Back to Journals » Drug Design, Development and Therapy » Volume 17
A Phase I Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Novel Intravenous Formulation of Meloxicam (QP001) in Healthy Chinese Subjects
Authors Ma J, Huang J, Zou C, Wu Q, Xie J, Zhang X, Yang X, Yang S, Wu Z, Jiang Y, Yu S, Zhang X, Yang G , Li M
Received 26 April 2023
Accepted for publication 28 July 2023
Published 3 August 2023 Volume 2023:17 Pages 2303—2313
DOI https://doi.org/10.2147/DDDT.S418730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Junlong Ma,1,2,* Jie Huang,1,* Chan Zou,1 Qian Wu,1 Jinlian Xie,1 Xingfei Zhang,1 Xiaoyan Yang,1 Shuang Yang,1 Ziteng Wu,3 Yan Jiang,3 Sen Yu,3 Xuqing Zhang,4 Guoping Yang,1,5 Mingyuan Li1
1The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 2Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000, People’s Republic of China; 3Nanjing Delova Biotech Co., Ltd., Nanjing, Jiangsu, 210042, People’s Republic of China; 4King-eagle Med Co., Ltd., Changsha, Hunan, 410013, People’s Republic of China; 5Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, Hunan, 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingyuan Li, Email [email protected]
Background: Meloxicam is a selective cyclooxygenase-2 inhibitor used for pain relief, but its poor solubility limits its clinical applications. QP001 is a novel intravenous formulation of meloxicam developed with PEG and pH regulator to improve its solubility. This study aimed to evaluate the safety, tolerability, and pharmacokinetics of QP001 in Chinese healthy subjects.
Methods: The trial consisted of three parts. Part I was a two-period crossover study to evaluate bioavailability, in which 10 healthy were either intravenously infused with 15mg QP001 (test) or orally given 15mg MobicⓇ (reference). Part II was a single-arm design to assess the pharmacokinetic (PK) characteristics after 30 mg single- and multiple-dose QP001 in 10 subjects. In part III, we investigated the PKs and tolerability of QP001 at a high dose (60 mg) in another 10 subjects. The PK parameters and treatment-emergent adverse events (TEAEs) were evaluated.
Results: A total of 30 subjects were enrolled in the study. QP001 was well tolerated and safe without significant TEAEs in all three study parts. The PK characteristics of QP001 were linear following a single-dose range of 15– 60 mg (Cmax and AUC0-t were 5.82– 17.66 μg/mL and 58.08– 251.17 μg∙h/mL, respectively). After five consecutive daily 30 mg doses, the accumulation index was around 1.98, which indicated a minimal accumulation of QP001. Compared to the tablet dosage form, the relative bioavailability of QP001 reached 116.85%. Additionally, the PK profile of QP001 showed no gender difference.
Conclusion: QP001 was well tolerated in healthy Chinese subjects after single ascending doses up to 60 mg and multiple-dose of 30 mg. Based on the PK and safety results, QP001 is a promising once-daily intravenous COX-2 inhibitor candidate for managing pain.
Trial Registration: The trial is registered at chinadrugtrials.org.cn (ChiCTR2100047884).
Keywords: meloxicam, phase I trial, pharmacokinetic, safety, novel formulation
Introduction
Pain and inflammation are common symptoms of many diseases and can greatly impact a patient’s quality of life.1,2 Non-steroidal anti-inflammatory drugs (NSAIDs) can help alleviate these symptoms by inhibiting the activity of regulatory enzymes such as cyclooxygenase (COX) which are responsible for the synthesis of prostaglandin. Meloxicam is a COX-2 inhibitor, widely used to treat rheumatoid arthritis, osteoarthritis, as well as various pain syndromes.3,4 Its selectivity for COX-2 over COX-1 within the therapeutic dose range means that meloxicam only reduces prostaglandin at the site of inflammation but does not significantly affect the physiological prostaglandin synthesis, resulting in fewer gastrointestinal adverse effects compared to non-selective NSAIDs.5 Meloxicam is available in tablet form around the world with good market value and was approved for marketing in China in 2014 (MobicⓇ, 7.5 mg tablets).
