Back to Journals » The Application of Clinical Genetics » Volume 16
A New de novo BRCA1 Mutation in a Young Breast Cancer Patient: A Case Report
Authors Scherz A , Stoll S, Rothlisberger B, Rabaglio M
Received 24 January 2023
Accepted for publication 13 April 2023
Published 11 May 2023 Volume 2023:16 Pages 83—87
DOI https://doi.org/10.2147/TACG.S405120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Amina Scherz,1 Susanna Stoll,2 Benno Rothlisberger,3 Manuela Rabaglio1
1Department of Medical Oncology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; 2Department of Medical Oncology, University Hospital and Stadtspital Triemli, Zurich, Switzerland; 3GENETICA AG, Zurich, Switzerland
Correspondence: Manuela Rabaglio, Email [email protected]
Background: BRCA1 and BRCA2 genes represent the most investigated breast and ovarian cancer predisposition genes. Ten cases of pathogenic de novo BRCA1 variations and six cases of pathogenic de novo BRCA2 variation have been reported at present. Here, we report a new case of a de novo BRCA1 gene mutation.
Case Presentation: A 30-year-old woman with no health issues and no family history for hereditary breast and ovarian cancer was diagnosed with a hormone receptor positive/HER2 negative invasive breast cancer. Genetic testing revealed a pathogenic variant in BRCA1 (c.4065_4068delTCAA) which was not found in her parents or sister.
Conclusion: We report a new case of de novo BRCA1 mutation, confirmed by repeated germline testing of the index patient and her parents. The published BRCA1/2 de novo mutation rate is low. This is probably due – in part – to the strict testing criteria.
Keywords: BRCA1 gene, breast cancer, de novo mutation, early onset, case report
Background
Hereditary cancer results from mutations in specific genes, such as those involved in regulating cell growth and DNA repair. Some of these mutations are inherited from one parent.1 De novo mutations are genetic alterations arising for the first time in a germ cell (ie, ovum or sperm) or in the fertilized egg itself during early embryogenesis. The majority of hereditary breast and ovarian cancer cases are due to pathogenic or likely pathogenic variants in the BRCA1/2 genes.2 Pathogenic germline variants in the BRCA1 or BRCA2 gene lead to an increased lifetime risk of breast, ovarian and further less frequently present cancers in women and an increased lifetime risk of breast, prostate and other tumors in men.
BRCA1 and BRCA2 genes were discovered in 1994 and 1995, respectively,3,4 and represent the most investigated breast and ovarian cancer predisposition genes. In recent years, numerous mutations have been described in these genes, of which very few cases involved de novo BRCA1/2 alterations. In fact, only ten cases of de novo BRCA1 mutations and six cases of de novo BRCA2 mutations have been reported at present.5–15 The BRCA1/2 de novo mutation rate was previously estimated to be 0.3% (0.1–0.7%). In a French study population of 12805 patients diagnosed with breast and/or ovarian cancer, 1527 were found to be BRCA1/2 mutated (12%), of whom three BRCA1 mutations and one BRCA2 mutation were de novo.6 This is in contrast with other genes, ie, TP53, NF1 and RB1.
We report a new case of a de novo BRCA1 gene mutation in a woman with an early onset breast cancer without a relevant family history.
Case Presentation
A 30-year-old woman with no health issues so far was diagnosed with a hormone receptor positive/HER2 negative invasive breast cancer. Her family history was not suggestive of a hereditary cancer syndrome (Figure 1). Only a cousin of her father had been diagnosed with a breast cancer at the age of 55 years. She underwent a neoadjuvant chemotherapy, followed by bilateral mastectomy and salpingo-ovarectomy. Regarding the early onset of her cancer, genetic testing was offered to the patient. It revealed a pathogenic variant (c.4065_4068delTCAA) and a variant of unknown significance (VUS) in the BRCA1 gene. Her healthy sister and her father were tested thereafter and both were found to be carriers of the VUS – but not the pathogenic variant – in the BRCA1 gene. In her mother neither the pathogenic BRCA1 mutation (c.4065_4068delTCAA) nor the described BRCA1 VUS was detected. The findings were confirmed by an analysis of additional blood samples taken from each parent and the patient. In the absence of a germline mutation in both parents, we assume a de novo origin of the mutation during parenteral germ cell gametogenesis. A non-paternity event could be excluded by the detection of the VUS in the BRCA1 gene, which was first reported in the patient and later in her father and sister. We concluded that our patient must be a carrier of a de novo BRCA1 mutation.
