Back to Journals » Cancer Management and Research » Volume 12
A Conceptual Model of an Oncology Information System
Authors Yazdanian A, Ayatollahi H , Nahvijou A
Received 29 April 2020
Accepted for publication 10 July 2020
Published 27 July 2020 Volume 2020:12 Pages 6341—6352
DOI https://doi.org/10.2147/CMAR.S259013
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Azadeh Yazdanian,1 Haleh Ayatollahi,2,3 Azin Nahvijou4
1Department of Medical Records and Health Information Technology, School of Allied Medical Sciences, Mazandaran University of Medical Sciences, Sari, Iran; 2Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran; 3Department of Health Information Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran; 4Cancer Research Center of Cancer Institute, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Haleh Ayatollahi Email [email protected]
Introduction: Oncologists are usually faced with a huge amount of diagnostic and therapeutic data in the process of cancer care. However, they do not have access to the integrated data. This research aimed to present a conceptual model of an oncology information system based on the users’ requirements.
Methods: This study was conducted in 2019 and composed of two phases. Initially, a questionnaire was designed, and clinical experts (n=34) were asked to identify the most important data elements and functional requirements in an oncology information system. In the second phase, conceptual, structural and behavioral diagrams of the system were drawn based on the results of the first phase. These diagrams were also reviewed and validated by five experts.
Results: Most of the data elements and all functional requirements were found important by the experts. The data elements were related to different phases of cancer care including screening, prevention, diagnosis, treatment, mental care and pain relief, and end-of-life care. Then, conceptual, structural and behavioral diagrams of the system were designed and approved by the experts or revised based on their comments.
Conclusion: The conceptual model and the diagrams presented in the current study can be used for developing an oncology information system. This system will be able to manage patients’ cancer data from screening to the end-of-life care. However, the system needs to be designed and implemented in a real healthcare setting to see how it can meet users’ requirements.
Keywords: oncology information system, hospital, neoplasm, functional requirements, cancer data
Introduction
Cancer is one of the most significant causes of mortality and disability across the world, especially in developing countries. In some cases, cancer occurs due to some changes in the individual’s genes, or due to the environment in which people live.1–3 Recently, the International Agency for Research on Cancer (IARC) reported a rise in global cancer burden. According to their report, 18.1 million new cases and 9.6 deaths occurred due to cancer in 2018. The most common types of cancer were lung, breast, and colorectal cancer.2 In 2015, about 360,000 mortalities occurred in Iran, among them, 14.9% were due to cancer. This figure shows that the number of deaths due to cancer has doubled from 1990 to 2015. Similar to the rest of the world, the common cancers were lung, colorectal, and breast cancer.4
With regard to the increasing number of patients, the large amount of clinical and non-clinical data of cancer patients and the necessity to optimize cancer information management, designing and implementing oncology information systems (OIS) seem quite necessary.5,6 This system can support the process of data integration in different stages, such as screening, prevention, diagnosis, treatment, palliative and end-of-life care. Moreover, the needed data will be available electronically to improve cancer care quality and to conduct clinical research.7 An oncology information system needs to be integrated with and interfaced to other hospital systems, such as hospital information systems (HIS) to harmonize processes, data, and reporting, improve patient safety, and facilitate clinical research. In this case, non-clinical data, such as financial data can be collected by the relevant subsystems of the HIS. This approach will prevent duplication of efforts or parallel investment in designing information systems.6
According to the literature, there have been numerous studies on the application of information systems in the field of oncology. For example, in 2014, Ando designed an oncology information system to record physicians’ instructions and to manage treatment plans and outcomes.8 In another study conducted by Evans et al, a geographical oncology information system was implemented in four medical care organizations. The results showed that the system success depended upon the commitment and active cooperation of top managers, designing the system based on the current clinical workflows, defining new workflows, and using appropriate technologies.9 Similarly, Urda et al developed an oncology information system in Spain which improved access to patient information. The availability of data analysis tools, the integration of the system with the clinical workflows, and user-friendliness were some of the benefits of the designed system.10 Other researchers, Hara and Ikushima highlighted the benefits of implementing a comprehensive oncology information system in Japan. These benefits included sharing patient information with other departments, electronic data entry, and reducing medical errors. They also noted that workflow reengineering helped physicians to have more time to care for patients and facilitated using the system.11
Prior to design a successful system, users’ requirements need to be identified and analyzed.10 Following this approach, designing models and diagrams based on the users’ requirements can help to represent them in a more clear way.12,13 The unified modeling language (UML) is one of the common tools for developing conceptual models and diagrams in a standard modelling language. In other words, it is a method for describing features and documenting the elements of a system in different diagrams. In the modeling process, all components are displayed as graphic figures to be conceptualized by all users.14
Currently, hospital information systems and paper-based records both are used to collect cancer data in Iran. However, different issues, such as data incompleteness, difficulty in getting access to the required data and challenges in sharing data between different departments15 have hindered effective use of cancer data. As a result, it seems that identifying users’ requirements and designing models and diagrams of an oncology information system can be a starting point to design this system in the near future to improve cancer care documentation. It is notable that users’ requirements of an oncology information system were previously identified in a qualitative study.16 However, in the current study, the researchers aimed to validate the results of the previous study in a bigger sample size by using a quantitative method.