The pharmacokinetic (PK) parameters in humans of meloxicam tablets were well studied in clinical trials and post-clinical studies. The maximum meloxicam plasma concentration under fasting conditions was achieved after approximately 5 hours for the most part.6 It is metabolized in the liver and has a long half-life of 20 to 24 hours, allowing for once-daily dosing and long-lasting effects.7 And the plasma elimination rate was 7 to 9 mL/min.6 But due to its poor aqueous solubility and high permeability,8 Meloxicam is classified as a Biopharmaceutical Classification System II drug. Oral administration of meloxicam has a long absorption time.9,10 Furthermore, the traditional injection of meloxicam (MobicⓇ) has the drawbacks of low drug loading (only 10 mg/mL) due to its poor solubility. Consequently, this delayed plasma peak and low drug loading limit its application in the treatment of acute pain.11 Given the well-established safety and efficacy of NSAIDs in managing perioperative pain without opioid-related adverse effects,12 it is necessary to overcome current limitations and explore novel formulations that can help improve patient satisfaction and reduce opioid requirements.
Recently, a novel intravenous nanocrystal formulation of meloxicam (AnjesoⓇ) with decreasing particle size was approved by the US Food and Drug Administration (FDA) for the management of moderate to severe pain.13,14 This novel formulation has the advantage of rapid onset of analgesic activity (within 15 minutes after administration) and a long-lasting period (throughout 24 hours).13 Administered once daily at doses of 30 mg or 60 mg, AnjesoⓇ resulted in a low incidence of adverse events.15 However, small particles of nanocrystal drugs can lead to thermodynamic instability and tend to cause aggregation,16 which hinders the large-scale production of nanocrystal formulations.
To overcome the liability of nanocrystal formulation, QP001 (novel intravenous formulation classified as 2.2 new drug), prepared with polyethylene glycol (PEG) and pH regulator to improve the aqueous solubility of meloxicam, was developed by Nanjing Delova Biotech Co., Ltd. This novel formulation significantly improves the solubility of meloxicam and provides excellent stability, which can be used directly for intravenous administration and rapidly achieve effective therapeutic concentrations for postoperative analgesia. However, meloxicam is metabolized to its inactive form by cytochrome P450 (CYP) 2C9, the allelic distribution of which varies among the population.17 There are currently few clinical trials of the intravenous formulation of meloxicam,13–15 especially the studies in East Asian populations are lacking. It’s necessary to investigate the PK profile and safety of QP001 in the East Asian population. Therefore, the main aim of this study was to evaluate the safety, tolerability, and PKs of single-ascending and multiple daily doses of QP001 in healthy Chinese subjects.
Subjects and Methods
Ethics Statement
This phase I trial was conducted in the Third Xiangya Hospital of Central South University and followed the Declaration of Helsinki and Good Clinical Practice. The study was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (approval no.21087) and registered with the Chinese Clinical Trial Registry (ChiCTR2100047884). All subjects have provided written informed consent forms prior to their participation in the study.
Subjects
Healthy male and female Chinese subjects aged 18–45 years with a body mass index (BMI) between 19 and 26 kg/m2 (weight >50 kg for males and >45 kg for females) were included. The physical examination and laboratory tests were carried out for all subjects during medical screening. Eligibility criteria included a history of serious clinical illness, drug abuse, digestive ulcers, or hemorrhagic events. The subjects who had taken any products or drugs that affected the metabolism of meloxicam 2 weeks before and during the study period were excluded. And those with an allergic constitution or known allergies to the study drugs or their ingredients were also not suitable to take part in the trial. Additionally, the CYP2C9 gene was detected to exclude the poor metabolizers (CYP2C9 *2/*3, *2/*2, and *3/*3).
