Back to Journals » Cancer Management and Research » Volume 11
YAP/TAZ: a promising target for squamous cell carcinoma treatment
Authors Dong X, Meng L , Liu P, Ji R , Su X, Xin Y , Jiang X
Received 12 December 2018
Accepted for publication 4 June 2019
Published 8 July 2019 Volume 2019:11 Pages 6245—6252
DOI https://doi.org/10.2147/CMAR.S197921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Xiaoming Dong,1,2 Lingbin Meng,3 Pinyi Liu,2 Rui Ji,4 Xuling Su,2 Ying Xin,2 Xin Jiang1
1Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, People’s Republic of China; 2Key Laboratory of Pathobiology, Ministry of Education, Jilin University, Changchun 130021, People’s Republic of China; 3Department of Internal Medicine, Florida Hospital, Orlando, FL 32804, USA; 4Department of Biology, Valencia College, Orlando, FL 32804, USA
Abstract: Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are two homologous transcriptional coactivators and the final effectors of the Hippo signaling transduction pathway. The transcriptional activity of YAP/TAZ is dependent on their recruitment to the nucleus, which promotes binding to the transcription factor of TEA domain family members 1–4 (TEAD1-4). In Hippo-signaling pathway, YAP/TAZ is inactivated and its translocation to the nucleus is blocked via a core kinase cascade stimulated by a variety of upstream signals, such as G-protein-coupled receptor signaling, mechanical pressure, and adherens junction signaling. This pathway plays a very important role in regulating organ size, tissue homeostasis, and tumor development. In recent years, many studies have reported upregulation or nuclear localization of YAP/TAZ in a number of human malignancies, such as breast cancer, melanoma, lung cancer, especially squamous cell carcinoma in different organs. A large number of experiments demonstrate that YAP/TAZ activation promotes cancer formation, progression, and metastasis. Therefore, in this review, we summarize the evidence of regulation and function of YAP/TAZ and discuss its role in squamous cell carcinoma. Collectively, this summary strongly suggests that targeting aberrant YAP/TAZ activation is a promising strategy for the suppression of squamous cell carcinoma.
Keywords: Hippo pathway, YAP/TAZ, squamous cell carcinoma
Introduction
The Hippo pathway was first discovered in Drosophila during the 1990s. This signal pathway is highly conserved. It regulates organ size and maintains homeostasis during cell proliferation and apoptosis.1,2 As the major downstream effectors of the Hippo pathway, YAP and its paralog TAZ3 are negatively regulated by the Hippo pathway, owing to their phosphorylation2 and their inability to enter the nucleus.4 Over the past two decades, a number of studies uncovered four proteins including Wart (Wts),5 scaffold protein Salvador (Sav),6,7 Hippo (Hpo),1,8–10 and Mats,11 as the central part of the Drosophila Hippo pathway. Based on this, Yorkie (Yki), a transcriptional co-activator acting as a Wts-binding partner, was discovered in 2005.2 In Drosophila, the core kinase Hippo binds and phosphorylates Salvador, then the downstream kinases Warts and Mats, which promote the phosphorylation of Yki and localize it in the cytoplasm, inhibit transcription of the target genes.10 On the contrary, unphosphorylated Yki is transferred to the nucleus and binds with scalloped (Sd),12,13 which induces transcription of the downstream genes, including cell growth and apoptosis-regulating genes such as cyclin E, cell-death inhibitor Diap1, and dmyc.8,14 The Yki homologous protein in mammals is the transcriptionally active YAP identified in 1994.15 TAZ, YAP’s paralog was identified in 2000 as a protein that binds with 14-3-3 protein.3 Since the discovery of YAP/TAZ, they have emerged as the critical regulators of early embryonic development and adult organs by driving the transcription of genes that promote cell proliferation, cell survival, and stem cell maintenance.16,17 Therefore, low activity of YAP/TAZ can lead to developmental defects, defective repair, and organ atrophy. On the contrary, aberrantly high activity of YAP/TAZ promotes cell overgrowth and tumor formation. In this review, we will provide an overview of the Hippo pathway network, the functions of YAP/TAZ, and its regulation. We will also discuss the roles of YAP/TAZ on squamous cell carcinoma.
