Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
A Novel Metabolic Score for Predicting the Acute Exacerbation in Patients with Chronic Obstructive Pulmonary Disease
Authors Peng L , You H, Xu MY, Dong ZY, Liu M, Jin WJ, Zhou C
Received 20 January 2023
Accepted for publication 30 April 2023
Published 5 May 2023 Volume 2023:18 Pages 785—795
DOI https://doi.org/10.2147/COPD.S405547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ling Peng,1,2,* Hong You,2,* Mei-yu Xu,2 Zhou-yu Dong,2 Min Liu,2 Wen-jing Jin,2 Chao Zhou2
1Department of Critical Care Medicine, Qiannan Buyi and Miao Autonomous Prefecture People’s Hospital, Guizhou, People’s Republic of China; 2Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Zhou, Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area, No. 43 DongMen Street. Pudong new District, Shanghai, 201399, People’s Republic of China, Tel +86-21-68019069, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) has higher mortality when developing to acute exacerbation (AECOPD); hence, the early intervention of COPD is critical for preventing AECOPD. Exploring the serum metabolites associated with acute exacerbation in patients with COPD will contribute to the early intervention of COPD.
Methods: In the study, a non-targeted metabolomics strategy combined with multivariate statistical methods was performed to explore the metabolic profiling of COPD developing acute exacerbation, to screen the potential metabolites associated with AECOPD and to analyze the potential value of these metabolites in predicting the development of COPD.
Results: Serum lysine, glutamine, 3-hydroxybutyrate, pyruvate and glutamate levels were significantly higher, while 1-methylhistidine, isoleucine, choline, valine, alanine, histidine and leucine levels were significantly lower in AECOPD patients, compared with stable COPD patients after normalization based on the healthy controls. Moreover, eight metabolic pathways were significantly altered (P< 0.05) in the serum of AECOPD patients compared with the stable COPD population, including purine metabolism, glutamine and glutamate metabolism, arginine biosynthesis, butyrate metabolism, ketone body synthesis and degradation, and linoleic acid metabolism. In addition, the correlation analysis between metabolites and AECOPD patients demonstrated that an M-score based on a weighted sum of concentrations of four metabolites including pyruvate, isoleucine, 1-methylhistidine and glutamine were significantly associated with the acute exacerbation of pulmonary ventilation function in COPD patients.
Conclusion: Altogether, the metabolite score based on a weighted sum of concentrations of four serum metabolites was associated with an increased risk of COPD developing acute exacerbation, which will provide a new insight for the understanding of COPD development.
Keywords: COPD, AECOPD, acute exacerbation, metabolomics, metabolites
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic airway inflammatory disease, which seriously affect the patient’s exercise tolerance and quality of life;1,2 meanwhile has a heavy economic burden to the society.3 According to the data from the World Health Organization, COPD is the third leading cause of death and the fifth disease burden globally in 2020.4 In China, an overall morbidity of COPD is about 8.2%, suggesting an emergent need for prevention and treatment.5
COPD is characterized by persistent respiratory symptoms and airflow, usually as a result of high exposure to harmful particles or gases.6,7 The acute exacerbation of COPD (AECOPD) often leads to clinical death and the aggravation of economic burden in these patients.8,9 Hence, early diagnosis and intervention are crucial for preventing AECOPD.10 Previously, FEV1/FVC and FEV1 were used as predicted values to assess the degree of airflow limitation in AECOPD.11,12 However, in real world, patients with AECOPD can often not complete the pulmonary function test or even though they are reluctant to complete the test, the results are not credible.13 Given the fact, it is particularly urgent to explore the serum biological indicators to evaluate the development of COPD.
In recent years, the development of omics technologies has revolutionized biomedical research, particularly the metabolomics provides a powerful tool for discovering the novel biomarkers in various clinical fields.14,15 A variety of metabolite panels have been used in screening, assessing and monitoring the disease development and therapeutic efficacy16,17 and metabolomics-based panels make different studies more comparable and available.18,19 At present, the application of metabolomics in the field of COPD has made great progress in these years. Recently, a study based on a targeted metabolomics suggested dysregulated amino acid metabolism in community-acquired pneumonia and some metabolites such as asparagine/threonine may be recognized as biomarker candidates for the infection-triggered COPD exacerbation.20 Moreover, abnormalities in lipid metabolism may underlie AECOPD as early-diagnostic biomarkers.21,22 In addition, a comparative study reported numerous plasma metabolites may be considered as candidate biomarkers for diagnosis or prognosis of COPD.23 Nevertheless, metabolites indicating the acute exacerbation of COPD are still to be more clarified; meanwhile an available evaluation approach based on these candidate metabolites should be explored. In the study, we performed a non-targeted metabolomics strategy combined with multivariate statistical methods to screen potential serum metabolites associated with AECOPD and identified the metabolism profiling of AECOPD, the associated metabolic pathways and a novel metabolite score (M-score) based on a weighted sum of concentrations of four serum metabolites which was associated with the increased risk of COPD developing acute exacerbation. The findings which will provide a new insight for the understanding of COPD development.