Study Drugs
The test drug (QP001 injection) was manufactured by Hefei Noratech Pharmaceuticals Co., Ltd. (specification: 1 mL:30 mg; batch number: 0420001), and the reference product (meloxicam tablets, MobicⓇ) was manufactured by Shanghai Boehringer Ingelheim Shanghai Pharmaceuticals Co., Ltd. (specification: 7.5 mg per tablet; batch number: C46124).
Study Design
This Phase I, single-ascending and multiple-dose study consisted of three parts, designed to investigate the safety, tolerability and PKs of QP001 injection in healthy Chinese subjects. The study drugs were all administered under fasting condition to a total of 30 healthy subjects, between June 2021 and October 2021. The trail flow chart is shown in Figure 1.
 |
Figure 1 The trial flow chart. |
The study was conducted in three parts with dose-escalating groups. In Part I (15 mg group), a two-period crossover design trial was performed to compare the bioavailability of QP001 injection (intravenous injection) and meloxicam tablets (MobicⓇ) among 10 eligible volunteers. The volunteers were randomly divided into two sequence groups: 15 mg QP001 injection followed by 15mg MobicⓇ (group A) or 15 mg MobicⓇ followed by 15 mg QP001 (group B). The groups were with a washout period of 7 days, and blood samples were collected at pre-determined time points. In Part II (30 mg group), a single-arm design was conducted to assess the PK characteristics and drug accumulation after multiple-dose administration at 30 mg. Ten subjects received a single dose of QP001, followed by five consecutive daily 30 mg doses of QP001 after a 7-day washout period. As for part III (60 mg group), we investigated the PKs and tolerability of QP001 injection with a high dose (60 mg). Blood samples were collected at pre-determined time points.
PK Evaluations
Blood samples for PK analysis of QP001 were collected at pre-dose, and 1, 2, 5, 10, 20, 30 min and 1, 2, 4, 6, 8, 12, 24, 48, 72, 96 h post-dose. The trough concentration measurement in part II was performed on day 11 and day 12 (prior to daily administration). Blood samples of MobicⓇ were collected at pre-dose, and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 24, 48, 72, 96 h post-dose. The blood samples were centrifuged at 1700 g for 10 min at 4 °C, and the plasma was stored at −80°C for further analysis.
Safety and Tolerability
Safety profile and tolerability were assessed through continuous observation of adverse events, vital signs (ie, body temperature, blood pressure, and heart and respiratory rates), clinical laboratory tests (ie, hematology, blood biochemistry, urinalysis, and liver and kidney functions), 12-lead ECG, and physical examination throughout the study period.
Adverse events (AEs) were coded and determined by the principal investigator and clinicians, based on the Medical Dictionary for Regulatory Activities (version 24.0) and the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). And all the AEs were monitored until the symptoms returned to normal or the conditions were stable.
Statistical Analyses
The major PK parameters, including time to reach maximum plasma concentration (Tmax), peak plasma concentration (Cmax), the area under the concentration versus time from zero to infinity (AUC0-∞), the area under the concentration versus time from zero to the last quantifiable concentration time (AUC0-t), and half elimination time (t1/2), were calculated using the non-compartmental model (NCA module) on WinNonlin 8.2 (Pharsight Corporation, Mountain View, CA, USA). The statistical analysis used SAS Enterprise Guide 9.4 and CPhaMAS platform (version 1.0; https://cphamas.com/; Changsha Xutong Technology Co., Ltd, China). The natural log-transformed AUC0-t, AUC0-∞, Cmax and trough concentrations were analyzed by analysis of variance with a mixed-effects model. The relative bioavailability and drug accumulation index were evaluated based on average bioequivalence. Differences between dose groups were determined using either t-tests or non-parametric Wilcoxon-Mann–Whitney test. Subgroup analyses by gender were also conducted. A p-value less than 0.05 was considered statistically significant. Additionally, dose proportionality was analyzed by ln[PK parameter] = β0+β2ln[dose]. Descriptive analysis was reported as means with standard deviation (SD). The accumulation index (Rac) was defined as the AUCTau, multiple / AUCTau, single, where AUCTau, multiple is AUC from time zero to the end of the dosing period at steady state and AUCTau, single is equal to AUC0-∞ for a single-dose.