The Hippo pathway in mammals
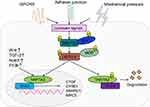
Similar to the Drosophila Hippo pathway, the mammalian Hippo pathway is also considered as a tumor suppressor pathway, regulated by a phosphorylation-dependent protein kinase cascade. In Drosophila, the kinase cascade mainly includes mammalian STE20-like protein kinase (MST1/2), large tumor suppressor 1/2 (LATS1/2), human Salvador homology1 (SAV1), and MOB kinase activator (MOB), which are homologous to Hpo, Wts, Sav, and Mats, respectively.1 The kinase chain can control the activity of the downstream effecter YAP/TAZ (Figure 1). Activated MST1/2 binds to the regulatory protein SAV1, which promotes the phosphorylation of LATS1/2 and MOB, and subsequently, phosphorylation of YAP/TAZ at serine residue-127.18,19 This phosphorylation-dependent process leads to the cytoplasmic retention of YAP/TAZvia 14-3-3 protein interaction,7,20–25 and its ubiquitination and degradation (Figure 1).26 Upon inactivation of the Hippo pathway, dephosphorylated YAP/TAZ translocates to the nucleus and binds to the transcription factor TEAD to induce expression of the genes involved in cell proliferation, anti-apoptosis, and epithelial–mesenchymal cell transformation (Figure 1).27–29 Recent studies by Lamar et al showed that the binding to TEAD family is an important step for YAP to perform its functions.30 Mutation of key sites associated with TEAD or YAP binding domains will inhibit YAP-induced gene expression and its functions.30 In addition to binding TEAD, YAP/TAZ can also bind to other transcription factors, such as Smad, RUNT-related transcription factors (Runx1/2), P63/P73, erythroblastic oncogene B4 (ErbB4),31 T-box transcription factor5 (TBX5),32 and Pax3.33,34 Finally, it participates in cell proliferation, differentiation, and apoptosis. Another study found that various cytokines including connective tissue growth factor (CTGF), cysteine-richangiogenic inducer 61 (CYR61), ankyrin Repeat Domain 1 (ANKRD1), baculoviral IAP repeat-containing protein 5 (BIRC5),18 brain-derived neurotrophic factor, and fibroblast growth factor1 worked as downstream substrates for YAP stimulation. CTGF acts as a direct target gene for YAP, promoting cell proliferation and anchorage for independent growth.35 Recently, Kim et al indicated that YAP/TAZ also functioned as a transcriptional co-repressor to promote cell survival by repressing DNA damage-inducible transcript 4 (DDIT4) and TNF-related apoptosis-inducing ligand (Trail) mediated by histone deacetylase (NuRD) complex.36
The Hippo pathway observed in mammals is mainly regulated by multiple upstream signal input factors, such as WW, C2 domain-containing protein 1 (KIBRA/WWC1), neurofibromin2 (Nf2), and FERM domain-containing protein 6 (willin/FRMD6).37 Recently, G protein-coupled receptor (GPCR) signaling was found to act as an upstream signal of the Hippo pathway. It was shown that stimulation of G12/13-coupled receptors or Gs-coupled receptors could inhibit or activate Lats1/2, thereby activating or inhibiting YAP/TAZ to influence cell proliferation and migration.38
YAP/TAZ and its regulation
The structure of YAP/TAZ
YAP is a transcriptional coactivator of the Hippo pathway and promotes gene expression by enhancing the activity of transcription factors. It has two subtypes, YAP1 and YAP2. YAP contains multiple domains and specific amino acid sequences, including a TEAD-binding region (formed by the 94th serine,S94), two WW domains (consist of amino acids 172–204 and 231–263), an N-terminal proline-rich domain, a C-terminal PDZ-binding motif, an SH3-binding motif, a coiled-coil domain and a transcription activation domain (Figure 2). TAZ is homologous to YAP and has similar domains and functions as YAP.3 However, it lacks a proline-rich domain, a second WW domain and an SH3-binding motif (Figure 2). A previous study demonstrated that the WW domain specifically recognizes the PpxY (P is proline, x is any amino acid and y is tyrosine) motifs, thereby controlling the localization and activity of YAP/TAZ.39 The C-terminal of YAP/TAZ can accurately identify the PDZ domain, which is composed of 80–90 amino acids and is present in many proteins.40
The effect of YAP/TAZ on cell proliferation and apoptosis
