Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Fibrinogen, a Promising Marker to Evaluate Severity and Prognosis of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Retrospective Observational Study
Authors Sun W, Cao Z, Ma Y, Wang J, Zhang L, Luo Z
Received 11 February 2022
Accepted for publication 19 May 2022
Published 3 June 2022 Volume 2022:17 Pages 1299—1310
DOI https://doi.org/10.2147/COPD.S361929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Wei Sun,1 Zhixin Cao,1 Yingmin Ma,2 Jing Wang,1 Liming Zhang,1 Zujin Luo1
1Department of Respiratory and Critical Care Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Zujin Luo, Department of Respiratory and Critical Care Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China, Email [email protected]
Background: Fibrinogen is increasingly being studied as an inflammatory biomarker in chronic obstructive pulmonary disease (COPD), but there are limited data on the role of fibrinogen in assessing the severity of acute exacerbation of COPD (AECOPD). This study aimed to explore whether circulating fibrinogen could be used as a surrogate to measure the severity and predict the prognosis of AECOPD.
Methods: A total of 535 AECOPD patients diagnosed at our center from January 2016 to June 2021 were retrospectively enrolled in this study. The electronic medical record of each patient was retrieved to collect data on baseline characteristics and laboratory parameters, as well as the use of noninvasive positive-pressure ventilation (NPPV) and prognosis. Multiple linear regression analysis was used to identify independent factors associated with circulating fibrinogen values. Receiver-operating characteristic curve and multivariate logistic regression analysis were applied to further verify the use of fibrinogen to predict NPPV failure.
Results: Compared to patients with fibrinogen < 4 g/L, patients with increased fibrinogen levels (> 4 g/L) tended to have elevated inflammatory response and higher incidence of DVT/PTE, emphysema, pneumonia, and atherosclerosis. In addition, fibrinogen levels in NPPV-failure patients were significantly higher than non-NPPV patients and NPPV-success ones. The presence of emphysema, pneumonia, and history of long-term oxygen therapy and higher CRP levels and leukocyte counts were independent risk factors associated with increased fibrinogen levels in AECOPD. Furthermore, our data indicated that fibrinogen could be considered as a reliable biomarker to predict NPPV failure (AUC, 0.899, 95% CI 0.846– 0.952), with an OR of 7.702 (95% CI 2.984– 19.875; P< 0.001).
Conclusion: The level of circulating fibrinogen can be used to measure severity of AECOPD, and among AECOPD patients managed with NPPV, fibrinogen > 3.55 g/L can independently predict NPPV failure.
Keywords: COPD, noninvasive positive-pressure ventilation, acute exacerbation, fibrinogen, predictor
Background
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation that is not fully reversible.1 It is associated with chronic inflammation, both locally and systemically, which increases further during acute exacerbations (AEs).2 It has been known that some inflammatory biomarkers are associated with AE,3 disease progression, and severity of airflow obstruction.4–6 Identification of these biomarkers not only provides a method of predicting prognosis but also helps with better understanding of the pathogenesis of COPD.
A key modulator of inflammation and fibrosis development, as well as tissue injury,7 fibrinogenhas been approved by the US Food and Drug Administration as a COPD biomarker for severity assessment.8 Higher baseline fibrinogen is associated with increasing incidence of COPD, COPD hospitalization, and all-cause mortality9 and related to severity of COPD.10 One study found that fibrinogen level was higher during AE of COPD (AECOPD) and returned to baseline 40 days after exacerbation.11 Fifteen-year follow-up data from the CARDIA study of 2,132 individuals showed an association between higher fibrinogen and greater loss of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), regardless of smoking status.12 Other studies have suggested that increasing fibrinogen levels are associated with the occurrence of COPD complications.13,14 However, there has been little research on the role of fibrinogen during AECOPD and its association with noninvasive positive-pressure ventilation (NPPV). Our study aimed to explore whether circulating fibrinogen could be used as a surrogate to measure the severity and predict the prognosis of AECOPD.