Methods
This research was conducted in 2019 and composed of two phases. Initially, the clinical experts (oncologists, pathologists, radiotherapists, chemotherapy nurses) (n=34), who had at least 3 years of work experience in cancer care, took part in the study to identify the most important data elements and functional requirements in an oncology information system. The research settings were eight teaching hospitals across the country in which cancer patients were provided with different types of healthcare services.
In order to collect data, the Delphi Method was used in two rounds. The research instrument was a five-point Likert scale questionnaire, which was designed based on the literature review5–15 and the results of a previous qualitative study.16 In the first round of the Delphi study, the questionnaire consisted of three main sections including a) participants’ characteristics (five items), b) data elements required for cancer screening (18 items), prevention (16 items), diagnosis (19 items), treatment (40 items), mental care and pain relief (eight items), and end-of-life cancer care (five items), and c) functional requirements of an oncology information system (22 items). In the second round of the Delphi study, the questionnaire consisted of two main sections including a) participants’ characteristics (five items) and b) data elements required for the oncology information system (17 items). These 17 items did not reach a consensus in the first round of the Delphi study and were asked again in the second round. The content and face validity of the questionnaires were approved by three experts.
To analyze data resulted from the Delphi study, descriptive statistics (frequency, mean value, standard deviation, median, and interquartile range) were calculated for each of the questionnaire’s item by using SPSS version 21.0. If 75% of the participants rated an item 4 or 5, it showed that the consensus was reached. In addition to this, the mean value of (3.75) was regarded as a criteria to show the level of agreement and those items with a mean value of 3.75 or more were included in the final list of users’ requirements.
In a Delphi study, decision rules must be established to assemble and organize the judgments provided by Delphi subjects. As the Delphi study consists of a series of rounds and the rounds repeat with the goal of reducing the range of responses until “consensus” is achieved, we decided to set the mean value of (2.5) as a cut-off point to remove the items from the final list (agreement level of 50% or less). As a result, items with the mean values between 2.5 and 3.75 were entered into the second round of the Delphi study. The confidence interval was 95%.
In the second phase of the research, a conceptual model and the structural and behavioral diagrams (UML diagrams) including use case, activity, workflow and sequence diagrams were drawn by using Visual Paradigm software. Then, the model and diagrams along with a questionnaire which included 38 questions were presented to five experts (oncologist, radiotherapist, pathologist, chemotherapy nurse, and a software engineer). The questionnaire had three responses for each question (acceptable (2), relatively acceptable (1), and not acceptable (0)). The content and face validity of the questionnaire were confirmed by three experts. Descriptive statistics (frequency and percentage) were used for data analysis and the experts’ comments were applied to revise the conceptual model and the UML diagrams.
Results
In the first round of the Delphi study, 34 out of 40 clinicians (85%) including oncologists, pathologists, radiotherapists, chemotherapy nurses completed the questionnaire. Over half of the participants were male (n=20, 58.8%) and the highest frequency (n=15, 41.1%) was related to the age range of 40–49 years old. In terms of education, the highest frequency (n=11, 32.3%) was related to the bachelor's degree of nursing. About half of the participants were nurses (n=15, 44.1%), and the highest frequency of work experience (n=15, 44.1%) was between 11 and 15 years. In the second round of the Delphi study, a similar pattern with different figures was repeated. The participants’ characteristics in the first and second rounds of the Delphi study are presented in Table 1.
