. These data were stored within the electronic module in each device and were downloaded directly from the devices at the end of the patients’ treatment period by appropriately designated and trained personnel using extraction software. A companion Digihaler smart phone application (app) is currently available; however, this was not used during this study due to the potential influence on patient behavior. Future studies will assess the usability and benefit of the app in terms of inhaler usage and technique.
As per informed consent, patients were aware of the recording of measurements by the Digihaler. Patients were contacted monthly by phone for collection of information regarding exacerbations, maintenance medication, and adverse events. Instructions for use were also discussed during the monthly call.
Throughout the study period, asthma maintenance medication was continued or altered as per the treating physician’s judgement. Although the Digihaler recorded measurements in real-time, data were only downloaded at the end and were not available during the study; therefore, any patient treatment modifications during the study were based on patient report and not Digihaler data. The use of nebulized albuterol was permitted for the treatment of acute exacerbations at home or at the hospital only, if deemed necessary by the patient or their physician. Additionally, patients could continue use of other medications, with any modifications at the discretion of their treating physician.
Predictive Model
Enrolled patients who completed the study with at least one valid inhalation using the Digihaler were eligible for inclusion in the predictive analysis dataset. A valid inhalation was defined as an inhaler event with a detected PIF ≤120 L/min and no errors in use. Patients who experienced an exacerbation during the first 10 days of the study or made no inhalation during the 4-day period preceding an exacerbation were excluded from the predictive analysis dataset.
The target of the predictive model was defined as the prediction of an exacerbation within the following 5 days. The selection of five days for the predictive window was based on balancing the accuracy of the prediction against having adequate time for treatment intervention. A 3-day model would have been slightly more accurate but would restrict time to intervene before the predicted exacerbation, whereas a 7-day model would allow more time for intervention but would likely be less accurate.
The primary measure for the predictive analysis was albuterol use, and parameters of interest included total number of inhalations in the days preceding an exacerbation peak (defined as the day on which the patient began using SCS), the number of days prior to exacerbation peak during which albuterol use increased, and the amount of albuterol use in the 24 hours prior to a moderate or severe exacerbation.
The model was used each day to predict whether the patient would have an exacerbation within the following 5 days. Predictions where an exacerbation was anticipated to occur within the following 5 days were described as positive predictions and negative predictions were those where no exacerbation was anticipated to occur within the following 5 days. Each day’s prediction was based on features input into the model, including comparisons of data on numbers of inhalations and inhalation parameters during the preceding days (“days prior to prediction”) with the baseline features (Figure 2). Data on actual ACQ-5 scores and lung function, which could have been important additional variables, were not collected during the study. Respiratory symptoms were not collected after enrollment and thus were not used in the predictive model.
To develop the predictive model, machine learning techniques were applied to a combination of case report form data taken on study Day 1 (age, body mass index, blood pressure, previous exacerbations, and the number of exacerbations and hospitalizations in the previous 12 months), data from the Digihaler prior to (and including) the day of the prediction, and patient baseline characteristics from the Digihaler (timestamp of inhalation, inhalation status, PIF, inhalation volume, time to PIF, and inhalation duration). The number of inhalations and mean (standard deviation [SD]) of each inhalation parameter during the first 10 days of the study were considered as baseline features for the predictive model. A feature engineering process was conducted to determine the most relevant features for the model.
As the goal of the model was to predict cases of impending exacerbations rather than the probability of an exacerbation occurring, gradient-boosting trees24 were identified as the most appropriate algorithm to be implemented in the predictive model. Specifically, the XG-Boost30 implementation of gradient-boosting – which utilizes a tree learning algorithm optimized for the handling of sparse data to iteratively combine trees and thereby optimize the predictive model – displayed the strongest performance on the test set and was subsequently evaluated on the validation set.