Previous studies have suggested that YAP/TAZ is an oncoprotein resulting in cell proliferation. In Drosophila, overexpression of yki (YAP homologue in Drosophila) leads to overgrowth of the third instar wing discs.2 Researchers have established that mice that overexpresses YAP had a higher mitogenic activity in keratinocytes and thicker basal layer, superstratum, and stratum corneum present on the epidermis than those in wild type mice. On the contrary, mutant YAP gene knockout mice showed marked epidermis shrinkage and delayed wound healing.41 When the skin was wounded, YAP protein level in the keratinocyte increased and subsequently induced cell proliferation.41 Another study showed that the proliferation was inhibited in lung cancer cell line A549 transfected with MST1 gene, which was due to high level of YAP phosphorylation.42 It was also observed that in liver injury caused by cholestasis, high expression of YAP promoted the proliferation of hepatocytes and bile duct epithelial cells, and resulted in the lesser of liver damage.43 Conditional knockout of YAP/TAZ gene leads to inhibition of hepatocyte regeneration and liver necrosis.43 Interestingly, high concentrations of bile acid can act as upstream activators of the Hippo pathway, activate YAP transcriptional activity, and stimulate hepatocyte proliferation and tumorigenesis.44
On the contrary, YAP/TAZ has an inhibitory effect on apoptosis. In Drosophila, Yki protein inhibits cell apoptosis by reducing the activity of apoptosis-related gene reaper (rpr), the binding of P53 with the apoptosis-stimulating protein of P53-1 (ASPP1), and activation of head involution defective (hid).45 A previous study also confirmed the anti-apoptosis effect of YAP/TAZ in carcinoma cells, showing that the nuclei of hepatocellular carcinoma and intrahepatic cholangiocarcinoma cellsare rich in YAP and apoptotic protein inhibitor, survivin, whose transcription is dependent on YAP/TAZ.46
The signaling pathways of YAP/TAZ
YAP/TAZ in the Hippo pathway can play a role in cell proliferation and programmed death by regulating or being regulated by various signaling pathways. YAP/TAZ is closely related to the Wnt/β-catenin,47 TGF-β/SMAD,48 Notch49,50 and P13/AKT pathway.51,52
Barry et al found that YAP restricted nuclear translocation of disheveled (DVL) and the regeneration of intestinal cells.47 This inhibition was caused by DVL-mediated inactivation of the Wnt signaling pathway.47 In a study based on tumorigenesis and development, it was found that Smad3/YAP/TEAD/p300 could form complexes to increase the expression of CTGF, promote proliferation of malignant stromal cells, and secretion of extracellular matrix.48 YAP/TAZ can also interact with the Notch signaling pathway to regulate intestinal homeostasis. Under normal conditions, only Hes-1, a target gene of the Notch signaling pathway, was expressed in the intestinal crypts.49 Activation of YAP and the expression of Hes-1 was widened and distributed in all chorio-epithelium, which suggested that YAP-mediated expansion of intestinal progenitor cells was at least partially dependent on the activation of the Notch signal.49,50 PI3K/AKT pathway can cooperate with the Hippo/YAP signaling pathway to regulate cell proliferation and programmed cell death.51 It was reported that Pik3cb, one of the genes encoding PI3k, could be directly activated by YAP/TAZ through TEAD to promote PI3K transcription and AKT activation.52 This mechanism has also been confirmed in rat cardiomyocytes as YAP-mediated PI3K/AKT activation promotes its proliferation and reduces programed cell death.52
The roles of YAP/TAZ in squamous cell carcinoma
As a downstream function of the Hippo pathway, YAP/TAZ can induce the activation of target genes related to cancer cell proliferation, invasion, and metastasis. Therefore, YAP/TAZ plays an oncogene role in a number of malignant tumors, such as lung,53 liver,54 gynecological,55,56 gastrointestinal,57,58 and skin malignant tumors.59 Among skin malignancies, squamous cell carcinoma (SCC), basal cell carcinoma (BBC), and melanoma are the most common forms. Studies show that in both human and mice BBC, YAP and TAZ were nuclear and highly expressed.59,60 Meanwhile, the conditional deletion of YAP and TAZ in mouse models of BCC prevented tumor formation.59 YAP signaling also accelerated BBC development via the c-JUN/AP1 axis,60 the positive regulatory interactions with Hedgehog/GLI2 signaling,61 and its downstream of cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2).62 For melanoma, recent papers described high expression of TAZ/YAP promoted its progression associated with stimulation of low-density lipoprotein receptor-related protein 1 (LRP1) and affects the postoperative survival of patients.63,64 Emerging evidence also suggested that YAP directly mediated evasion of cytotoxic T-cell immune responses in BRAFi-resistant melanoma cells by upregulating PD-L1, and targeting of YAP-mediated immune evasion may improve prognosis of melanoma patients.65 More and more evidences show that squamous cell carcinoma (SCC) that occurs in different organs covered with squamous epithelium not only restrict to skin, was initiated and determined by YAP/TAZ. Here, we will focus on SCCs and summarize the effect of YAP/TAZ on tumorigenesis and progression of several common SCCs.