Methods
Study Design and Participants
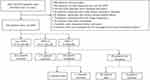
A total of 535 patients diagnosed with AECOPD at Beijing Chao-Yang Hospital (west campus) from January 2016 to June 2021 were retrospectively enrolled in this study. The patient-selection process is shown in Figure 1. The hospital’s ethics committee determined that this study qualified for waiving patient consent according to its policies, because it analyzed a large data set without patient identifiers, which is in compliance with the Declaration of Helsinki regarding patient-data confidentiality (2016-KE-95).
Inclusion criteria were age ≥45 years, primary diagnosis of COPD determined by spirometry data of airflow obstruction with bronchodilator (FEV1/FVC <0.7, previous spirometry also considered, since a minority of patients had had pulmonary function tests during AE period), and admission to hospital due to AECOPD (defined as an acute worsening of respiratory symptoms requiring additional treatment). Indications for NPPV use during hospitalization were arterial blood pH <7.35 and/or PaCO2 >45 mmHg and/or presence of dyspnea at rest assessed using accessory respiratory muscles or paradoxical abdominal breathing.
Exclusion criteria were presence of other severe pulmonary diseases (such as severe bronchiectasis or pulmonary tuberculosis), end-stage chronic diseases (eg, chronic kidney failure, chronic heart failure, and malignancy) with <1 year of expected survival, requiring intubation before admission, incomplete data, and endotracheal intubation for other diseases (such as acute heart failure, kidney failure, and shock). For patients with multiple admissions during the study period, only the last was selected.
NIPPV failure was defined as worsening of pH and PaCO2 in arterial blood (defined as arterial pH <7.25 with PaCO2 increased by >20% compared with baseline or PaO2 <60 mmHg, despite maximum tolerated supplemental oxygen), clinical signs suggestive of severely decreased consciousness (eg, coma, delirium), excessive respiratory secretions with weak cough, use of accessory respiratory muscles or paradoxical thoracoabdominal movement, severe upper gastrointestinal bleeding with aspiration or vomiting, and severe hemodynamic instability despite fluid repletion and use of vasoactive agents.15
Data Collection
Baseline characteristics of age, sex, length of stay (LOS), heart rate, systolic pressure, diastolic pressure, temperature, respiratory rate, history of smoking, history of long-term oxygen therapy (LTOT), and history of domestic noninvasive ventilation (DNV) were recorded. In addition, data on comorbidities, ie, deep-vein thrombosis/pulmonary thromboembolism, emphysema, pneumonia, hypertension, diabetes, Cor pulmonale, chronic heart disease, atherosclerosis, chronic kidney disease, cerebrovascular disease, and other malignancies were collected. Arterial blood gas and peripheral venous blood (routine blood, CRP, and coagulation index <24 hours after admission) and the use of antibiotics and management of NPPV were also reviewed.
Concentration of serum fibrinogen was measured using immuno-scatter turbidimetry with a Werfen ACL Top 700. Normal fibrinogen levels are 2–4 g/L. Concentration of serum CRP were measured using immunoscatter turbidimetry with a Goldsite Aristo. Normal CRP levels are 0–5 mg/L. Parameters for noninvasive ventilation were set according to clinical practice and patients’ tolerance. An oronasal mask was used for all subjects. Arterial blood gas was intermittently analyzed by physicians according to clinical needs. When the patient reached the criteria for NPPV failure, a physician made the clinical decision (intubation or continuation of NPPV) based on laboratory data, symptoms, signs, and the inclination of the patients and their family members. The prognosis of each patient was recorded.
Statistical Analysis
Descriptive data are expressed as medians with IQRs or numbers with percentages as appropriate. Differences between groups were measured with the Mann–Whitney U test for continuous variables and x2 test for categorical variables. Spearman correlations were used for correlation analysis, and the results are displayed as correlation coefficients with P values. Multiple linear regression models were applied to identify independent risk factors of increasing fibrinogen levels. Differences in laboratory parameters among non-NPPV, NPPV-success (NPPV-S), and NPPV-failure (NPPF-F) groups were examined using the Kruskal–Wallis H test. Receiver-operating characteristic (ROC) curves were constructed to evaluate the ability of inflammatory markers to predict NPPV failure. For each ROC curve, the optimal cutoff, sensitivity, specificity, Youden’s index, area under the curve (AUC), and 95% CI were calculated. Logistic regression analyses with a conditional forward stepwise–regression model were used to determine whether any factors were independently associated with NPPV failure. All analyses were two-tailed, and differences were considered statistically significant at P<0.05. SPSS 21.0 was utilized for all statistical analysis.