Methods and Materials
Study Population
This study intends to select patients who were diagnosed as COPD during July 2020 to December 2021 in the Department of Respiratory Medicine, Guangming Hospital of Traditional Chinese Medicine, Shanghai. A total of 60 cases with COPD were included in the study, among who included 30 cases with stable COPD and 30 cases with acute exacerbation of COPD (AECOPD), defined as the phenomenon of sudden worsening in airway function and respiratory symptoms in patients with COPD; meanwhile, additional 30 matched normal volunteers were also collected as a healthy control group. All subjects signed informed consent. The diagnostic and staging criteria of COPD referred to the Guidelines (version 2021) from the Chronic Obstructive Pulmonary Disease Group of the Respiratory Disease Branch of the Chinese Medical Association. The period of acute worsening, including continue aggravation of cough, expectoration and dyspnea accompanied by fever or aggravated inflammation, is known as exacerbation. Moreover, gender, age, disease duration, smoking status (including current smokers and former smokers), BMI and pulmonary function parameters including forced expiratory volume in first second (FEV), percentage of FEVl in predicted value LL (FEVl%pred), forced expiratory volume in first second occupied forced vital capacity ratio (FEVl/FVC%) of patients were collected. In particular, for patients with AECOPD, the individual medical history records were collected to analysis and assess the development from stable stage to acute exacerbation of COPD. Exclusion criteria included patients with bronchial asthma, bronchiectasis, pulmonary fibrosis, lung tumors, tuberculosis, and other respiratory diseases that caused chronic cough or airflow limitation and those with severe heart disease, endocrine disease, autoimmune or other chronic wasting disease. In addition, those with comorbidities associated with liver, kidney, hematopoietic system and other serious primary diseases or mental illness or pregnant or lactating women or losing follow-up were excluded. According to the requirements of clinical design and multi-dimensional statistics, the population was divided into groups: 30 patients with AECOPD as the case group, 30 patients with stable chronic obstructive pulmonary disease as the case–control group, normal patients 30 cases were used as normal control group. The gender and age among the three groups were comparable.
Metabolomics-Based Sample Analysis and Data Processing
In strict accordance with the relevant regulations on the collection of clinical biological samples, the research subjects were required to stop taking drugs 2 days before collecting these samples, fasted after 8 pm on the last day; then 10 mL serum per patient was collected. After centrifuged for 10 min, the supernatant was separated and stored at −80°C in freezers. Serum per patient was divided with a duplicate copy and one was shipped to Applied Protein Technology Co., Ltd. (Shanghai) on dry ice for the metabolomics analysis by using a UHPLC (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight mass spectrometer (AB Sciex TEIPLE TOF 6600).
For centrifuged ×9000g at 4°C for 10 min, samples were injected directly onto a 2.1 × 100 mm ACQUITY UPLC BEH 1.7 μm column (Waters, Ireland). The column was eluted isocratically at a flow rate of 250 lL/min with the mobile phase A (25 mM ammonium acetate and 25 mM ammonium hydroxide in water) for 0.5 min, followed by B (acetonitrile with 0.1% formic acid) for 10 min. A 5 min re-equilibration period was employed. Mass spectrometry analyses were performed by using AB Sciex TEIPLE TOF 6600, set for acquisition over an m/z range of 60–1000 Da, for accumulation at 0.20 s/spectrum. The parameters were set as follows: collision energy (CE) fixed at 35 V with ±15 eV; declustering potential (DP), 60 V (+) and −60 V (−); exclusion of isotopes within 4 Da; candidate ions monitored per cycle, 10. The raw MS data was processed using XCMS software. Metabolites were identified using authentic reference standards. The metabolites with a VIP >1.0 and P-values <0.05 were considered statistically significant.