 |
Table 1 Participants’ Characteristics in the First and Second Rounds of the Delphi Study |
The experts were asked to identify the most important data elements and functional requirements of an oncology information system. The first part of the questionnaire was related to the data elements required for cancer screening (Table 2).
 |
Table 2 Distribution of the Participant’s Responses Regarding the Importance of the Data Elements Required for Cancer Screening |
As Table 2 shows, most of the data elements were found necessary for cancer screening. However, some data elements, such as being exposed to chemicals (3.23±0.3), exercising (3.55±1.13), nutritional status (3.70±1.03), and sonography (3.73±0.81) did not reach a consensus and they were transferred to the second round of the Delphi study. Among the data elements required for cancer screening, patient’s health status (4.85±0.35) and mammography (4.79±0.41) had the highest mean values.
Among the data elements required for cancer prevention (Table 3), body mass index (4.61±0.55) and patient history (4.55±0.66) had the highest mean values. However, data elements, such as exercising (3.5±1.18) and nutritional status (3.73±1.16) did not reach a consensus and were transferred to the second round of the Delphi study.
 |
Table 3 Distribution of the Participant’s Responses Regarding the Importance of the Data Elements Required for Cancer Prevention |
Regarding cancer diagnosis, most of the data elements, such as symptoms (4.50±0.56), results of clinical examinations (4.94±0.23), patient history (4.20±0.94), current medications (4.11±0.72) were found important (Appendix I). However, some data elements, such as drug allergies (3.14±1.15) and job-induced risk factors (3.67±1.22) did not reach a consensus and were transferred to the second round of the Delphi study. One item, i.e., “food allergies” (2.38±1.15) was removed from the final list as its mean value was lower than 2.5.
In terms of cancer treatment (Appendix I), most of the data elements, such as patients’ clinical history (4.29±0.87), medical consultation (4.20±0.68), suggested surgery (4.52±0.56), laboratory tests results during radiotherapy (4.14±0.89), tumor position (4.38±0.77), and other data elements were found important to be included in the system. Nevertheless, a number of data elements, such as patient consent (3.73±1.28), radiotherapy complications (3.73±1.05), body surface area (3.73±1.30), pharmacist’s approval (3.5±1.10), vital signs control (3.73±1.46), consciousness assessment (3.73±1.37), disease progress report (3.73±1.10), and discharge plan (3.58±1.39) did not reach a consensus and were asked again in the second round of the Delphi study.
Regarding mental care and pain relief (Appendix I), prescribed medications for mental care (4.05±0.77), training patients and their caregivers (4.20±0.64), prescribed medications for pain relief (4.44±0.89) and other data elements reached a consensus. However, training to improve nutritional status (3.73±1.13) was asked again in the second round of the Delphi study. Another part of the questionnaire was related to the data required for the end-of-life care (Appendix I) and included spiritual challenges of patients and their families (3.91±0.86), procedures to overcome these challenges (4.02±0.75), prescribed medications for pain relief (4.2±0.76), and training to improve patient’s nutritional status (3.94±0.95). These data elements along with all functional requirements of an oncology information system (Appendix I), such as obtaining an electronic patient consent for treatment (4.47±0.50), applying DICOM-RT standard in radiotherapy (4.20±0.72), treatment scheduling (3.91±1.05), and scanning medication bar codes (4.26±0.75) were found important.
In the second round of the Delphi study, all data elements required for an oncology information system, that did not reach a consensus in the previous round, were asked again, and finally, all of them were approved by the experts.
In the second phase of the research, the data elements and functional requirements found in the Delphi study along with the cancer care workflows reported in the previous qualitative study16 were used together to provide a conceptual model (Figure 1) and the structural and behavioral diagrams of the system by using Unified Modelling Language (UML). The UML diagrams included use case, activity, sequence, and workflow diagram. A sample of these diagrams is presented in Figures 2–5.
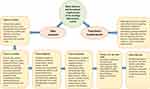 |
Figure 1 Data elements and functional requirements of an oncology information system. |
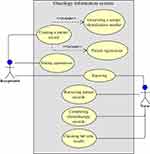 |
Figure 2 Use case diagram for the chemotherapy department. |
 |
Figure 3 Workflow diagram for the chemotherapy department. |
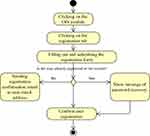 |
Figure 4 Activity diagram of user registration. |
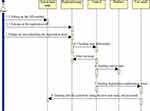 |
Figure 5 Sequence diagram of user registration. |
The UML diagrams were given to five experts to review and comment on them. Most of the participants were male (n=3, 60%), and their age range was between 40 and 49 years old. Moreover, the highest frequency (n=3, 60%) belonged to the work experience of 11 to 15 years. Most of the diagrams were approved by the participants. However, four diagrams including two use case diagrams and two workflow diagrams (chemotherapy and radiotherapy) required revisions. These diagrams were revised based on the experts’ comments.