Result
Baseline-Characteristic and Laboratory-Data Comparison Between Higher and Lower Fibrinogen Values
In sum, 1,925 AECOPD patients were screened and 535 selected. Among the latter, 312 (58.3%) were not managed with NPPV and 223 (41.7%) received NPPV management. Of all patients managed with NPPV, 177 (79.4%) were categorized as NPPV-S and 46 (20.6%) NPPV-F (Figure 1). In the NPPV-F group, 20 patients were intubated and 26 not, with three and 18, respectively, dying (Figure 1).
No significant differences in terms of age, sex, LOS, systolic pressure, temperature, smoking history, pH, PaCO2, HCO3–, or BMI were identified between patients with low (≤4 g/L) and high (>4 g/L) fibrinogen levels. However, as suggested in Table 1, patients with higher fibrinogen (>4 g/L) presented faster heart beats and respiratory rates. There were more patients managed with LTOT and DNV in the high-fibrinogen group than the low-fibrinogen group. DVT/PTE, emphysema, pneumonia, and atherosclerosis were more commonly observed among patients with >4 g/L fibrinogen. In addition, increased CRP levels and leukocyte and neutrophil counts, decreased lymphocyte counts and PaO2/FiO2, and more frequent use of antibiotics were observed among patients with increased fibrinogen.
 |
Table 1 Baseline characteristics of AECOPD subjects according to fibrinogen value |
Next, we examined differences among the non-NPPV, NPPV-S, and NPPV-F groups and discovered that patients in the NPPV-F group had higher levels of fibrinogen, CRP, leukocytes, and neutrophils including those in the non-NPPV and NPPV-S groups (Figure 2). In addition, there were significant differences among the non-NPPV, NPPV-S, and NPPV-F groups in terms of lymphocytes, pH, PaCO2, and PaO2/FiO2. For HCO3– levels, there were differences between the non-NPPV and NPPV-S groups and non-NPPV and NPPV-F groups, but none between the NPPV-S and NPPV-F groups.
Risk Factors of Increased Fibrinogen Levels During AECOPD
Table 2 shows positive correlations between fibrinogen levels and heart rate (x=0.130, P=0.003), respiratory rate (x=0.098, P=0.023), CRP (x=0.461, P<0.001), leukocytes (x=0.280, P<0.001), neutrophils (x=0.316, P<0.001), and PaCO2 (x=0.098, P=0.024). Negative correlations between fibrinogen and lymphocytes (x=−0.144, P=0.001) and PaO2/FiO2 (x=−0.147, P=0.001) were identified. In the multiple linear regression model, we included emphysema, pneumonia, atherosclerosis, LTOT, DNV, HR, RR, CRP, leukocytes, neutrophils, lymphocytes, PaCO2, and PaO2/FiO2 as covariates and found that emphysema, pneumonia LTOT, CRP, and leukocytes were independent factors associated with circulating fibrinogen levels during AECOPD (R2=0.302, P<0.001; Table 3).
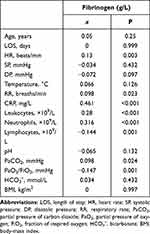 |
Table 2 Correlations between fibrinogen levels and baseline characteristics/laboratory results |
 |
Table 3 Multiple linear regression analysis of fibrinogen |
Risk Factors of NPPV Failure
The ROC curves for fibrinogen, CRP, leukocytes, neutrophils, and lymphocytes in the NPPV-F group are presented in Figure 3. As shown in Table 4, the AUC for fibrinogen (0.899, 95% CI 0.846–0.952) was higher than those for CRP (0.71, 95% CI 0.625–0.795), leukocytes (0.724, 95% CI 0.637–0.812), neutrophils (0.795, 95% CI 0.724–0.867), and lymphocytes (0.792, 95% CI 0.707–0.876). Cutoffs for predicting NPPV-F were fibrinogen >3.55 g/L, CRP >17 mg/L, leukocytes >8.05×109/L, neutrophils >6.72×109/L, and lymphocytes <0.68×109/L. Associations between NPPV-F and clinical parameters were analyzed by multivariate logistic regression, and the results showed that fibrinogen (OR 7.702, 95% CI 2.984–19.875) was independently associated with NPPV-F (Figure 4).