Quantitative Analysis of Metabolites and Identification of Markers
Firstly, the unsupervised principal component analysis method-PCA was used to analyze the serum metabolic profiles of different groups, respectively, to provide the general trend of all samples, to find clusters and singularities, and to observe the natural distribution and group distribution of the samples. In order to further distinguish the differences between different groups, the supervised analysis methods such as Partial Least Squares Discriminant Analysis (PLS-DA) and Orthogonal Partial Least Squares (OPLS) modeling were used to find the main difference variables that cause sample aggregation and dispersion. Through analyzing quantitatively the significantly differential metabolites, metabolites were identified to be associated with AECOPD. Pearson analysis was used to calculate the correlation between clinical parameters and significant metabolites, and t-test was used to verify the significance of the correlation. Finally, the differential metabolites were queried against the online Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp/) and mapped to KEGG pathways (Figure 1).
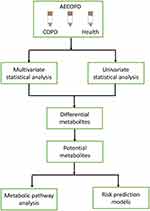 |
Figure 1 Metabolomics-based serum metabolite screening workflow. |
Statistical Analysis
SPSS 25.0 software was used for statistical analysis and normalization of data. The normal distribution of general clinical measurement data was expressed as mean ± standard deviation (±s), independent sample t-test was used for comparison between two groups, and one-way analysis of variance was used for comparison between multiple groups. In the AECOPD cohort, a logistic regression analysis to estimate hazard ratios (HR) and their 95% confidence intervals (CIs) for predicting the development of COPD, from stable stage to acute exacerbation; in the model, the variants included age, BMI, smoking history, C reactive protein (CRP), white blood cell count (WBC), peripheral eosinophil count (PEC), peripheral neutrophil count (PNC) and comorbidity (such as CVD, diabetes). Similarly, a logistic regression model was also used to clarify the acute exacerbation-associated metabolites; in the model, independent variables included differential metabolites and outcome variable was disease acute exacerbation. Inverse normal transformation was applied to raw values of metabolites and a weighted sum of concentrations of four metabolites including pyruvate, isoleucine, 1-methylhistidine and glutamine was used to build the M-score. The correlation analysis between M-score and COPD grades was performed by using a Pearson correlation coefficient. P<0.05 was considered a statistically significant difference, and the data are presented as the median ± standard errors of at least three independent replicates.
Results
Characteristics of Participants
The study included 30 patients with stable COPD and 30 patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), as well as 30 age-matched normal subjects. As shown in Table 1, for all COPD patients, 50% (30/60) suffered from CVD, but no particular CVD was associated with an increased percent of AECOPD. Moreover, compared to the healthy controls, patients with COPD presented a relatively increased risk of CVD and were more likely to smoke (all P<0.05). The number of WBC and the CRP indicator of inflammation were significantly increased in the AECOPD patients whose airway function was worse, reflected by the significant decreases of FVC(L), FVC%pred, FEV1(L), PEV1%pred and PEV1/FVC%, when compared with stable COPD patients which displayed a significant increase, compared to the healthy controls (all P<0.05), however no differences in the BMI were observed between the three groups (all P>0.05). Notably, PNC was also significantly increased in AECOPD patients when compared with the stable COPD patients (P<0.05). In addition, the result from a logistic regression analysis showed that CRP, WBC count and PNC were the independent factors for predicting the acute exacerbation of COPD (Table 2), suggesting a higher risk of COPD exacerbation among patients with elevated airway inflammation.
 |
Table 1 Clinical Characteristics of Patients with COPD and Control Population in the Study |
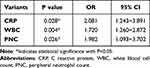 |
Table 2 A Multivariable Logistic Regression Analysis on the Acute Exacerbation of COPD |
Metabolism Profiling and Identification of AECOPD-Associated Metabolites
Based on the characteristic ions from UHPLC-QTOF/MS detection, a total of 127 metabolites were identified by 1HNMR metabolomic analysis combined with the NMR database (HMDB (http://www.hmdb.ca/) and BMRB (http://bmrb.wisc.edu/) to match the metabolites in the serum NMR maps of the three populations, mainly including lipids, basal acids, sugars and other substances (Figure 2A). Among these metabolites, there are 40 differential metabolites between control and COPD, in which 22 upregulated and 18 downregulated metabolites in COPD compared to control as well as 16 upregulated and 18 downregulated metabolites in AECOPD compared to COPD (Figure 2B). Of note, it showed that COPD patients had a dysregulated TCA cycle and nucleotide metabolism compared to the healthy controls; while the relative high level of TCA cycle and low level of nucleotide metabolism after the standardization of all metabolites was observed in AECOPD patients than that in stable COPD patients (Figure 2A). Among these different metabolites, COPD patients hold higher serum levels of isoleucine, valine, alanine and leucine but lower levels of lactate and AMP, FAD, uric acid compared to the healthy controls. Moreover, serum lysine, glutamine, 3-hydroxybutyrate, pyruvate and glutamate levels were significantly higher, while 1-methylhistidine, isoleucine, choline, valine, alanine, histidine, leucine and AMP levels were significantly lower in AECOPD patients compared with stable COPD patients after normalization based on the healthy controls, indicating that ACOPD patients have severe disorders of energy and nucleotide metabolism.