Analyses of the safety were conducted in subjects receiving at least one dose of the drug. All safety summaries were descriptive without statistical tests.
Results
Demographic Characteristics
From June 23, 2021, to September 2, 2021, a total of 30 healthy Chinese subjects were enrolled in this study (Part I: n = 10; Part II: n = 10; Part III: n = 10). Except for one subject who dropped out during the multiple-dose study (Part II) due to AEs, all the other 29 subjects completed the study. The safety and PK analysis sets for the single-dose study and multiple-dose study consisted of 30 subjects and 9 subjects, respectively. Their baseline demographic characteristics are presented in Table 1. Half of the subjects were female, and the mean age was 30.00, 31.20, and 25.00 years for the three parts.
 |
Table 1 Demographic Characteristics of Subjects |
Safety
During single- and multiple-dose studies, a total of 10 treatment-related adverse events (TRAEs) were reported in 7 (23.3%) subjects (Table 2). Among them, there was one event in one (10.0%) subject in the 15 mg QP001 group, two events in two (20.0%) subjects in the 30 mg QP001 group, four events in two (20.0%) subjects in the 45 mg QP001 group, two events in two (22.2%) subjects in the 30 mg multiple-dose QP001 group, and one event in one (10.0%) subject in the 15 mg MobicⓇ group. There was no significant difference in the incidence of TRAEs among subjects receiving different doses of QP001, indicating no clear dose-related trends. The incidence of TRAEs did not show a trend to increase after multiple-dose treatment, suggesting that QP001 was safe and well tolerated.
 |
Table 2 Treatment-Related Adverse Events Sorted by SOC and PT |
Among 7 subjects who experienced TRAEs, 1 subject respectively experienced TRAEs of increased creatinine and administration site pain after single- and multiple-dose of 30 mg QP001. Except for one TRAE that was grade II in severity (blood creatinine increased) and lead to the withdrawal of the subject from the trial, all other TRAEs were mild in severity and spontaneously resolved (Table S1). This withdrawn subject recovered 2 days after reporting grade II TEAE, without any medical intervention. After being classified by system organ class (SOC) code, most of the TRAEs (70%) belong to investigations (Table 2). All the TRAEs were reviewed by the local ethics committees and resolved without sequelae.
Pharmacokinetic Characteristics
In part I, we compared the PK parameters of intravenous 15 mg QP001 and oral 15 mg MobicⓇ tablet. As shown in Table S2, the Cmax and AUC0-t of QP001 were higher than MobicⓇ. The Cmax and AUC0-t were 5.82 ± 1.33 μg/mL and 58.08 ± 13.95 μg·h/mL for QP001 and 1.79 ± 0.52 μg/mL and 49.37 ± 11.57 μg·h/mL for MobicⓇ, respectively. The relative bioavailability reached 116.85%, indicating that QP001 exhibited a higher exposure level than MobicⓇ at 15 mg single-dose. The concentration-time profiles are shown in Figure 2. The median Tmax of QP001 was 0.03 h. After 6 hours of administration, the plasma concentration-time curves of QP001 injection and the control drug MobicⓇ tended to overlap, showing similar clearance rates.