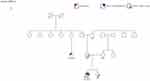 |
Figure 1 Family pedigree chart for case. |
Materials and Methods
DNA extraction from blood leucocytes was done followed by PCR amplification and DNA sequencing (Cancer Panel V2, Illumina, MiSeq-Tec (Illumina), Alignment: NextGene V2.4.1.1 (Softgenetics)). A pathogenic mutation (NM_007294.3:c.4065_4068delTCAA/p.(Asn1355Lysfs*10)) and a VUS (NM_007294.3:c.1868T>C)/p.Leu623Pro) were found in the BRCA1 gene. The detected pathogenic variant was already reported by Friedman et al.16 Recently, it has been also described by Rashid et al as founder mutation in breast cancer patients from Pakistan.17
This pathogenic variant NM_007294.3:c.4065_4068delTCAA/p.(Asn1355Lysfs*10) results in a frameshift of the reading frame and, presumably, a premature stop codon. Thus, this deletion leads to a shortened protein and/or degradation of the transcript by NMD (nonsense mediated mRNA decay).
PCR amplification and Sanger sequencing were performed in the healthy sister and parents.
Results and Discussion
Our patient with a pathogenic BRCA1 variant is a Swiss citizen without a migration background. Neither the mother nor the father or sister are carriers of the pathogenic BRCA mutation, providing additional evidence for the de novo occurrence of this variant.
A search in PubMed (Search Terms: BRCA/germline/mutation/de novo) yielded 13 publications reporting 16 de novo BRCA1/2 mutations.6–15,18–20,21 The largest published clinical trial – from Golmard et al – detected – on a series of 12805 consecutive unrelated patients diagnosed with breast and/or ovarian cancer who met the inclusion criteria for BRCA1/2 gene analysis according to French guidelines – a BRCA1 or BRCA2 variation in 1527 (12%) patients. A total of 801 (6.3%) BRCA1 and 726 (5.7%) BRCA2 mutation carriers were identified. In this study, de novo status was found for three BRCA1 mutations and one BRCA2 mutation, resulting in de novo mutation rates of 0.4% and 0.1% for BRCA1 and BRCA2 genes, respectively, and an overall BRCA1/2 de novo mutation rate of 0.3%.
de novo mutations are generally identified in sporadic cases in most genetic diseases. However, in the 2015 study by Golmard et al, cases with a family history of breast cancer significantly outnumbered, while in the other published cases, half were found to have a family history of breast cancer (Table 1). The authors attribute this observation to the high incidence of breast cancer in the normal population, bias for family cancer cases due to the strong impact of family history as a criterion for BRCA1/2 genetic testing, and genetic heterogeneity in breast cancer.
 |
Table 1 BRCA1 and BRCA2 de Novo Mutations Reported in the Literature and in This Case Report Updated and Revised from L Golmard et al 2015 |
Conclusion
We report a new case of de novo BRCA1 mutation, confirmed by repeated germline testing of the index patient and her parents. The published BRCA1/2 de novo mutation rate is low. This is probably due – in part – to the strict testing criteria, which are not uniform internationally and the restrictions regarding the cost coverage by the insurance companies. Most described de novo BRCA1/2 variations were found in patients with early-stage breast cancer and without a family history of the disease. Genetic testing of breast cancer patients based on their young age or family history does not allow detection of all BRCA1/2 carriers who may benefit from preventive interventions. In our opinion, we should focus on identifying as many carriers of a pathogenic BRCA variant as possible. Genetic testing based on selection criteria mainly considering family history, age of onset and tumor characteristics may underestimate the prevalence of de novo mutations. By detecting a larger number of de novo mutations, more patients could benefit from preventive interventions and tailored treatments. Genetic testing for a pathogenic variant in offspring of a carrier of a de novo variant is useful in identifying individuals at high risk for malignancies.
Abbreviations
BRCA1, BReast Cancer Gene 1; BRCA2, BReast Cancer Gene 2; NF1, Neurofibromatosis Gene 1; RB1, Retinoblastoma Gene 1; TP53, Tumor Protein P53 Gene; VUS, Variant of unclear significance; PCR, Polymerase chain reaction; DNA, Deoxyribonucleic acid; HER2, Human epidermal growth factor receptor 2.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Patient/Proband gave informed consent to tests as well as to use data/material for further anonymous use according to local and national guidelines.
Consent for Publication
Patient/Proband gave informed consent to tests as well as to use data/material for further anonymous use including publication according to local and national guidelines. Institutional approval was given (KEK – Kantonale Ethikkommission für die Forschung am Menschen, Bern, Switzerland).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work”.
Funding
No funding was received for this case report.
Disclosure
The authors declare no conflicts of interest.