Discussion
An oncology information system supports cancer care documentation by collecting data related to different stages of cancer care.7 These data assist clinicians, researchers, and healthcare organizations to work on different types of cancer more effectively. Moreover, cancer modelling, monitoring the disease and treatment plans, allocating resources for cancer care, and conducting clinical research can be facilitated by collecting data via oncology information systems.11,17
The usefulness of oncology information systems has been highlighted in different studies. For example, Yang showed that this system helped to improve quality of care and assisted the users to spend more time on the important tasks rather than spending time on the simple and repetitive tasks.18 Yu et al believed that the use of oncology information systems may lead to better cancer care management, reduces human errors, saves costs, and increases the quality of care.19 The results of the study carried out by Poulter et al showed that system users were satisfied with the system, as it helped with keeping information up-to-date, enhancing the quality of care, making better decisions, and reducing time to search for patient information.20
Given the importance and benefits of using oncology information systems, the current research focused on the data elements and functions necessary for developing this system. As the clinicians’ perspectives might be different due to their departmental requirements and workflow, clinicians in the oncology, pathology, radiotherapy, and chemotherapy departments were asked to take part in the study. Moreover, cancer care documentation includes different stages of screening, prevention, diagnosis, treatment, mental care, pain relief, and end-of-life care. Therefore, in the current research, data related to each stage were investigated.
In a similar study, Kabukye et al focused on the users’ requirements of an oncology electronic medical records. They found that data elements, such as demographic, clinical, diagnostic, and treatment data (e.g., cancer stage, tumor size, and chemotherapy progress), and functions, such as using a clinical decision support system to prescribe correct dosage of chemotherapy drugs and electronic scheduling for care plans were necessary to be included in the system.21 In another study, the use of DICOM-RT standard (an extension of digital imaging and communications in medicine (DICOM) standard used in radiotherapy), radiotherapy treatment plan, radiation dosage, CT scan images, treatment documents, and a summary of treatment documents were suggested to be included in the system.22
In the report published by the Canadian Institute for Health Information in 2018, providing a minimum dataset of diagnostic, topographic, and morphological cancer data, as well as progress report and initial date of diagnosis were recommended.23 Similarly, other research studies highlighted the radiotherapy18,24-27 and chemotherapy data required to be included in an oncology information system.3,25,28 It is notable that although there is a variety of data elements that can be considered in an oncology information system, users’ requirements might be different in each setting and need to be investigated to prevent designing a system which is not able to meet their needs.
The research findings also showed that similar to the numerous data elements that can be considered in an oncology information system, a number of functions needs to be taken into account to facilitate users’ daily tasks. For example, presenting and reporting statistical analyses, controlling data confidentiality, sharing information with other clinical systems, using DICOM-RT in radiotherapy, scanning barcodes of medications and patient wristbands, and treatment scheduling are some of the functions recommended to be included in an oncology information system.10,12,29,30
Liu and Wen used a drug injection system in a chemotherapy department which scanned the barcodes of medications and patients’ wristbands to check the correctness of five criteria (patient, medication, dosage, method of injection, and time) and the users were satisfied with the system efficiency.31 After determining data elements and functional requirements of an oncology information system, like other information system, designing a conceptual model and UML diagrams is another step towards developing an actual system.32 In the current study, a conceptual model and the UML diagrams including use case, activity, sequence, and workflow diagrams were designed based on the results derived from the Delphi study and the previous qualitative study.16
In a similar study, Shiki et al presented a conceptual model of a hospital-based oncology information system. The conceptual model of this system included use case, activity, and class diagrams.33 In another study, Lyalin and Williams presented the activity diagrams to enhance the workflow of the admission process.34 Therefore, with regard to the fact that the conceptual models help with designing information systems, it is expected that the conceptual model and the UML diagrams presented in the current study can facilitate the process of system design in the future.