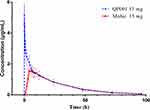 |
Figure 2 Mean plasma concentration-time curves in healthy subjects following single-dose of 15mg of QP001 injection and MobicⓇ. |
After a repeated dose of 30 mg QP001 for 5 days in part II and comparison of trough concentration changes from day 10 to day 12 (Figure S1), there was no statistically significant difference between trough concentrations (p = 0.42), indicating achievement of steady-state after 2 days of continuous dosing. Mean plasma concentration-time profiles of QP001 on day 1 and day 12 are shown in Figure 3a, and the corresponding PK parameters are summarized in Table 3. Certain multiple-dose PK characteristics were consistent with single-dose administration, such as t1/2 (22.34 h vs 17.26 h, p > 0.05), Vz (7.50 L vs 7.47 L, p > 0.05), and CL (247.89 mL·h−1 vs 312.13 mL·h−1, p > 0.05). A significant difference in Cmax and AUC was observed between the multiple-dose and single-dose groups (p < 0.05). Furthermore, the accumulation index (Rac) was around 1.98.
 |
Table 3 Pharmacokinetic Parameters of QP001 After Single and Multiple Administrations at 30 mg |
 |
Figure 3 Mean plasma concentration-time curves in healthy subjects following (a) multiple-dose and single-dose of 30 mg QP001 injection or (b) single-dose of 15, 30 and 60 mg of QP001 injection. |
Given that baseline characteristics of the included subjects were similar, mean plasma concentration-time profiles of single-dose treatments of QP001 from three parts were uniformly described (Figure 3b). Peak concentrations were observed immediately and mean plasma concentrations increased with increasing dose. Details of PK parameters are presented in Table 4, where Cmax (mean ± SD) valued 5.82 ± 1.33, 9.34 ± 2.13, and 17.66 ± 3.63 μg/mL, respectively; AUC0–t (mean ± SD) values were 58.08 ±13.95, 101.50 ± 29.61, and 251.17 ± 42.01 μg/mL, respectively; and AUC0-∞ (mean ± SD) values were 60.13 ± 14.91, 104.42 ± 31.96, and 264.51 ± 47.61 μg/mL, respectively, showing a linear increase proportional to the dose range of 15–60 mg. The proportionality coefficients for Cmax, AUC0-t and AUC0-∞ were 0.90, 0.93 and 0.92, respectively (Table S3). Other PK parameters of QP001 including Tmax, λz, t1/2, CL and Vz, were not statistically significant across the dose range. Therefore, QP001 exhibited linear PKs across the dose range studied. Subgroup analysis by gender showed that only the Vz parameters in the 30 mg and 60 mg groups and the Cmax parameters in the 60 mg group differed (p < 0.05), indicating no gender differences in the range of 15 mg to 60 mg single injections of QP001 (Table S4).
 |
Table 4 Pharmacokinetic Parameters of QP001 After Single-Ascending-Dose |
Discussion
This phase I study was conducted on 30 healthy Chinese subjects to investigate the safety, tolerability and PKs of QP001 injection, which is a novel intravenous formulation of meloxicam for pain relief. First, based on our findings, a single-dose at 15–60 mg (15, 30, 60 mg) and multiple-dose at 30 mg of QP001 injection were generally well-tolerated with no severe AEs. And AEs among different dose levels did not appear to be dose-dependent. Second, QP001 presented linear pharmacokinetics features following the single-dose (15, 30, 60 mg). A repeated dose of QP001 30 mg for five days (once-daily dosing) reached a steady state and presented minimal accumulation. Third, the PK profile of QP001 did not differ by gender. Fourth, the exposure level of QP001 was slightly higher than Mobic®, but the clearance profiles were similar. Collectively, these findings support the further clinical development of QP001 for pain management.