To investigate the metabolic pathways involved in the differential metabolites in serum from COPD patients, the various differential metabolites screened were imported into the MetPA database, and the metabolic pathways involved in the differentials were analyzed. The metabolic pathways with an effect factor >0.10 and P<0.05 were selected as the significant metabolic pathways. As shown in Figure 2C, compared with healthy controls, 12 metabolic pathways were significantly altered in COPD patients (P<0.05), including pyruvate metabolism, alanine, aspartate and glutamate metabolism, glycolysis or gluconeogenesis pathway, central carbon metabolism, riboflavin metabolism and so on. Eight metabolic pathways were significantly altered (P < 0.05) in the serum of AECOPD patients compared with the stable COPD population, including purine metabolism, glutamine and glutamate metabolism, arginine biosynthesis, butyrate metabolism, ketone body synthesis and degradation, and linoleic acid metabolism (Figure 2D). In addition, further enrichment analysis on these differential metabolites showed they can also be expectedly enriched in energy metabolism and nucleotide metabolism (Figure 2E and F). The metabolite-pathway network was constructed using Gephi software, and the results are shown in Figure 2G, with high centrality and intermediary centrality of tRNA biosynthesis, biosynthesis of alanine, glutamate and aspartate etc.
Correlation Analysis
A multivariate logistic regression model was established to estimate the COPD exacerbation probability of COPD patients. The results show that lysine, glutamine, 3-hydroxybutyrate, pyruvate, glutamate and 1-methylhistidine, isoleucine, choline, valine, alanine, histidine and leucine show non-normal distribution and the magnitude difference is large, so it needs to be normalized, and Matlab is used to perform Box-Cox transformation. Regression coefficients (beta-coefficients), odds ratios (95% confidence intervals) and standard errors (SE) were used for covariate logistic regression model probabilities. We compared quantitively the levels of pyruvate, isoleucine, 1-methylhistidine and glutamine between AECOPD and COPD and found that significant difference existed (Figure 3A–D). Subsequently, the related level index among the four metabolites were calculated and a metabolite score based on the related level index was constructed through applying a weighted sum of concentrations of 4 metabolites (Figure 3E). Moreover, a person correlation analysis demonstrated the different values of M-score including pyruvate, isoleucine, 1-methylhistidine and glutamine were significantly correlated with GOLD grade in COPD patients (r=0.4229; 95% CI:0.1893–0.6112; Figure 3F) (Table 3), suggesting a prognostic value in predicting the risk of COPD exacerbation.
 |
Table 3 Analysis for the Association Between M-Score and Characteristics of Patients with COPD |
Discussion
As an important part of systems biology, metabolomics expresses the functional changes brought about by exogenous disturbances in real time through the changes of metabolites. Metabolomics technology has been widely used in many disease fields such as circulatory system, digestive system, respiratory system, etc., especially in the research of TCM syndromes, some progress has been made.24,25 Metabolomics exploration has already been carried out in the diagnosis, molecular mechanism, syndrome diagnosis and pharmacodynamic mechanism of COPD; however, the associated metabolomics characteristics and clinical application need still to be improved in the future.26,27 At present, some researches based on a targeted or untargeted metabolomics have suggested that dysregulated metabolism including amino acid, lipid and glycerophospholipid may be recognized as biomarker candidates for the acute exacerbation of COPD,20–23 but the early diagnosis of COPD based on the serum or plasma metabolites or panels is also still insufficient. With the help of metabolomics, relying on a high-throughput and high-sensitivity metabolic analysis platform, the endogenous small molecule metabolites in COPD patients can be qualitatively or quantitatively analyzed, and specific biomarkers can be found.21 In researches to discover and identify potential biomarkers in body fluids, once compounds have been identified as potential biomarkers, further studies should be conducted to develop well-validated analytical procedures to confirm that these analytes are indeed accurate.28 Among these procedures, mathematical models are promising, which will be available and feasible for clinical evaluation for predicting the development of COPD in real world. In view of the above problems, in this study, we performed a non-targeted metabolomics strategy combined with multivariate statistical methods to screen potential serum metabolites associated with COPD and identified the metabolism characteristics of the acute exacerbation of COPD, the associated metabolic pathways and constructed a novel metabolite score based on a weighted sum of concentrations of four serum metabolites including pyruvate, isoleucine, 1-methylhistidine and glutamine significantly associated with the acute exacerbation of COPD. The findings which will provide a new insight for the understanding of COPD development.