Research Limitations
This research had some limitations. First of all, although clinicians from eight teaching hospitals took part in the study, the number of the participants in the first and second rounds of the Delphi study was limited. However, as there is no well-defined rule for selecting a specific number of the participants in a Delphi study and representation is assessed by the quality of the expert panel rather than its number, we can conclude that the participants were well-experienced clinicians in cancer care and the results might be generalized to a bigger sample size. Moreover, the logical and physical data models of the system were not designed in this study. The logical data model deals with the structure of the data elements and the relationships between them, and the physical data model describes the database-specific implementation of the data model. As it is expected to continue this research in the near future and develop an oncology information system, designing the logical and physical data models of the system will be part of the future projects.
Another issue might be related to the level of details associated with each data element. Although we reached a large number of data elements necessary for designing an oncology information system, it was not possible to include all of them in the questionnaire. Therefore, we decided to focus on the main items. Moreover, the current study aimed to present a conceptual model of an oncology information system. Therefore, more details about other data elements, which might not be mentioned in this study, should be investigated before or during designing a real system.
Conclusion
The process of cancer care consists of screening, prevention, diagnosis, treatment, mental care and pain relief, and end-of-life care. To be able to manage these processes effectively, reliable and up-to-date data can be available by using oncology information systems. In the present study, data elements and functional requirements of an oncology information system were identified from the clinicians’ perspectives. The results showed that the data elements varied in different stages of cancer care and different departments. Regarding the system functions, statistical analyses and the use of clinical decision support systems for determining medication or radiation dosage were found important. A conceptual model and the UML diagrams designed in the current study can facilitate designing the actual system. However, this system needs to be implemented and used in practice to see how it can meet users’ requirements.
Acknowledgment
This study was funded and supported by Iran University of Medical Sciences, Tehran, Iran (IUMS/SHMIS_94-36).
Disclosure
The authors declare that they have no competing interests.
References
1. Amereh F, Jahangiri-rad M, Mazloomi S, Rafiee M. The role of environmental and lifestyle factors in the incidence and prevalence of cancer. J Environ Health Eng. 2016;4(1):30–42.
2. Brey F, Ferlay J, Soerjomataram I, Siegel R, Torre I, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi:10.3322/canjclin.55.2.74
4. Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. doi:10.1002/ijc.29670
5. Rouhollahi MR, Mohagheghi MA, Mohamadrezaie N, et al. Situation analysis of the national comprehensive cancer control program in the IR of Iran; assessment and recommendations based on the IAEA imPACT mission. Arch Iran Med. 2014;17(4):222–231.
6. Yazdanian A, Ayatollahi H, Nahvijou A. A review of oncology clinical information systems- what are the critical success factors and reasons for system failure? J Evol Med Dent Sci. 2018;7(47):5118–5127. doi:10.14260/jemds/2018/1137
7. Kukreti V, Manzan A, Habib S, et al. Ontario Oncology Information System Standards: Defining Its Meaningful Use. Toronto: Cancer Care Ontario; 2014.
8. Ando Y. Oncology Information System. In: Hirohiko T, Tadashi K, Toshiyuki S, Koji N, Hiroshi T, Kumiko K, editors. Carbon-Ion Radiotherapy: Principles, Practices, and Treatment Planning. Tokyo: Springer; 2014:110–117.
9. Evans WK, Ashbury FD, Hogue GL, Smithe A, Pun J. Implementing a regional oncology information system: approach and lessons learned. J Curr Oncol. 2014;21(5):224–233. doi:10.3747/co.21.1923
10. Urda D, Ribelles N, Subirats JL, Franco L, Alba E, Jerez JM. Addressing critical issues in the development of an oncology information system. Int J Med Inform. 2013;82(5):398–407. doi:10.1016/j.ijmedinf.2012.08.001
11. Hara Y, Ikushima H. Implementing a Comprehensive Oncology Information System in a Multi-Vendor Environment at Tokushima University Hospital [Pamphlet]. Stockholm: ELEKTA; 2012.
12. Kahouei M, Eskrootchi R, Ebadi Fard AF. Conceptual model designing of clinical staff’s information needs of emergency information system. Payavard Salamat. 2013;7(3):217–227.
13. Booch G, Rumbaugh H, Jacobson J. The Unified Modelling Language Reference Manual. Delhi: Pearson Higher Education; 2004.