 |
Table 4 ROC-curve data |
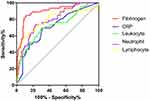 |
Figure 3 ROC curve of fibrinogen, CRP, leukocytes, neutrophils, and lymphocytes for predicting NPPV failure. Abbreviation: ROC, receiver-operating characteristic. |
Discussion
In this study, we found that 16.1% (n=86) of patients in the current study had fibrinogen >4 g/L, which was associated with a more robust inflammatory response. The fibrinogen level during AECOPD was related to CRP expression and leukocyte counts, as well as the presence of LTOT, emphysema, and pneumonia. Among those managed with NPPV, circulating fibrinogen (OR 7.702, 95% CI 2.984–19.875; P<0.001) was the independent factor predicting NPPV-F in the laboratory data.
Synthesized mainly by the liver and converted into fibrin by thrombin during blood coagulation,16 fibrinogen is a major acute-phase reactant, and its synthesis is upregulated in response to inflammation. Polatli et al discovered that the level of fibrinogen during AECOPD (4.48±1.28 mg/L) was higher than in stable periods (3.49±0.92 mg/L).17 However, Valipour et al found no difference in fibrinogen between stable COPD (4.24 g/L) and AECOPD (4.19 g/L).18 Since the current study did not include data prior to hospitalization and focused on the AE period only, we were not able to compare differences in fibrinogen levels between stable COPD and AECOPD; therefore, conclusions drawn from this study should be applied to the AECOPD population only.
Elevated fibrinogen induces a state of hypercoagulability that may lead to the progression of thrombosis.19 In this study, the incidence of DVT/PET during AECOPD was 6.7% overall, and more patients developed DVT/PTE in the high-fibrinogen group than the low-fibrinogen group. Furthermore, the incidence of atherosclerosis was much higher in the >4 g/L fibrinogen group. A multicenter prospective observational study based in China of 1,144 AECOPD patients showed that 78 (6.8%) were diagnosed with VTE, including 24 PE, 64 DVT, and ten combined PE and DVT,20 which is consistent with our results. A meta-analysis involving 3,170 AECOPD patients showed that the prevalence of PTE and DVT in AE-COPD patients was 17.2% and 7.1%, respectively.21 However, the link between fibrinogen and DVT/PTE is unclear, since Watz et al found that fibrinogen was inversely associated with levels of physical activity,14 and the lack of physical exertion is itself a risk factor of the development of DVT/PTE. AECOPD patients tend to have reduced physical exercise due to respiratory dysfunction and/or muscle weakness, which could partly contribute to higher levels of fibrinogen.22 Also, COPD is characterized by excessive activation of neutrophils in both stable and AECOPD periods, and neutrophil extracellular traps (NETs), a defense mechanism of neutrophils, have been demonstrated to play an important role in atherosclerosis and thrombosis.23 In the current study, there was a significant positive correlation between neutrophil counts and fibrinogen, which might explain the presence of atherosclerosis and DVT/PTE being higher in the AECOPD individuals with increased levels of fibrinogen. Further study will be required to elucidate associations among the incidence of COPD comorbidity, fibrinogen levels, and neutrophil counts.
It has been suggested that fibrinogen levels >3.93 g/L can predict COPD-related hospitalization. In a COPD-related study of 20,192 subjects, mean fibrinogen was 3.07 g/L and 10% of the sample had levels >3.93 g/L.9 Singh et al used 3.5 g/L as a cutoff to predict AE, and the ratios were 1.03 for moderate exacerbations, 1.08 for moderate/severe exacerbations, and 1.30 for severe exacerbations.24 In the current study, we grouped the patients using a cutoff of 4 g/L. Mean fibrinogen was 3.33 g/L, and 16.1% of the patients had levels >4 g/L. Differences in mean numbers and percentages of patients with high fibrinogen between this and other studies might be due to the fact that the current study included only AECOPD individuals. Valvi et al9 included not only AECOPD subjects but also stable ones, individuals with respiratory symptoms in the absence of any lung-function abnormality, and even healthy controls. The current study demonstrated that AECOPD patients with >4 g/L fibrinogen had a more robust inflammatory response and that it is possible that fibrinogen could be used as a marker of ongoing airway inflammation.