It is well known that a portion of COPD patients can progress into acute exacerbations due to viral or bacterial infections, specific environment exposures, or other predisposing factors.29 The finding from our study showed that, during the acute exacerbation period, the relative high level of TCA cycle and low level of anaerobic glycolysis after the standardization of all metabolites were observed in AECOPD patients than that in stable COPD patients. However, this does not mean that energy consumption increases in patients with acute pulmonary exacerbations. Furthermore, it was found that COPD patients hold higher serum levels of isoleucine, valine, alanine and leucine but lower levels of lactate and uric acid compared to the healthy controls. It has been reported that serum amino acids and lipoproteins are decreased and choline glycerate is increased in COPD patients compared to normal subjects by NMR analysis; creatinine and lactate levels are decreased and alpha-ketoglutarate, ketone bodies, and pyruvate levels are increased in urine.30 Our study and these studies suggest the value of metabolomics in exploring the disease process and potential mechanisms of COPD. In addition, in the study, for COPD patients, serum lysine, glutamine, 3-hydroxybutyrate, pyruvate and glutamate levels were significantly higher, while 1-methylhistidine, isoleucine, choline, valine, alanine, histidine and leucine levels were significantly lower in AECOPD patients compared with stable COPD patients after normalization based on the healthy controls. The finding is consistent with a previous report31,32 and indicated that ACOPD patients have severe disorders of energy and protein metabolism. Following that, we identified AECOPD-associated metabolite pathways including pyruvate metabolism, alanine, aspartate and glutamate metabolism, glycolysis or gluconeogenesis pathway, central carbon metabolism, riboflavin metabolism and so on. As mentioned above, an increase in the nucleotide metabolic pathway of COPD patients in the acute phase can be reflected in the accumulation of nucleotide synthesis, suggesting a high energy consumption state.23,33 In addition, we constructed a metabolite score based on the related level index through applying a weighted sum of concentrations of 4 metabolites including pyruvate, isoleucine, 1-methylhistidine and glutamine and confirmed its potential by demonstrating the association between it and pulmonary ventilation function and symptoms and GOLD grade in COPD patients. The result suggested a prognostic value of the M-score in predicting the risk of COPD exacerbation.
There are several limitations in our current study. The observation could be limited by sample size. Moreover, we did not track the data in the clinically stable patients following hospital-based treatment, nor in the patients who had been stable for an indicated period and, therefore, we were unable to do a longitudinal level analysis. In addition, inflammation in lung and radiograph-based pneumonia status have been recognized as important influencing factors of metabolomics in patients with COPD.34 Although some inflammation factors were analyzed in the recurrent study, the association between metabolomics and imaging-based evidence needs to be explored in the future. Nevertheless, based on our studies, we believe that metabolomics can provide accurate disease assessment, early intervention, accelerated target drug development, and real-time monitoring of treatment effects. Furthermore, the eventual application of metabolic biomarkers may help to determine the pathogenesis of COPD diversity, and researchers should pay more attention to COPD to provide more meaningful information to guide clinical diagnosis and treatment based on previous studies.
Conclusion
Altogether, the metabolite score based on a weighted sum of concentrations of four serum metabolites was associated with an increased risk of COPD developing acute exacerbation, which will provide a new insight for the understanding of COPD development.