Generally, opioids have traditionally served as the cornerstone of pain management; however, excessive reliance on these medications has been associated with a plethora of opioid-induced adverse effects, including but not limited to respiratory depression, nausea, vomiting, and constipation.18,19 Additionally, the risk of opioid dependence and abuse necessitates the exploration of alternative non-opioid analgesic therapies. Non-opioid rapid analgesics have been demonstrated to effectively provide rapid pain relief by intravenous injections. Meloxicam, a preferential COX-2 inhibitor, shows potent and long-acting effects on pain relief. However, poor solubility is the main limitation in the development of meloxicam intravenous injection. Recently, nanocrystal formulations have been found to achieve nanometer-sized drug particles, significantly improving solubility and drug loading.20,21 Nevertheless, the quality control and large-scale production of nanocrystal formulations are challenging, and their safety is still under scrutiny.13,15
In our study, QP001 is a novel intravenous formulation of meloxicam prepared with PEG and pH regulator to increase solubility and stability. The preparation process is simple and can be completed by stirring and dispersing at room temperature, facilitating the development of meloxicam intravenous injection for pain relief. And the results in part I indicate that QP001 has a higher exposure level than MobicⓇ (oral meloxicam tablet), supporting the effectiveness of this novel formulation in improving solubility. And QP001 reached peak concentration immediately, and the value of Cmax was nearly twice higher as MobicⓇ, indicating its potential for fast pain relief. This effect may be attributed to the synergistic effect of PEG and pH regulator on the dissolution of meloxicam. And this novel formulation did not affect the clearance of meloxicam, which is similar to oral tables.
The previous study22 has shown that single-dose intravenous meloxicam of 15–60 mg exhibits better pain control than a placebo or a morphine dose of 0.15 mg/kg. And based on the results of several Phase 2 clinical trials,13,15,23 no significant difference in the effect of pain relief was found between the 30 mg and 60 mg groups. Therefore, we chose 30 mg as the repeated dose in our study. Compared to previous PK studies of 30 mg single-dose AnjesoⓇ injection with nanocrystal technology,24 AUC0-∞ of QP001 was similar at the same dose (101.50 ± 29.61 μg∙h/mL vs 107.51 ± 34.44 μg∙h/mL). But the Rac of 1.98 in our 30 mg repeated administration group is lower than it is in Caucasians. This may be due to variations in the frequency of CYP2C9 between East Asians and Caucasians, which is mainly responsible for the metabolism of meloxicam.17,25 In further clinical research, attention should be paid to the differences in the metabolic rate of this novel meloxicam injection in different populations. Although both QP001 and AnjesoⓇ are administered intravenously, QP001 appears to reach Cmax more quickly and with a higher value than AnjesoⓇ(Tmax: 0.03 h vs 0.12 h, Cmax: 9.34 ± 2.13 ug/mL vs 5.64 ± 1.01 ug/mL). Furthermore, the median Tmax of QP001 at doses of 15–60 mg were all in 2 minutes, indicating a rapid onset of action and supporting QP001 as a suitable medication for the treatment of acute pain.
In the past, sex-specific disparities regarding PKs of various NSAIDs have been observed. A survey of patients with osteoarthritis found that plasma concentrations of naproxen (a non-selective COX inhibitor) were significantly higher in females than in males.26 In a crossover study conducted in healthy volunteers, a faster intestinal absorption rate with an earlier Tmax of ketoprofen was observed in males.27 This may be due to the higher body-fat content in females, which may affect the distribution of drugs.28 Therefore, it’s essential to ensure the representation of female volunteers in the clinical study of NSAIDs. In our study, the proportion of male and female volunteers was balanced, and gender subgroups were compared, which is different from the usual Phase I trial that only includes male subjects. The sex-stratified data revealed that QP001 had no significant sex difference in the PK profile. Considering that gender factor on drug effects is generally at the PK level,29 there is no need to especially describe efficacy or side effects by sex in further research.
In terms of safety, QP001 demonstrated a favorable safety profile and was well tolerated by healthy Chinese subjects, with single dose up to 60 mg and multiple doses of 30 mg. No serious AEs were observed in our study. The most common TRAEs were hepatotoxicity and renal toxicity, which resulted in elevations of ALT, AST, blood bilirubin or serum creatinine. These TRAEs have been commonly reported in patients with NSAIDs.30,31 Overall, our study did not observe potential chronic toxicity associated with injection surfactants,32 which underscores the advantages of this novel formulation of QP001 with a reduced amount of excipients.