This study revealed that emphysematous AECOPD patients tended to have higher levels of fibrinogen and that the presence of emphysema can significantly affect fibrinogen level. It has been proved that the end product of fibrinogen is elevated in emphysematous stable COPD compared to patients without emphysema.25 Papaioannou et al reached a similar conclusion that among stable COPD individuals, those with emphysema were prone to have higher plasma-fibrinogen levels.26 Fibrinogen combined with other biomarkers is highly predictive of emphysema and associated with progression of emphysema.27 Emphysema is a key contributor to airflow limitation in COPD individuals, and fibrinogen itself is closely related to faster decline in lung function;28 therefore, there might be an association between fibrinogen and emphysema, but the mechanism and causality of the association is not well established. In the current study, patients with elevated ibrinogen (>4 g/L) had significantly lower PaO2/FiO2, and patients managed with LTOT and DNV at baseline had increased fibrinogen, which could be partially attributable to the fact that higher circulating fibrinogen correlated with the emphysema. According to the natural disease progression of COPD, individuals with progressive emphysema were prone to have an advanced stage of airway and lung-parenchyma inflammatory response, which could lead to worse gas-exchange functioning, and were more likely to require oxygen therapy and noninvasive ventilation support. In the future, studies on whether anti-fibrinogen agents could slow the progression of emphysema should be conducted for further evaluation of the association between fibrinogen and emphysema.
Another independent factor that can affect fibrinogen levelse during AECOPD is the presence of pneumonia. One study demonstrated that inflammatory response was different between infection-induced AECOPD and uninfectious AECOPD.29 Patients with moderate–severe COPD who have pathogenic microorganisms (PPMs) in their sputum have an exaggerated airway inflammatory response and higher levels of plasma fibrinogen than subjects with non-PPMs in their sputum.30 It has been found that fibrinogen was significantly higher in the presence of purulent sputum, a symptomatic cold, or increased cough among AECOPD patients.31 Furthermore, it has been demonstrated that fibrinogen increases threefold during acute-phase stimulation in response to increased IL6 production,32,33 which is commonly observed in community-acquired pneumonia-associated AECOPD patients.34,35 The fact that the presence of pneumonia contributed to a higher level of fibrinogen makes it a reasonable assumption that patients with fibrinogen >4 g/L had higher inflammatory response and more frequent use of antibiotics.
Failure of noninvasive ventilation was associated with increased mortality among AECOPD patients, and the current study showed that fibrinogen might be used in identifying those at great risk and who may therefore benefit from more aggressive treatment like early intubation. It has been demonstrated that low molecular–weight heparin significantly reduces fibrinogen and mean duration of mechanical ventilation among AECOPD individuals.36 We found that fibrinogen is an independent risk factor of NPPV-F with an AUC value higher than other traditional inflammatory markers, including CRP, leukocytes, neutrophils, and lymphocytes. This finding was not surprising, considering the fact that an inverse correlation between circulating fibrinogen and FEV1 has been proved in previous studies already,28,37,38 and the fact that fibrinogen can reflect the severity of AECOPD.24 Correlations between the incidence of domestic noninvasive ventilation and fibrinogen value and between PaCO2 and fibrinogen in this analysis showed that AECOPD individuals with higher fibrinogen tended to have worse ventilation function. Pathogens of airway colonization were associated with higher fibrinogen than those with no detectable airway pathogens in two small studies.30,39
Pillay et al found that increased circulating concentrations of fibrinogen during the acute-phase response can act as a natural antagonist of neutrophil recruitment by inhibiting neutrophil adhesion,40 which is an essential step in its antimicrobial function. This imbalance between pathogen overload and suppression of neutrophil immunofunction made the inflammatory response during AECOPD more intense, which was reflected by higher CRP, leukocytes, and neutrophils. Although the cutoff value for fibrinogen to predict NPPV failure was 3.55 g/L, less than 4 g/L, in Table 2 the median fibrinogen value of NPPV-F patients was 4.10 g/L, which was higher than that of non-NPPV and NPPV-S individuals. The NPPV-F population included in this study was relatively small (n=46), and maybe the cutoff for fibrinogen deduced from a large population would be different. Collectively, fibrinogen levels suggested reduced ventilation functioning together with more intense inflammation, which might eventually contribute to NPPV-F among AECOPD individuals.