Following feature engineering to convert the data inputs into explanatory variables structured suitably for predictive modeling, several supervised machine learning algorithms, including logistic regression, random forest, and gradient-boosting trees, were applied. Patients were randomly divided into three groups to train the model (“training set”), test and optimize the model (“test set”), and validate the chosen model (“validation set”). A 4-fold cross validation technique was used to compare the predictive performance metrics of the algorithms. Patients in the training set were randomly partitioned into four mutually exclusive and collectively exhaustive subsets, and the algorithm was trained in four distinct repeats. In each repeat, the algorithm was trained on data from three of the subsets of patients. The predictive performance of the algorithms was evaluated using the receiver operating characteristics (ROC) curve of sensitivity versus specificity. The ROC area under the curve (AUC) value represents the capability of the model to separate between classes. Values for ROC AUC fall between 0 and 1, with 1 representing perfect performance of the model.31 The ROC AUC was computed separately for each group and then averaged over the group to provide a single quality measure for the model. The relevance of the features used for the model is given as a percentage. This percentage relates to the variance reduced by data splits in the feature engineering that used this variable among all trees of the model. A large percentage variance reduction when data are split indicates that the feature has a large amount of relevance to the model and, therefore, contributes to the model performance.
Statistical analyses and algorithm development were performed using R Statistical Software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria), on a Windows operating system. Descriptive statistics were used to report demographics and outcome measures, but no between-group statistical comparisons were made.
Ethics
This study was conducted in full accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. Written informed consent was obtained from each patient before study participation. All study documents and procedures were approved by Schulman Institutional Review Board (IRB #201606556). The choice of ethics committee was the decision of the clinical research organization who conducted the study.
Results
Study Populations
Overall, 449 patients with asthma were screened and 397 patients were enrolled (intention-to-treat population). Of these, 16 (4%) patients were excluded due to early termination and 21 (5%) were excluded because they did not make at least one valid inhalation using the Digihaler. Patients who completed the study with at least one valid inhalation using the Digihaler (n=360 [91%]) were eligible for inclusion in the predictive analysis dataset. Excluded from the predictive analysis dataset were patients who experienced an exacerbation during the first 10 days of the study (n=6), and patients who made no inhalations from the Digihaler during this period (n=47), during the period after the first 10 days (n=2) or during the 4-day period preceding an exacerbation (n=7). The predictive analysis population, therefore, comprised 298 patients (Figure 3).
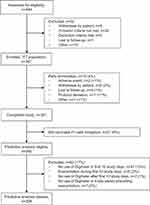 |
Figure 3 Patient disposition. Abbreviation: ITT, intention-to-treat. |
Baseline demographics and maintenance medication use of the predictive analysis population are shown in Table 1; 81.2% of patients were female and the mean age was 50.5 years. Patients experienced a mean of 1.5 exacerbations in the 12 months prior to the study. The estimated annualized exacerbation rate for patients during the study was 0.91 exacerbations per patient per year. Baseline demographics and maintenance medication use of the intention-to-treat population are shown in Supplementary Table 1.
 |
Table 1 Baseline Demographics and Maintenance Medication Use |
Inhalation Parameters: Predictive Analysis Dataset
Of the 360 patients who made ≥1 valid inhalation and completed the study, and so were eligible for inclusion in the predictive analysis, 64 (18%) experienced a total of 78 moderate/severe exacerbations. Over the full study period, the mean PIF (SD) for all analysis eligible patients was 73.2 L/min (21.0 L/min) (Table 2). For patients with and without exacerbations, the mean PIF (SD) was 73.4 L/min (23.1 L/min) and 73.2 L/min (20.3 L/min), respectively. The mean inhalation volume, inhalation duration and time to PIF were similar in all patients, patients with exacerbations and patients without exacerbations. Among non-exacerbators, the mean number of daily albuterol inhalations over the study duration was 1.14 (SD 2.35). For exacerbators, the mean number of daily albuterol inhalations was 1.87 (SD 2.78) outside of the ±14-day window around an exacerbation and 2.43 (SD 3.67) during the exacerbation window. During the exacerbation window, the mean daily albuterol use increased in the days leading to the exacerbation peak and decreased in the days following (Figure 4). Analysis of percentage changes from baseline showed that PIF and inhalation volume decreased in the days prior to the exacerbation peak and increased after (Figure 5). A representative example of a patient who experienced an exacerbation is shown in Figure 2. This particular patient had an exacerbation on Day 76 and this figure demonstrates the predictive periods leading up to this exacerbation and the changes in inhalation parameters before and after the exacerbation.