This study was associated with several limitations. First, although the small sample size is generally acceptable for a Phase I trial, rare AEs may be difficult to detect. And further large-scale clinical trials are needed to precisely demonstrate the safety of QP001. Second, because healthy subjects are not representative of a patient population, it is important to consider that PK values may differ when QP001 is applied in clinical practice.
Conclusion
QP001, a novel intravenous formulation of meloxicam, exhibited favorable safety and PK profile following both single-ascending-dose and multiple-dose studies in healthy Chinese subjects. The PK profile of QP001 suggested the potential for once-daily administration. Exposure levels were generally dose-proportional and no severe AEs were observed. Moreover, QP001 was well-tolerated, and no significant accumulation was observed in the Chinese population following repeated dosing of QP001 at 30 mg for five days. Overall, our findings support further clinical investigation of QP001 as a potential treatment option for pain relief.
Data Sharing Statement
The clinical raw data in this paper will be available upon reasonable request through an e-mail to the corresponding author (Mingyuan Li).
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81803639); Hunan Provincial Natural Science Foundation of China (No. 2020JJ5852); the Key Research and Development Project of Hunan Province (2020SK2010).
Disclosure
Mrs Ziteng Wu, Dr Yan Jiang and Dr Sen Yu are employees of Nanjing Delova Biotech Co., Ltd. Xuqing Zhang is affiliated with King-eagle Med Co., Ltd. The authors have no other relevant conflicts of interest in this work.
References
1. Manion J, Waller MA, Clark T, Massingham JN, Neely GG. Developing modern pain therapies. Front Neurosci. 2019;13:1370. doi:10.3389/fnins.2019.01370
2. Ducharme J. Acute pain and pain control: state of the art. Ann Emerg Med. 2000;35(6):592–603. doi:10.1016/S0196-0644(00)70033-3
3. Engelhardt G. Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol. 1996;35(Suppl 1):4–12. doi:10.1093/rheumatology/35.suppl_1.4
4. Ahmed M, Khanna D, Furst DE. Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol. 2005;1(4):739–751. doi:10.1517/17425255.1.4.739
5. Hasan SM, Shoaib MH, Hassan F, Rehman IU. Bioequivalence studies of two brands of meloxicam tablets in healthy Pakistani volunteers. Pak J Pharm Sci. 2009;22(2):199–204.
6. Turck D, Busch U, Heinzel G, Narjes H. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung. 1997;47(3):253–258.
7. Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(Suppl 1):13–16. doi:10.1093/rheumatology/35.suppl_1.13
8. Ambrus R, Kocbek P, Kristl J, Sibanc R, Rajko R, Szabo-Revesz P. Investigation of preparation parameters to improve the dissolution of poorly water-soluble meloxicam. Int J Pharm. 2009;381(2):153–159. doi:10.1016/j.ijpharm.2009.07.009
9. Distel M, Mueller C, Bluhmki E, Fries J. Safety of meloxicam: a global analysis of clinical trials. Br J Rheumatol. 1996;35(Suppl 1):68–77. doi:10.1093/rheumatology/35.suppl_1.68
10. Busch U, Heinzel G, Narjes H. The effect of cholestyramine on the pharmacokinetics of meloxicam, a new non-steroidal anti-inflammatory drug (NSAID), in man. Eur J Clin Pharmacol. 1995;48(3–4):269–272. doi:10.1007/BF00198310
11. Yu J, Wang Y, Wu Y, et al. Pharmacokinetics of meloxicam tablets in healthy Chinese adults in the fasting and fed states: a single-site, single-dose, randomized, open, 2-period, 2-sequence, crossover bioequivalence study. Clin Pharmacol Drug Dev. 2022;11(1):71–79. doi:10.1002/cpdd.965
12. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi:10.1007/s11916-016-0591-7