14. Zendehdel K, Sedighi Z, Hassanloo H, Nahvijou A. Improving quality of cancer registration in Iran. Part1: evaluation and comparison of cancer registration results in the country. Hakim Res J. 2010;12(4):42–49.
15. Mohammadi G, Akbari ME, Mehrabi Y, Motlagh AG. Quality assessment of the national cancer registry in Iran: completeness and validity. Iran J Cancer Prev. 2016;9(6):e8479.
16. Yazdanian A, Ayatollahi H, Nahvijou A. Oncology information system: a qualitative study of users’ requirements. Asian Pac J Cancer Prev. 2019;20(10):3085–3091. doi:10.31557/APJCP.2019.20.10.3085
17. Blayney DW, McNiff K, Hanauer D, Miela G, Markstrom D, Neuss M. Implementation of the quality oncology practice initiative at a university comprehensive cancer center. Int J Clin Oncol. 2009;27(23):3802–3807. doi:10.1200/JCO.2008.21.6770
18. Yang OY A Case Study to Investigate the Feasibility of Supporting Radiotherapy Workflows Through the Use of Mobile Devices [M.Sc. Thesis]. Stockholm: Karolinska institute; 2016.
19. Yu P, Gandhidasan S, Miller A. Different usage of the same oncology information system in two hospitals in Sydney—lessons go beyond the initial introduction. Int J Med Inform. 2010;79(6):422–429. doi:10.1016/j.ijmedinf.2010.03.003
20. Poulter T, Gannon B, Bath P. An analysis of electronic document management in oncology care. Health Informatics J. 2012;18(2):135–146. doi:10.1177/1460458211435514
21. Kabukye J User Requirements for an Electronic Medical Records System for a Cancer Hospital in Uganda [M.Sc. Thesis]. Stockholm: Stockholm University; 2016.
22. Oliveira CM, Rodrigues P. The relevance of DICOM-RT in radiotherapy information systems.
23. Pan-Canadian oncology drug data minimum data set [Internet]. 2018. [Cited 10 June 2020]. Ontario: Canadian Institute for Health Information. Available from: https://secure.cihi.ca/estore/productSeries.htm?pc=PCC1715.
24. Levy MA, Giuse DA, Eck C, Holder G, Lippard G, Cartwright J. Integrated information systems for electronic chemotherapy medication administration. J Oncol Pract. 2011;7(4):226–230. doi:10.1200/JOP.2011.000259
25. Mukai M, Ando Y, Yokooka Y, et al. Development of clinical database system specialized for heavy particle therapy. Stud Health Technol Inform. 2015;216:933.
26. Estathiou JA, Nassf D, Mcnutt T, et al. Practice-based evidence to evidence-based practice: building the national radiation oncology registry. J Oncol Pract. 2013;9(3):e90–e95. doi:10.1200/JOP.2013.001003
27. Yang D, Wu Y, Brame RS, et al. Technical note: electronic chart checks in a paperless radiation therapy clinic. Med Phys. 2012;39(8):726–732. doi:10.1118/1.4736825
28. Pirnejad H, Niazkhani Z, Aarts J, Bal R. What makes an information system more preferable for clinicians? A qualitative comparison of two systems. Stud Health Technol Inform. 2011;169:392–396.
29. Montouchet C, Thomas M, Anderson J, Fostor S. Report on oncology health data in Europe [Internet]. 2018. [Cited 20 May 2020]. Available from: https://www.efpia.eu/media/412128/efpia-oncology-data-report.pdf.
30. Brack C Data management best practices in radiation oncology [Internet]. 2010. [Cited 5 June 2020]. Available from: https://www.aapm.org/meetings/amos2/pdf/49-14581-70649-324.pdf.
31. Liu Y, Wen P. Establishment and evaluation of information system for chemotherapy care. Int J Med Health Sci. 2014;8(5):226–229.
32. Lau E, Mowat F, Kelsh M, et al. Use of electronic medical records (EMR) for oncology outcomes research: assessing the comparability of EMR information to patient registry and health claims data. J Clin Epidemiol. 2011;3(3):259–272.
33. Shiki N, Ohno Y, Murata T, Matsumara Y. Unified modelling language for hospital-based cancer registration process. Asian Pac J Cancer Prev. 2008;9(4):789–796.
34. Lyalin D, Williams W. Modelling cancer registration processes with an enhanced activity diagram. Methods Inf Med. 2005;44(1):3–11.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
