Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Pregnancy Associated Invasive Apocrine Carcinoma of the Breast: Case Report from Ethiopia
Authors Alemu HK, Hammad N, Tola MA, Vanderpuye V
Received 4 February 2023
Accepted for publication 14 June 2023
Published 23 June 2023 Volume 2023:15 Pages 429—433
DOI https://doi.org/10.2147/BCTT.S405612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Haimanot Kasahun Alemu,1 Nazik Hammad,2 Mesfin Asefa Tola,3 Verna Vanderpuye4
1Department of Internal Medicine, Oncology Unit; Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 2Department of Medical Oncology, Queen’s University, Kingston, Ontario, Canada; 3Department of Pathology; Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 4National Centre for Radiotherapy, Oncology and Nuclear Medicine, Korle Bu Teaching Hospital, Accra, Ghana
Correspondence: Haimanot Kasahun Alemu, Clinical Oncologist, Saint Paul’s Hospital Millennium Medical College, Swaziland Street, PO Box: 13920/1271, Addis Ababa, Ethiopia, Tel +251 930594404, Email [email protected]
Abstract: We present a case report on a case of invasive apocrine carcinoma of breast during pregnancy at a tertiary referral hospital in Ethiopia. The patient’s case in this report signifies the challenging clinical situation that the patient, developing fetus and treating physicians have to go through and the need to improve maternal–fetal medicine and oncologic setup and treatment guidelines in Ethiopia. Our case also illustrates the huge disparity between the management of both breast cancer and its occurrence during pregnancy in low-income countries like Ethiopia and developed nations elsewhere. Our case report shows a rare histological finding. The patient has invasive apocrine carcinoma of the breast. To our knowledge, it is the first case to be reported in the country.
Keywords: breast cancer, pregnancy, apocrine carcinoma, disparity, case report
Background
Pregnancy Associated Breast Cancer (PABC) is defined as breast cancer diagnosed during pregnancy or in the first year of post-partum period with incidence of 1 per 3000 to 10,000 pregnant women.1 PABC is the second most common malignancy diagnosed during pregnancy following cervical cancer.1,2 We report the first case in Ethiopia of PABC with invasive apocrine carcinoma histology features. This case highlights the challenging clinical situation that the patient, fetus and treating physicians have to face both in the diagnosis and management and the need for the establishment of improved Maternal-Fetal Medicine (MFM), oncologic setup and treatment guidelines and, moreover, international collaborative efforts to reduce disparity and close the gap in cancer care.3
The patient in this case presents a rare type of apocrine carcinoma histology occurring in PABC. The clinical relevance of this pathology in PABC is yet unknown, therefore, further studies are needed.4
Patient and Case Report
A 30-year-old woman presented with a 4-cm left breast lump for three months. She has a 5-year-old son. She has five siblings, four brothers and a sister who died of breast cancer at the age of 33. Her parents are deceased of unknown causes. She has a history of using injectable contraceptive for 2 years without a history of smoking or alcohol intake. She is a school teacher with no health insurance coverage and struggles to support her treatment. The patient presented to a non-oncology public hospital and was investigated by breast ultrasound, an excisional breast biopsy, chest x-ray (CXR) and abdominal ultrasound. The biopsy revealed Invasive Apocrine Carcinoma (IAC) histology of tumor size less than 2 cm, originating from high-grade apocrine Ductal Carcinoma In Situ (DCIS) lesion with calcifications and fibrocystic changes without necrosis (Figures 1 and 2). Excisional margin is involved by high-grade DCIS, no lymphovascular invasion or perineural invasion seen.