 |
Table 2 Inhalation Parameters and Albuterol Use Over the Full Study Period Captured by the Digihaler in Patients Who Completed the Study with ≥1 valid Inhalation |
Predictive Factors and Model Validation
The training set comprised 184 patients, the test set comprised 60 patients and the validation set comprised 54 patients. In the validation set, the gradient-boosting model predicted an exacerbation within the following 5 days with an ROC AUC value of 0.83 (95% confidence interval: 0.77–0.90) (Figure 6). Of the features included in the predictive model, the strongest predictive factors for a future exacerbation were the mean number of daily albuterol inhalations during the 4 days prior to the prediction (47%), the inhalation parameters in the four days prior to prediction (PIF, inhalation volume, and inhalation duration; 11%), and comparison to the baseline values for these inhalation parameters (14%; Figure 7). Other features were also input to the model but were not found to contribute to the model performance.
Discussion
Data from adult patients with poorly controlled asthma were obtained via the use of a reliever medication inhaler device equipped with an integrated sensor and, along with demographic and clinical information, were used to develop a predictive model for exacerbations within 5 days. To our knowledge, this represents the first successful attempt to identify clinical deterioration of asthma using data from a reliever medication inhaler device with integrated sensors.
Despite available therapies, many patients report daily symptoms associated with partly- or uncontrolled asthma per the Global Initiative for Asthma 2020 report.1 Respiratory symptoms in patients with asthma are known to be predictive of exacerbations32 and patients with poorly controlled asthma have an increased risk of exacerbations, poorer outcomes and reduced quality of life.1 Similarly, it is well known that increased SABA use is an indicator of poor asthma control and exacerbation risk,33,34 and is widespread among patients with poorly controlled asthma.35 These patients often underestimate their symptom control,36,37 which may be one barrier to the identification of early deterioration and the implementation of asthma action plans that depend on this recognition.38
The Digihaler pressure sensor was originally designed with the intention of facilitating correct DPI technique and recording medication use. When using DPIs, the delivery of the inhaled particles to the lungs relies on generation of an adequate inspiratory flow to deagglomerate the powder into an emitted dose. The time taken to achieve PIF, duration of inhalation, and inhaled volume are also important considerations39 as a rapid and deep inhalation has been shown to be the most appropriate for using DPIs.6 Consequently, measurement of these inhalation parameters can give important information regarding inhaler technique. Furthermore, PIF has been shown to deteriorate with hyperinflation around the time of an exacerbation when assessed in relation to changes in other inhalation parameters.9,40 Changes in PIF patterns may indicate an impending exacerbation, particularly if identified in combination with other signals such as changes in albuterol use.
Sensor-derived data enabled the observation of SABA use and inhalation characteristics of poorly controlled asthma patients who did and did not experience an exacerbation over the 12 weeks of the study. Despite high baseline SABA use, which was apparent across the study population, increases in daily inhalations during the days preceding an exacerbation were evident. Consistent with previously reported observations, PIF, inhalation volume and inhalation duration were all observed to decrease in the days prior to an exacerbation. Together, these observations suggest that exacerbations are preceded by predictable changes in patient behaviors and inhalation attributes, and that it may be feasible to pre-empt more severe exacerbations through intervention upon detection of these changes.
Achieving a shift towards personalized medicine in asthma will necessitate accurate measurement of physiologic parameters, combined with powerful diagnostic tools that take into account individual variability.41 Machine learning algorithms designed to classify and optimize large volumes of heterogeneous data are ideally placed to contribute to this shift,42 and the adaptive nature of these algorithms is key for personalized predictive modeling.43 Gradient-boosting24 is well-suited for this task; it offers classification and structured prediction approaches with a high degree of flexibility.31,44
By combining Digihaler data with patient information and applying the gradient-boosting trees algorithm, we were able to develop a model predictive of an impending exacerbation within the following 5 days with an ROC AUC value of 0.83. The most important predictive feature was found to be the mean number of daily inhalations during the 4 days prior to the day the prediction was made. It is, however, important to note the breadth of other features which were utilized in the model and contributed to its overall power. These reflect the highly personalized nature of the exacerbation progression within each patient and their patterns of inhalation parameters. Multiple factors may be predictive of asthma exacerbation risk, including clinical, behavioral, and social factors. When developing this model, a feature engineering process was conducted to determine which features would be most relevant for the model. For this data set, baseline systolic blood pressure was found to add accuracy to the model. It is possible that this would not be the case with a different data set, but the inclusion of systolic blood pressure here highlights the importance of predictive modelling. With predictive modelling, trends may become apparent that are counter-intuitive or unexpected. Lung function and ACQ-5 score data, along with respiratory symptoms data and data on other physiologic measures such as heart rate and respiratory rate, were not used in this model and may have been important additional variables, if available. Future research that builds on this study will help to further understand the role these factors play in predicting asthma exacerbations.