Abbreviations
COPD, chronic obstructive pulmonary disease; AECOPD, the acute exacerbation of COPD; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; UHPLC, ultra high performance liquid chromatography; CE, collision energy; DP, declustering potential; PLS-DA, partial least squares discriminant analysis; OPLS, orthogonal partial least squares; KEGG, Kyoto Encyclopedia of Genes and Genomes; BMI, Body mass index; FEV, forced expiratory volume in first second; FEVl%pred, percentage of FEVl in predicted value LL; FEVl/FVC%, forced expiratory volume in first second occupied forced vital capacity ratio; ICS, inhaled corticosteroids; LAMA, long-acting muscarine anticholinergic; CVD, cerebrovascular disease; CRP, C reactive protein; WBC, white blood cell count; PEC, Peripheral eosinophil count; PNC, peripheral neutrophil count; SE, standard errors; TCM, Traditional Chinese Medicine; TCA, tricarboxylic acid cycle.
Data Sharing Statement
The data will not be shared as the data are considered property of Guangming Traditional Chinese Medicine Hospital of Pudong New Area, which does not allow sharing of data with third party.
Ethics Approval and Informed Consent
The study was approved by the Institutional Review Board (IRB) of Guangming Traditional Chinese Medicine Hospital of Pudong New Area. The requirement for informed consent was waived by the IRB (reference number: GM 2022-33). The study was conducted in compliance with the Declaration of Helsinki. Patient data confidentiality was maintained throughout the study. All participants provided informed consent for this study.
Author Contributions
Ling Peng and Hong You should be regarded as co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Pudong new area Science, Technology and Economy Commission, Shanghai (No: PKJ2020-Y29).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–972. doi:10.1016/S0140-6736(22)01273-9
2. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
3. Lareau SC, Fahy B, Meek P, et al. Chronic Obstructive Pulmonary Disease (COPD). Am J Respir Crit Care Med. 2019;199(1):P1–P2. doi:10.1164/rccm.1991P1
4. Jeyachandran V, Hurst JR. Advances in chronic obstructive pulmonary disease: management of exacerbations. Br J Hosp Med. 2022;83(7):1–7. doi:10.12968/hmed.2022.0275
5. Zhou J, Zhang J, Zhou M, et al. The role of long-acting muscarinic antagonist/long-acting beta agonist fixed-dose combination treatment for chronic obstructive pulmonary disease in China: a narrative review. J Thorac Dis. 2021;13(11):6453–6467. doi:10.21037/jtd-21-961
6. Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med. 2019;381(11):1023–1034. doi:10.1056/NEJMoa1905248
7. Stoeppel CM, Eriksson EA, Diaz-Flores R, et al. Guiding the management of intubated patients with pneumonia and ventilator-associated events using serial catheter-directed bronchoalveolar lavage. J Trauma Acute Care Surg. 2014;76(5):1264–1269. doi:10.1097/TA.0000000000000219
8. Feng Z, Zhang L, Yu H, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for AECOPD patients after extubation: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2022;17:1987–1999. doi:10.2147/COPD.S375107
9. Kohnlein T, Schwarz SB, Nagel S, et al. Home Non-invasive positive pressure ventilation in chronic obstructive pulmonary disease: why, who, and how?. Respiration. 2022;101(8):709–716. doi:10.1159/000525015
10. Hu H, Ji Z, Qiang X, et al. Chinese medical injections for acute exacerbation of chronic obstructive pulmonary disease: a network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2021;16:3363–3386. doi:10.2147/COPD.S335579
11. Liang C, Mao X, Niu H, et al. characteristics, management and in-Hospital clinical outcomes among inpatients with acute exacerbation of chronic obstructive pulmonary disease in China: results from the Phase I data of ACURE study. Int J Chron Obstruct Pulmon Dis. 2021;16:451–465. doi:10.2147/COPD.S281957
12. Baqdunes MW, Leap J, Young M, et al. Acute exacerbation of chronic obstructive pulmonary disease. Crit Care Nurs Q. 2021;44(1):74–90. doi:10.1097/CNQ.0000000000000341
13. Jones SE, Barker RE, Nolan CM, et al. Pulmonary rehabilitation in patients with an acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2018;10(Suppl 12):S1390–S1399. doi:10.21037/jtd.2018.03.18