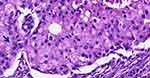 |
Figure 1 Photomicrograph showing tumor cells with abundant granular and eosinophilic cytoplasm, enlarged and pleomorphic nuclei with prominent nucleoli (Arrow head) (H&E 20X objective). |
 |
Figure 2 Low power photomicrograph showing variable histology mainly high grade DCIS with apocrine cribriform (circular box), and comedo (rectangular box) histology. (H&E 4X objective). |
She underwent modified radical mastectomy of the left breast which revealed multiple high-grade DCIS with negative margin, and no invasive carcinoma was noted which reflects its removal during prior excisional biopsy. Four lymph nodes were resected; all were negative to tumor deposit. Immunohistochemical analysis revealed Estrogen (ER) and Progesterone Receptor (PR) negative and Human Epidermal Growth Factor Receptor 2 (HER2) with Immunohistochemistry score 3+. Three weeks following the mastectomy, staging CT scan of the abdomen and CXR were done. The CT scan showed an incidental 1st trimester intrauterine viable pregnancy with an estimated gestational age (GA) of 5 weeks. Confirmed by urine HCG test.
After discussion with the patient, she terminated the pregnancy at GA of 9 weeks.
Discussion
Breast cancer (BC) is the most common cancer among Ethiopian women. Surprisingly, half of women diagnosed with BC are young with age less than 40 years.5,6 The median age of BC mortality is 37 years, reflecting a huge disparity between women diagnosed with BC here and high-income countries (HIC).7,8 In the USA, only 4% are under age 40 at presentation and median age at diagnosis and death from the disease is 62 and 68 years, respectively.8 Unfortunately, with such a degree of disparity, women with BC in Ethiopia live close to half the life span of women with the same disease in the USA.7,8 There is no national BC screening program, and due to various socioeconomic reasons, patients in Ethiopia present late in the disease course. There are significant human and material resource limitations in the management of BC which could contribute to poor oncologic outcomes.3,9
Breast cancer is the most common cancer occurring in 1 in 3000–10,000 pregnancies.2 The prevalence and management of PABC is unknown in Ethiopia.
Although recent studies show that stage-matched survival is similar between cases of PABC and non-PABC, because of larger tumor size and diagnoses delay at presentation, survival in PABC was shown to be significantly lower with cumulative survival of 59% compared to 79% in non-PABC cases.10
The management of PABC is complex, mostly attributed to the unique clinical challenges which treatment during pregnancy poses to developing fetus and the mother diagnosed with cancer. It also imposes significant challenges to treating physicians as one has to take into account the welfare of both the mother and fetus in all management decisions.11–13 If surgery is done as initial treatment and should there be radiation indication, we need to postpone it to avoid fetal toxicity.12,13 As a result, the appropriate time to administer radiation elapses and local recurrence risk increases.14 On the contrary, if we delay surgery, there is a risk of tumor growth and stage migration.12,13 Therefore, thorough discussion with the patient is recommended. Generally, surgery is considered safe, whereas radiotherapy should be delayed until post-delivery.12,13 Sentinel lymph node biopsy (SLB) in pregnancy is controversial, and if it has to be done, Technetium-99m colloid solution is preferred.13,15
Cytotoxic chemotherapy is associated with a high risk of malformation and miscarriage and should be avoided in 1st TM and is safe to administer during 2nd and 3rd TM of pregnancy. An Adriamycin-based strategy is the most well-studied regimen during pregnancy and is the preferred regimen.13,15 Tamoxifen and trastuzumab use during pregnancy are contraindicated due to fetal malformation and oligo-anhydramnios risks, respectively; their administration should be postponed until post-delivery.12,13,15
In addition to intrinsic clinical challenges in managing PABC cases, we faced even more difficulty managing our patient for several reasons related to resource-limited settings. We could not offer her optimal management recommendations. The pregnancy was wanted although unplanned, particularly with future infertility risk from chemotherapy.16 Baseline urine HCG was not done leading to accidental early TM fetal radiation exposure with abdominal CT scan and multiple unshielded CXR which could result in fetal malformation.17,18 Our patient has early-stage disease, and staging her disease with imaging modalities is not recommended. Even when staging is warranted, screening for pregnancy prior to CT scan is indicated in most pre-menopausal