Given the evidence of an association between fibrinogen and the incidence of COPD, presence of exacerbations, and mortality, certain anti-inflammatory drugs targeting fibrinogen may shed new light on the treatment of COPD. A p38 MAPK inhibitor was showed to reduce plasma fibrinogen by 11% in individuals with stable COPD after a 3-month treatment.41 Clinically relevant improvement accompanied by a decline of fibrinogen should be further determined, but the effect on the level of fibrinogen may suggest the potential utility of biomarkers in response to treatment and help the physician to identify individuals who might benefit from certain interventions.
Limitations
A number of limitations must be acknowledged in the current study. Firstly, it was a single-center retrospective observational study with a small sample, and thus our results may not be generalized to a broader population. Secondly, a substantial proportion of pulmonary function data was missing, because most AECOPD patients were not able to undergo a spirometry test during their hospital stay. Thirdly, NPPV-related data, such as pressure and tidal volume, were unavailable, since they are not collected routinely in clinical settings.
Conclusion
The current study suggested that circulating fibrinogen value during AECOPD strongly correlated with traditional inflammatory markers and can reflect the severity of systematic inflammatory response. Increased fibrinogen may indicate a need for antibiotics. Moreover, fibrinogen was a better marker for predicting NPPV-F than traditional inflammatory ones, and this indicated that it might be used for identifying AECOPD patients who may not benefit from NPPV. However, further study with a larger sample is needed to determine whether using fibrinogen as a biomarker to assess AECOPD individuals could actually result in clinical benefit.
Abbreviations
Fib, fibrinogen; LOS, length of stay; HR, heart rate; SP, systolic pressure; DP, diastolic pressure; RR, respiratory rate; LTOT, long-term oxygen therapy; DNV, domestic noninvasive ventilation; DVT/PTE, deep-vein thrombosis/pulmonary thromboembolism; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; HCO3–, bicarbonate; BMI, body-mass index; non-NPPV, no use of noninvasive ventilation; NPPV-S, noninvasive ventilation success; NPPV-F, noninvasive failure; ROC, receiver-operating characteristic; AUC, area under the curve.
Data Sharing
The data sets used and/or analyses during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board for Beijing Chao-Yang Hospital (2016-KE-95) and conducted according to the principles of the Declaration of Helsinki. The need to obtain informed consent was waived, due to the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Key Research and Development Program of China (grant 2019YFC0121700) and Beijing Hospitals Authority Youth Programme (grant QML20180303).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. doi:10.1016/j.ccm.2013.10.004
3. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
4. Celli B, Locantore N, Yates JC, et al. Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort. Eur Respir J. 2021;57(3):2001339. doi:10.1183/13993003.01339-2020
5. Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(6):1008–1011. doi:10.1164/ajrccm.164.6.2010067
6. Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(3):250–255. doi:10.1164/rccm.200605-713OC
7. Wang J, Pathak R, Garg S, Hauer-Jensen M. Fibrinogen deficiency suppresses the development of early and delayed radiation enteropathy. World J Gastroenterol. 2017;23(26):4701–4711. doi:10.3748/wjg.v23.i26.4701