References
1. Lynch HT, Watson P, Conway TA, Lynch JF. Clinical/genetic features in hereditary breast cancer. Breast Cancer Res Treat. 1990;15:63–71. doi:10.1007/BF01810778
2. Smith P, McGuffog L, Easton DF, et al. A genome wide linkage search for breast cancer susceptibility genes. Genes Chromosomes Cancer. 2006;45(7):646–655. doi:10.1002/gcc.20330
3. Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi:10.1126/science.7545954
4. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi:10.1038/378789a0
5. Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–822. doi:10.1093/jnci/djt095
6. Golmard L, Delnatte C, Laugé A, et al. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene. 2016;35(10):1324–1327. doi:10.1038/onc.2015.181
7. Tesoriero A, Andersen C, Southey M, et al. De novo BRCA1 mutation in a patient with breast cancer and an inherited BRCA2 mutation. Am J Hum Genet. 1999;65:567–569. doi:10.1086/302503
8. Edwards E, Yearwood C, Sillibourne J, Baralle D, Eccles D. Identification of a de novo BRCA1 mutation in a woman with early onset bilateral breast cancer. Fam Cancer. 2009;8:479–482. doi:10.1007/s10689-009-9270-8
9. Kwong A, Ng EK, Tang EY, et al. A novel de novo BRCA1 mutation in a Chinese woman with early onset breast cancer. Fam Cancer. 2011;10:233–237. doi:10.1007/s10689-011-9429-y
10. Garcia-Casado Z, Romero I, Fernandez-Serra A, et al. A de novo complete BRCA1 gene deletion identified in a Spanish woman with early bilateral breast cancer. BMC Med Genet. 2011;12:134. doi:10.1186/1471-2350-12-134
11. De Leeneer K, Coene I, Crombez B, et al. Prevalence of BRCA1/2 mutations in sporadic breast/ovarian cancer patients and identification of a novel de novo BRCA1 mutation in a patient diagnosed with late onset breast and ovarian cancer: implications for genetic testing. Breast Cancer Res Treat. 2012;132:87–95. doi:10.1007/s10549-011-1544-9
12. Delon I, Taylor A, Molenda A, et al. A germline mosaic BRCA1 exon deletion in a woman with bilateral basal-like breast cancer. Clin Genet. 2013;84:297–299. doi:10.1111/cge.12057
13. Van der Luijt RB, van Zon PH, Jansen RP, van der Sijs-Bos CJ, Wárlám-Rodenhuis CC, Ausems MG. De novo recurrent germline mutation of the BRCA2 gene in a patient with early onset breast cancer. J Med Genet. 2001;38:102–105. doi:10.1136/jmg.38.2.102
14. Robson M, Scheuer L, Nafa K, Ellis N, Offit K. Unique de novo mutation of BRCA2 in a woman with early onset breast cancer. J Med Genet. 2002;39:126–128. doi:10.1136/jmg.39.2.126
15. Hansen TV, Bisgaard ML, Jønson L, et al. Novel de novo BRCA2 mutation in a patient with a family history of breast cancer. BMC Med Genet. 2008;9:58. doi:10.1186/1471-2350-9-58
16. Friedman L, Ostermeyer E, Szabo C, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994;8:399–404. doi:10.1038/ng1294-399
17. Rashid M, Muhammad N, Naeemi H, et al. Chasing the origin of 23 recurrent BRCA1 mutations in Pakistani breast and ovarian cancer patients. Int J Cancer. 2022;151:402–411. doi:10.1002/ijc.34016
18. Marshall M, Solomon S, Lawrence Wickerham D. Case report: de novo BRCA2 gene mutation in a 35-year-old woman with breast cancer. Clin Genet. 2009;76:427–430. doi:10.1111/j.1399-0004.2009.01246.x
19. Diez O, Gutiérrez-Enríquez S, Mediano C, et al. A novel de novo BRCA2 mutation of paternal origin identified in a Spanish woman with early onset bilateral breast cancer. Breast Cancer Res Treat. 2010;121:221–225. doi:10.1007/s10549-009-0494-y
20. Bonaïti B, Alarcon F, Andrieu N, et al. A new scoring system in cancer genetics: application to criteria for BRCA1 and BRCA2 mutation screening. J Med Genet. 2014;51:114–121. doi:10.1136/jmedgenet-2013-101674
21. Van Minkelen R, van Bever Y, Kromosoeto JN, et al. A clinical and genetic overview of 18 years neurofibromatosis type 1 molecular diagnostics in the Netherlands. Clin Genet. 2014;85:318–327. doi:10.1111/cge.12187
22. Antonucci I, Provenzano M, Sorino L, Rodrigues M, Palka G, Stuppia L. A new case of “de novo” BRCA1 mutation in a patient with early-onset breast cancer. Clin Case Report. 2017;5(3):238–240. doi:10.1002/ccr3.718
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Pregnancy Associated Invasive Apocrine Carcinoma of the Breast: Case Report from Ethiopia
Alemu HK, Hammad N, Tola MA, Vanderpuye V
Breast Cancer: Targets and Therapy 2023, 15:429-433
Published Date: 23 June 2023