female cancer patients.15,18 This highlights the need for wise use of both medical resources and widely used guidelines in resource limited setting like ours. We do not practice SLB. The patient has only one child, and she could have been a candidate to keep her pregnancy and receive chemotherapy in 2nd and 3rd TM.12,13,15 However, our MFM lacks standard supportive care for both pregnant mother and developing fetus considering chemotherapy-associated adverse events in exposed infants.13,19,20 Furthermore, she was managed surgically at a non-oncology center and was not evaluated by a multidisciplinary team (MDT). Studies showed that MDT discussion led to management change in close to half of patients and it could have changed the course of our patient’s treatment with a better workup and management approach.21 She has unfavorable BC biology with HER2 enriched type, unfortunately, will not be treated with trastuzumab because of its unavailability in the country.22
After discussing with the patient the cons and pros of managing PABC at our setup, she terminated the pregnancy at GA of 9 weeks. We offered her chemotherapy with Adriamycin, Cyclophosphamide and Paclitaxel. Our patient wants to conceive, and we planned to put her on contraceptive at least 6 months post chemotherapy. Considering her age, 1st degree family history of BC and associated multifocal DCIS, we advise genetic testing; unfortunately, it is unavailable in the country. Genetic testing in Low- and Middle-Income Countries (LMIC) has complex issues with cultural, religious and social perspectives.23 Family values and disruption of beliefs, social stigmas associated with genetic disease, psychosocial consequences, religious and cultural values all pose barriers to a patient’s willingness to undergo genetic testing.23
The patient was diagnosed to have a rare type of apocrine carcinoma (AC) histology of the breast, infrequently seen in PABC.24 AC is characterized with apocrine differentiation of neoplastic cells and molecular expression of ER and PR negative, HER 2 overexpression and positive androgen receptor (AR).4 This is consistent with our patient’s histopathology examination although AR status is not determined due to resource-limited settings (Figures 1 and 2). Data are conflicting regarding the outcome of IAC of breast as compared to non-special-type ductal carcinoma. Some studies show that both have comparable oncologic outcome, while others showed IAC to be more aggressive with worse prognosis.25 Therefore, the definitive clinical relevance of such a histology is unknown, and future directions should explore individualized and tailored targeted approach.4,24,25
Conclusion
Given the high prevalence of BC in young women in Africa, PABC management protocol, improved MFM, optimal oncology centers with genetic counseling, testing, anticancer agents and MDT are warranted to direct better management and outcomes. This case illustrates the importance of patients’ education, workforce training and better referral system of cancer patients. Furthermore, our case highlights the huge disparity between the management of women with both BC and PABC in LMIC and HIC and the need for international collaboration to close the gap in cancer care. Future studies on the clinical relevance of invasive apocrine carcinoma of the breast and its management are recommended.
Patient Perspective
I wish I get all treatment available in the world that enables me give birth to my son’s sibling so that my son will not be alone if worst happens to me and if not, at least to live longer for my son who needs me most.
Patient Consent
Authors declare that written consent has been provided by the patient to have the case details and accompanying images published. Study participant informed consent included publication of anonymized responses. Institutional approval from the institution’s IRB has been provided to publish the case details.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Antonelli NM, Dotters DJ, Katz VL, Kuller JA. Cancer in pregnancy: a review of the literature. Part 1. Obstet Gynecol Surv. 1996;51(2):125–134. doi:10.1097/00006254-199602000-00022
2. Nicholas AP. Coexistence of pregnancy and malignancy. Oncologist. 2002;7(4):279–287. doi:10.1634/theoncologist.2002-0279
3. Taylor L, Harris C, Abebe T, Addissie A, Assefa M, Kantelhardt EJ. A decade of strengthening breast oncology in Ethiopia. Lancet. 2021;22:1219–1220.
4. Vranic S, Feldman R, Gatalica Z. Apocrine Carcinoma of the breast: a brief update on the molecular features and targetable biomarkers. BJBMS. 2017;17(1):9–11.
5. Memirie ST, Habtemariam MK, Asefa M, et al. Estimates of cancer incidence in Ethiopia in 2015 using population based registration data. JCO Glob Oncol. 2018;4:1–11.