8. Available from: http://www.copdfoundation.org/PressRoom/ArticlesPressReleases/News/187.aspx.
9. Valvi D, Mannino DM, Mullerova H, Tal-Singer R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis. 2012;7:173–182. doi:10.2147/COPD.S29892
10. Garcia-Rio F, Miravitlles M, Soriano JB, et al. Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res. 2010;11:63. doi:10.1186/1465-9921-11-63
11. Koutsokera A, Kiropoulos TS, Nikoulis DJ, et al. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;103(6):919–926. doi:10.1016/j.rmed.2008.12.006
12. Kalhan R, Tran BT, Colangelo LA, et al. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5(7):e11431. doi:10.1371/journal.pone.0011431
13. Fowkes FG, Anandan CL, Lee AJ, et al. Reduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular disease. J Vasc Surg. 2006;43(3):474–480. doi:10.1016/j.jvs.2005.11.018
14. Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136(4):1039–1046. doi:10.1378/chest.09-0393
15. Cao Z, Luo Z, Hou A, et al. Volume-targeted versus pressure-limited noninvasive ventilation in subjects with acute hypercapnic respiratory failure: a multicenter randomized controlled trial. Respir Care. 2016;61(11):1440–1450. doi:10.4187/respcare.04619
16. Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105(2):284–296. doi:10.3324/haematol.2019.236901
17. Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, Yenicerioglu Y. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis. 2008;26(2):97–102. doi:10.1007/s11239-007-0073-1
18. Valipour A, Schreder M, Wolzt M, et al. Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci. 2008;115(7):225–232. doi:10.1042/CS20070382
19. van Hylckama Vlieg A, Rosendaal FR. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thrombos Haemost. 2003;1(12):2677–2678. doi:10.1111/j.1538-7836.2003.0543b.x
20. Pang H, Wang L, Liu J, et al. The prevalence and risk factors of venous thromboembolism in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(11):2573–2580. doi:10.1111/crj.12959
21. Fu X, Zhong Y, Xu W, et al. The prevalence and clinical features of pulmonary embolism in patients with AE-COPD: a meta-analysis and systematic review. PLoS One. 2021;16(9):e0256480. doi:10.1371/journal.pone.0256480
22. Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi:10.1016/j.rmed.2011.10.022
23. Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16. doi:10.1016/j.atherosclerosis.2019.06.919
24. Singh D, Criner GJ, Dransfield MT, et al. InforMing the PAthway of COPD treatment (IMPACT) trial: fibrinogen levels predict risk of moderate or severe exacerbations. Respir Res. 2021;22(1):130. doi:10.1186/s12931-021-01706-y
25. Manon-Jensen T, Langholm LL, Rønnow SR, et al. End-product of fibrinogen is elevated in emphysematous chronic obstructive pulmonary disease and is predictive of mortality in the ECLIPSE cohort. Respir Med. 2019;160:105814. doi:10.1016/j.rmed.2019.105814
26. Papaioannou AI, Mazioti A, Kiropoulos T, et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir Med. 2010;104(2):275–282. doi:10.1016/j.rmed.2009.09.016
27. Zemans RL, Jacobson S, Keene J, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18(1):117. doi:10.1186/s12931-017-0597-7
28. Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168(6):602–610. doi:10.1093/aje/kwn174
29. Lieberman D, Lieberman D, Gelfer Y, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002;122(4):1264–1270. doi:10.1378/chest.122.4.1264
30. Banerjee D, Khair OA, Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J. 2004;23(5):685–691. doi:10.1183/09031936.04.00056804
31. Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(2):210–215. doi:10.1055/s-0037-1613998
32. Gabay C, Kushner I, Epstein FH. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi:10.1056/NEJM199902113400607
33. Mackiewicz A, Speroff T, Ganapathi MK, Kushner I. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol. 1991;146(9):3032–3037.
34. Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. doi:10.1378/chest.13-0488
35. Damera G, Pham TH, Zhang J, et al. A sputum proteomic signature that associates with increased IL-1β levels and bacterial exacerbations of COPD. Lung. 2016;194(3):363–369. doi:10.1007/s00408-016-9877-0
36. Qian Y, Xie H, Tian R, Yu K, Wang R. Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Copd. 2014;11(2):171–176. doi:10.3109/15412555.2013.831062
37. Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004. doi:10.1378/chest.128.4.1995
38. Shibata Y, Abe S, Inoue S, et al. Relationship between plasma fibrinogen levels and pulmonary function in the Japanese population: the Takahata study. Int J Med Sci. 2013;10(11):1530–1536. doi:10.7150/ijms.7256
39. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi:10.1164/ajrccm.164.9.2105011
40. Pillay J, Kamp VM, Pennings M, et al. Acute-phase concentrations of soluble fibrinogen inhibit neutrophil adhesion under flow conditions in vitro through interactions with ICAM-1 and MAC-1 (CD11b/CD18). J Thrombos Haemost. 2013;11(6):1172–1182. doi:10.1111/jth.12250
41. Lomas DA, Lipson DA, Miller BE, et al. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2012;52(3):416–424. doi:10.1177/0091270010397050
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.