This study represents early progress in the development of a predictive model based on data from a digital inhaler system. As this model is further developed and improved, additional work needs to explore how the predictive capabilities might best be optimized for a clinical setting. The use of a single universal risk threshold to guide decision-making is commonplace in clinical practice,16 whereas a predictive model has the potential to provide a personalized, dynamic measure of risk that is informed by day-by-day changes in parameters as well as patient-specific characteristics.16 In practice, however, it is anticipated that implementation will likely entail the prior selection of one or more risk thresholds, balancing sensitivity with specificity as deemed appropriate for the specific use-case.42 The algorithm offers the flexibility to support this. The relative impacts of false positives (which could result in inefficient use of healthcare resources) and false negatives (which could result in missed opportunities to intervene to prevent deteriorations) will depend on exactly how this or similar models are implemented.
The study had several limitations. Digihaler data were only downloaded and analyzed at the end of the study, and exacerbations were identified by monthly patient phone calls, potentially resulting in recall bias and unidentified exacerbations, which may have affected the precision of the model. Minor technical issues with the Digihaler may also have affected model precision, mostly as a result of timestamp errors from a later download of the data instead of real-time synchronization with a smart phone app. The digital inhaler system, designed with the capability to transmit data from the sensor to a smart phone app and/or internet dashboard might support real-time or near real-time identification of an exacerbation by a patient or HCP. Additionally, although the protocol discouraged nebulizer use, it is impossible to prohibit its use by all patients, even outside of an exacerbation, and this possible use was not recorded. Therefore, reliever medication use outside of the Digihaler data was not accounted for in the model. Lastly, there was an imbalance in the population demographic, with 80% of enrolled patients being female. Studies have shown that females are more likely to experience an asthma exacerbation45 and have lower PIF values on average than are typical in males.46 This imbalance in demographic could have had an effect on the overall findings of the study and the influence of sex in the model requires further exploration.
Conclusion
Our findings highlight the potential value of a model predictive of impending asthma exacerbations based on data from the Digihaler. Individualizing treatment regimens is key to optimal asthma management. This initial model represents the first step in refining the algorithm predictive of exacerbations and will inform future development and validation of predictive models from inhaler use data in respiratory care.
Study Conduct
This study was conducted by a contract research organization. All authors contributed to the study concept, study design, data analysis, or data interpretation.
Data Sharing Statement
The data sets used and/or analyzed for the study described in this manuscript are available upon reasonable request. Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please contact Guilherme Safioti ([email protected]) with any requests.
Acknowledgments
We thank the patients, caregivers, health care providers, and research staff who participated in this study. We also thank Thomas Ferro, Dilip Chary, Dan Buck, Enric Calderon and Mark Milton-Edwards for important contributions to the conception, design, and direction of the study, and Shai Fine and Shahar Cohen for their important contributions to the statistical analysis and machine learning modeling. We also acknowledge Melanie Francis, MSc, of Ashfield MedComms, an Inizio company, for her medical writing support in the preparation of the manuscript, under the direction of the authors and funded by Teva Branded Pharmaceutical Products R&D Inc. Data from this paper were presented as poster presentations at the 2019 Annual Scientific Meeting of the American College of Allergy, Asthma and Immunology [https://doi.org/10.1016/j.anai.2019.08.072], the American Thoracic Society 2019 International Conference [https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A7307], the 2019 Connected Health Conference [https://doi.org/10.2196/15173], the 2020 American Academy of Allergy, Asthma & Immunology Annual Meeting [https://doi.org/10.1016/j.jaci.2019.12.235], the 2020 CHEST Annual Meeting [https://doi.org/10.1016/j.chest.2020.08.079], and the 2021 European Respiratory Society International Congress [https://doi.org/10.1183/13993003.congress-2021.PA3397].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding was provided by Teva Branded Pharmaceutical Products R&D Inc. Teva employees were involved in the study design, data collection and analysis, and in the writing of this manuscript. All authors had full access to all the study data and had final responsibility for the decision to submit for publication.