14. Roverso M, Dogra R, Visentin S, et al. Mass spectrometry-based “omics” technologies for the study of gestational diabetes and the discovery of new biomarkers. Mass Spectrom Rev. 2022. doi:10.1002/mas.21777
15. Doni F, Suhaimi NSM, Mispan MS, et al. Microbial contributions for rice production: from conventional crop management to the use of “Omics” technologies. Int J Mol Sci. 2022;23(2). doi:10.3390/ijms23020737
16. Bansal N, Kumar M, Sankhwar SN, et al. Relevance of emerging metabolomics-based biomarkers of prostate cancer: a systematic review. Expert Rev Mol Med. 2022;24:e25. doi:10.1017/erm.2022.20
17. Di Minno A, Gelzo M, Caterino M, et al. Challenges in metabolomics-based tests, biomarkers revealed by metabolomic analysis, and the promise of the application of metabolomics in precision medicine. Int J Mol Sci. 2022;23(9):5213. doi:10.3390/ijms23095213
18. Benchoula K, Vohra MS, Parhar IS, et al. Metabolomics based biomarker identification of anti-diabetes and anti-obesity properties of Malaysian herbs. Metabolomics. 2022;18(2):12. doi:10.1007/s11306-022-01870-2
19. Aderemi AV, Ayeleso AO, Oyedapo OO, et al. Metabolomics: a scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites. 2021;11(7):418. doi:10.3390/metabo11070418
20. Arshad H, Siokis A, Franke R, et al. Reprogramming of amino acid metabolism differs between community-acquired pneumonia and infection-associated exacerbation of chronic obstructive pulmonary disease. Cells. 2022;11(15):2283. doi:10.3390/cells11152283
21. Liu X, Zhang H, Si Y, et al. High-coverage lipidomics analysis reveals biomarkers for diagnosis of acute exacerbation of chronic obstructive pulmonary disease. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1201–1202:123278. doi:10.1016/j.jchromb.2022.123278
22. Gai X, Guo C, Zhang L, et al. Serum glycerophospholipid profile in acute exacerbation of chronic obstructive pulmonary disease. Front Physiol. 2021;12:646010. doi:10.3389/fphys.2021.646010
23. Zhou J, Li Q, Liu C, et al. Plasma metabolomics and lipidomics reveal perturbed metabolites in different disease stages of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:553–565. doi:10.2147/COPD.S229505
24. Yang Q, Zhang AH, Miao JH, et al. Metabolomics biotechnology, applications, and future trends: a systematic review. RSC Adv. 2019;9(64):37245–37257. doi:10.1039/C9RA06697G
25. Shao M, Lu Y, Xiang H, et al. Application of metabolomics in the diagnosis of non-alcoholic fatty liver disease and the treatment of traditional Chinese medicine. Front Pharmacol. 2022;13:971561. doi:10.3389/fphar.2022.971561
26. Nambiar S, Bong How S, Gummer J, et al. Metabolomics in chronic lung diseases. Respirology. 2020;25(2):139–148. doi:10.1111/resp.13530
27. Ghosh N, Dutta M, Singh B, et al. Transcriptomics, proteomics and metabolomics driven biomarker discovery in COPD: an update. Expert Rev Mol Diagn. 2016;16(8):897–913. doi:10.1080/14737159.2016.1198258
28. Donaldson K, Buchanich JM, Grigson PS, et al.Abstracts of presentations at the association of clinical scientists 143(rd) meeting Louisville, KY May 11–14, 2022. Ann Clin Lab Sci. 2022;52(3):511–525.
29. Ruiz-Gonzalez A, Saez-Huerta E, Martinez-Alonso M, et al. A simple scoring system to differentiate bacterial from viral infections in acute exacerbations of COPD requiring hospitalization. Int J Chron Obstruct Pulmon Dis. 2022;17:773–779. doi:10.2147/COPD.S356950
30. MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45(3):291–300. doi:10.3109/07853890.2012.732703
31. Wei B, Tian T, Liu Y, et al. The diagnostic value of homocysteine for the occurrence and acute progression of chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20(1):237. doi:10.1186/s12890-020-01265-w
32. Adamko DJ, Nair P, Mayers I, et al. Metabolomic profiling of asthma and chronic obstructive pulmonary disease: a pilot study differentiating diseases. J Allergy Clin Immunol. 2015;136(3):571–580 e573. doi:10.1016/j.jaci.2015.05.022
33. Xue M, Zeng Y, Lin R, et al. Metabolomic profiling of anaerobic and aerobic energy metabolic pathways in chronic obstructive pulmonary disease. Exp Biol Med. 2021;246(14):1586–1596. doi:10.1177/15353702211008808
34. Mathioudakis AG, Janssens W, Sivapalan P, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax. 2020;75(6):520–527. doi:10.1136/thoraxjnl-2019-214484
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