13. Gottlieb IJ, Tunick DR, Mack RJ, et al. Evaluation of the safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate-to-severe pain after bunionectomy. J Pain Res. 2018;11:383–393. doi:10.2147/JPR.S149879
14. Christensen SE, Cooper SA, Mack RJ, McCallum SW, Du W, Freyer A. A randomized double-blind controlled trial of intravenous meloxicam in the treatment of pain following dental impaction surgery. J Clin Pharmacol. 2018;58(5):593–605. doi:10.1002/jcph.1058
15. Singla N, Bindewald M, Singla S, et al. Efficacy and safety of intravenous meloxicam in subjects with moderate-to-severe pain following abdominoplasty. Plast Reconstr Surg Glob Open. 2018;6(6):e1846. doi:10.1097/GOX.0000000000001846
16. Li J, Wang Z, Zhang H, Gao J, Zheng A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2021;28(1):19–36. doi:10.1080/10717544.2020.1856224
17. Hasunuma T, Tohkin M, Kaniwa N, et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br J Clin Pharmacol. 2016;81(6):1078–1090. doi:10.1111/bcp.12884
18. White PF. What are the advantages of non-opioid analgesic techniques in the management of acute and chronic pain? Expert Opin Pharmacother. 2017;18(4):329–333. doi:10.1080/14656566.2017.1289176
19. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. doi:10.36076/ppj.2008/11/S105
20. Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16(7–8):354–360. doi:10.1016/j.drudis.2010.02.009
21. Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. doi:10.1038/nrd1494
22. Ochi M, Kawachi T, Toita E, et al. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int J Pharm. 2014;474(1–2):151–156. doi:10.1016/j.ijpharm.2014.08.022
23. Mack R, Freyer A, Du W. (409) An evaluation of the efficacy and safety of N1539, a novel intra venous formulation of NanoCrystal Meloxicam, in subjects with moderate to severe pain following hysterectomy. J Pain. 2016;17(4):S77. doi:10.1016/j.jpain.2016.01.386
24. Clinical pharmacology review; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/210583Orig1s000ClinPharmR.pdf.
25. Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27(1):9–54. doi:10.2133/dmpk.DMPK-11-RV-111
26. Rugstad HE, Hundal O, Holme I, Herland OB, Husby G, Giercksky KE. Piroxicam and naproxen plasma concentrations in patients with osteoarthritis: relation to age, sex, efficacy and adverse events. Clin Rheumatol. 1986;5(3):389–398. doi:10.1007/BF02054259
27. Magallanes L, Lorier M, Ibarra M, Guevara N, Vazquez M, Fagiolino P. Sex and food influence on intestinal absorption of ketoprofen gastroresistant formulation. Clin Pharmacol Drug Dev. 2016;5(3):196–200. doi:10.1002/cpdd.208
28. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–157. doi:10.2165/00003088-200948030-00001
29. Farkouh A, Baumgartel C, Gottardi R, Hemetsberger M, Czejka M, Kautzky-Willer A. Sex-related differences in drugs with anti-inflammatory properties. J Clin Med. 2021;10(7):1441. doi:10.3390/jcm10071441
30. Sarges P, Steinberg JM, Lewis JH. Drug-induced liver injury: highlights from a review of the 2015 literature. Drug Saf. 2016;39(9):801–821. doi:10.1007/s40264-016-0427-8
31. Monteiro C, Silvestre S, Duarte AP, Alves G. Safety of non-steroidal anti-inflammatory drugs in the elderly: an analysis of published literature and reports sent to the Portuguese pharmacovigilance system. Int J Environ Res Public Health. 2022;19(6):3541. doi:10.3390/ijerph19063541
32. Suo Z, Sun Q, Peng X, et al. Lentinan as a natural stabilizer with bioactivities for preparation of drug-drug nanosuspensions. Int J Biol Macromol. 2021;184:101–108. doi:10.1016/j.ijbiomac.2021.06.056
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