Disclosure
NLL received consulting fees for advisory board participation from Amgen, AstraZeneca, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK and Astra Zeneca; and travel support from Astra Zeneca; grants from Novartis and Evidera; her institution received research support from Amgen, AstraZeneca, Avillion, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, and Teva Pharmaceuticals. MR, TL, TH, RWB, and GS are employees of Teva Pharmaceuticals. MD and LG are previous employees of Teva Pharmaceuticals. RM reports personal fees from AstraZeneca, Sanofi, Propeller Health, and Teva Pharmaceuticals. RP reports grants from Boehringer Ingelheim, Astra Zeneca and Adherium LTD; personal fees from Grifols, and Theravance; Clinical Research from Teva. HC reports grants and personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Innovata Biomed, Meda, Menarini, Mundipharma, Napp Pharmaceuticals, Nemera, NorPharma, Novartis, Orion, Sanofi, Teva Pharmaceuticals, Trudell Medical International, UCB, and Zentiva. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: www.ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
2. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi:10.1016/j.rmed.2011.01.005
3. Lavorini F, Usmani OS. Correct inhalation technique is critical in achieving good asthma control. Prim Care Respir J. 2013;22:385–386. doi:10.4104/pcrj.2013.00097
4. Wright J, Brocklebank D, Ram F. Inhaler devices for the treatment of asthma and chronic obstructive airways disease (COPD). Qual Saf Health Care. 2002;11:376–382. doi:10.1136/qhc.11.4.376
5. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
6. Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(1071–1081.e9). doi:10.1016/j.jaip.2017.01.004
7. Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–1331. doi:10.1183/09031936.00166410
8. Atkins PJ. Dry powder inhalers: an overview. Respir Care. 2005;50:1304–12; discussion 1312.
9. Broeders MEAC, Molema J, Hop WCJ, Vermue NA, Folgering H. The course of inhalation profiles during an exacerbation of obstructive lung disease. Resp Med. 2004;98:1173–1179. doi:10.1016/j.rmed.2004.04.010
10. Teva Pharmaceuticals Digihaler inhalers. Available from: https://www.digihaler.com.
11. Chrystyn H, Saralaya D, Shenoy A, et al. Investigating the accuracy of the digihaler, a new electronic multidose dry-powder inhaler, in measuring inhalation parameters. J Aerosol Med Pulm Drug Deliv. 2022;35(3):166–177. doi:10.1089/jamp.2021.0031
12. National Heart, Lung, and Blood Institute, Expert Panel Report 3. Guidelines for the diagnosis and management of asthma full report 2007. National Asthma Education and Prevention Program; 2007. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf.