6. Kantelhardt EJ, Mathewos A, Aynalem A, et al. The prevalence of estrogen receptor negative breast cancer in Ethiopia. BMC Cancer. 2014;14(1):895. doi:10.1186/1471-2407-14-895
7. Ayele W, Führer A, Braun GA, et al. Breast cancer morbidity and mortality in rural Ethiopia: data from 788 verbal autopsies. BMC Womens Health. 2022;22:89. doi:10.1186/s12905-022-01672-7
8. DeSantis CE, Ma J, Guadet MM, et al. Breast Cancer Statistics, 2019. CA. 2019;69:435–451. doi:10.3322/caac.21581
9. Tesfaw A, Alebachew W, Tiruneh M. why women with breast cancer present late to health care facility in North-West Ethiopia? A qualitative Study. PLoS One. 2020;15:e0243551. doi:10.1371/journal.pone.0243551
10. Ishida T, Yokoe T, Kasumi F, et al. Clinicopathologic Characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn J Cancer Res. 1992;83(11):1143. doi:10.1111/j.1349-7006.1992.tb02737.x
11. Jeanne A, Petrek MD. Breast cancer during pregnancy. Cancer. 1994;74(51):518–527. doi:10.1002/cncr.2820741341
12. Poggio F, Tagliamento M, Pirrone C, et al. Update on the management of breast cancer during pregnancy. Cancers. 2020;12(12):3616. doi:10.3390/cancers12123616
13. Amant F, Loibl S, Neven P, Calsteren KV. Malignancies in pregnancy 2: breast cancer in pregnancy. Lancet. 2012;379:570–579. doi:10.1016/S0140-6736(11)61092-1
14. Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21(3):555–563. doi:10.1200/JCO.2003.04.171
15. Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2013;24(6):160–170. doi:10.1093/annonc/mdt199
16. Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer study: a report from the childhood cancer survivor study. JNCI. 2006;98(13):890–896. doi:10.1093/jnci/djj243
17. Mccollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? RSNA. 2007;27(4):909–917.
18. Shetty MK. Abdominal computed tomography during pregnancy: a review indications and fetal radiation exposure issues. Semin Ultrasound CT MR. 2010;31:3–7. doi:10.1053/j.sult.2009.09.001
19. Giacalone PL, Laffargue F, Benos P. Chemotherapy for breast carcinoma during pregnancy: a French national survey. Cancer. 1999;86(11):2266. doi:10.1002/(SICI)1097-0142(19991201)86:11<2266::AID-CNCR14>3.0.CO;2-7
20. Zemelickis D, Lishner M, Dogendorfer P, et al. Maternal and Fetal outcome after in utero exposure to cancer chemotherapy. AMJ Obstet Gynecol. 1992;166(30):781. doi:10.1016/0002-9378(92)91334-7
21. Chang JH, Vines E, Bertsch H, et al. The impact of multidisciplinary breast cancer center on recommendations for patient management. Cancer. 2001;91(70):1231–1237. doi:10.1002/1097-0142(20010401)91:7<1231::AID-CNCR1123>3.0.CO;2-K
22. Gonzalez–Angulo AM, Litton JK, Broglio KR, et al. High Risk of recurrence for patients with breast cancer who have human epidermal growth factor 2-positive node negative tumors 1cm or smaller. JCO. 2009;34:5700–5706. doi:10.1200/JCO.2009.23.2025
23. Zhong A, Darren B, Loiseau B, et al. Ethical, Social and Cultural issues related to clinical genetic testing and counseling in low and middle-income countries: a systematic review. Genet Med. 2018;23:2270–2280.
24. Elledge RM, Ciocca DR, Langone G, McGuire WL. Estrogen receptor, progesterone receptor and HER-2/new protein in breast cancer from pregnant patients. Cancer. 1993;71(8):2499.
25. Dellapasqua S, Maisonneuve P, Viale G, et al. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin Breast Cancer. 2013;13(2):95–102.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