13. Gupta S, Wan FT, Hall SE, Straus SE. An asthma action plan created by physician, educator and patient online collaboration with usability and visual design optimization. Respiration. 2012;84:406–415. doi:10.1159/000338112
14. Kouri A, Boulet LP, Kaplan A, Gupta S. An evidence-based, point-of-care tool to guide completion of asthma action plans in practice. Eur Respir J. 2017;49:1602238. doi:10.1183/13993003.02238-2016
15. Gupta S, Kaplan A. Solving the mystery of the yellow zone of the asthma action plan. NPJ Prim Care Respir Med. 2018;28:1. doi:10.1038/s41533-017-0067-1
16. Than MP, Pickering JW, Sandoval Y, et al. Machine learning to predict the likelihood of acute myocardial infarction. Circulation. 2019;140:899–909. doi:10.1161/CIRCULATIONAHA.119.041980
17. Stelhick J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail. 2020;13(3):e006513. doi:10.1161/CIRCHEARTFAILURE.119.006513
18. Giannini HM, Ginestra JC, Chivers C, et al. A machine learning algorithm to predict severe sepsis and septic shock: development, implementation and impact on clinical practice. Crit Care Med. 2019;47(11):1485–1492. doi:10.1097/CCM.0000000000003891
19. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi:10.1038/nature21056
20. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402–2410. doi:10.1001/jama.2016.17216
21. Ghahramani Z. Probabilistic machine learning and artificial intelligence. Nature. 2015;521(7553):452–459. doi:10.1038/nature14541
22. Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi:10.1161/CIRCULATIONAHA.115.001593
23. Kingsford C, Salzberg SL. What are decision trees? Nat Biotechnol. 2008;26:1011–1013. doi:10.1038/nbt0908-1011
24. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001;29:1189–1232. doi:10.1214/aos/1013203451
25. Bateman ED, Buhl R, O’Byrne PM, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135(1457–1464.e4):1457–1464.e4. doi:10.1016/j.jaci.2014.08.015
26. Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci. 2017;1387:153–165. doi:10.1111/nyas.13218
27. Messinger AI, Deterding RR, Szefler SJ. Bringing technology to day-to-day asthma management. Am J Respir Crit Care Med. 2018;198:291–292. doi:10.1164/rccm.201805-0845ED
28. Korn S, Both J, Jung M, Hübner M, Taube C, Buhl R. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474–479. doi:10.1016/j.anai.2011.09.001
29. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi:10.1164/rccm.200801-060ST
30. Chen T, Guestrin C. Xgboost: a scalable tree boosting system.
31. Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006;27(8):861–874. doi:10.1016/j.patrec.2005.10.010
32. Honkoop PJ, Taylor DR, Smith AD, Snoeck-Stroband JB, Sont JK. Early detection of asthma exacerbations by using action points in self-management plans. Eur Respir J. 2013;41:53–59. doi:10.1183/09031936.00205911
33. Amin S, Soliman M, McIvor A, Cave A, Cabrera C. Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract. 2020;8(8):2556–2564.e8. doi:10.1016/j.jaip.2020.03.013
34. Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872. doi:10.1183/13993003.01872-2019
35. Azzi EA, Kritikos V, Peters MJ, et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta2 agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9:e028995. doi:10.1136/bmjopen-2019-028995
36. Centers for Disease Control and Prevention. Uncontrolled asthma among persons with current asthma; 2014. Available from: http://www.cdc.gov/asthma/asthma_stats/uncontrolled_asthma.htm.
37. European Lung White Book. The economic burden of lung disease; 2019. Available from: http://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease.
38. Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333. doi:10.1378/chest.121.2.329
39. Azouza W, Chrystyn H. Clarifying the dilemmas about inhalation techniques for dry powder inhalers: integrating science with clinical practice. Prim Care Respir J. 2012;21:208–213. doi:10.4104/pcrj.2012.00010
40. Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Crit Care. 2002;6:30–44. doi:10.1186/cc1451
41. Chung K-F. Personalised medicine in asthma: the time for action. Eur Respir Rev. 2017;26:170064. doi:10.1183/16000617.0064-2017
42. Kocsis O, Lalos A, Arvanitis G, Moustakas K. Multi-model short-term prediction schema for mHealth empowering asthma self-management. Electron Notes Theor Comput Sci. 2019;343:3–17. doi:10.1016/j.entcs.2019.04.007
43. FDA. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD). Available from: https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf.
44. Cosgriff CV, Celi LA, Sauer CM. Boosting clinical decision-making: machine learning for intensive care unit discharge. Ann Am Thorac Soc. 2018;15(7):804–805. doi:10.1513/AnnalsATS.201803-205ED
45. Patel M, Pilcher J, Reddel HK, et al. Predictors of severe exacerbations, poor asthma control, and β-agonist overuse for patients with asthma. J Allergy Clin Immunol Pract. 2014;2:751–758. doi:10.1016/j.jaip.2014.06.001
46. Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26:174–179. doi:10.1089/jamp.2012.0987
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