Cutaneous squamous cell carcinoma
The homozygous mouse mutant of the gene encoding 14-3-3σ displays severe skin defects such as thick epidermis, high proliferation of basal and spinous layers, and lack of granular and stratum corneum, accompanied with enhanced YAP nuclear localization in epidermal cells.66 This study demonstrated that YAP/TAZ as a downstream molecule of 14-3-3σ, blocked its binding with1 4-3-3 isoforms and failed to sequester in the cytoplasm due to the mutant, 14-3-3σ.66 Accumulation of YAP/TAZ in the nucleus causes continued progenitor expansion, inhibition of differentiation in the epidermis, and formation of cutaneous squamous cell carcinoma (CSCC).66 It was observed that YAP/TAZ is highly expressed in both mouse and human CSCCs and shows elevated expression in wounded skin.67 When YAP/TAZ was knocked out, proliferation of cells as marked by positive of Ki67 is reduced.67
YAP/TAZ can activate the EGFR/RAS signaling pathway by inducing the expression of amphiregulin (AREG) during transcription, thus activating its downstream P13K/AKT and ERK pathways.68 The two latter pathways are involved in the regulation of cell growth, survival, and migration on CSCC.68 The S100A7 protein is highly expressed in psoriasis, differentiated SCC, and keratoacanthoma lesions.69 In the SCC cell line A431, YAP can inhibit the expression of S100A7 protein by binding to TEAD1. Although YAP has lesser protein expression compared to S100A7, researchers believe that both the proteins can promote cell proliferation and inhibit cell differentiation. Moreover, S100A7 protein may be a substitute for YAP to maintain cell survival.69,70
Esophageal squamous cell carcinoma
Esophageal cancer is one of the most common gastrointestinal cancers.71 The pathological type of esophageal cancer is mainly esophageal squamous cell carcinoma (ESCC).72 Muramatsu et al found that the effect of YAP in ESCC depended on its expression level and depletion of YAP inhibited cell proliferation, which was accompanied with an increase in the transcription of CDKN1A/p21, and a decrease in the transcription of BIRC5/survivin.73 Clinical observation showed that nuclear accumulation or overexpression of YAP was associated with poor prognosis for patients with ESCCs.73 Another clinical study in ESCC 142 cases and 29 normal esophageal tissue cases found that the location of YAP in nuclear was much more in ESCC than in normal esophageal tissue.74 Moreover, univariate analyses showed that enrichment of nuclear YAP was associated with tumor diameter, Ki67 expression, histological grade (1–2 versus 3), pathological T category (1 versus 2–4), and pathological TNM stage (stage 1 versus 2–4) in patients with ESCC, indicating that nuclear accumulation of YAP/TAZ is correlated with the patients’ survival rate and prognosis.74
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is the most common of head and neck malignancy. Due to its anatomical location and the adjacent relationship, all aspects of tumor treatment are limited, which seriously affects the life quality of patients.75 Evaluation of YAP expression by immunohistochemical tissue chip technology showed that YAP expression in HNSCC was significantly higher than in benign tissue and precancerous lesions and the poorer differentiation of tumor was along with the higher of YAP expression.75 A recent study found that an ACTL6A/P63 complex suppressed WWC1 to activate YAP/TAZ and promote tumorigenesis in HNSCC. It was also observed that ACTL6A and an activated YAP/TAZ pathway conferred poor prognosis in primary HNSCC.76 An experiment by Wang et al showed that the level of nuclear YAP/TAZ in fibroblasts associated with perineural invasion was higher than those in the stroma of normal mucosa, suggesting that the transcription programs mediated by YAP/TAZ in the fibroblasts may contribute to perineural invasion in HNSCC.77 Interestingly, a recent study reported that upregulation of a circular RNA, circPVT1 together with TP53 mutation resulted in increased proliferation of HNSCC cell lines through the formation of mut-p53/YAP/TEAD complex.78
Oral squamous cell carcinoma
Genomic analysis showed that YAP/TAZ was amplified and overexpressed in oral squamous cell carcinoma (OSCC).79 Hiemer et al demonstrated that nuclear accumulation of YAP/TAZ promoted OSCC cell proliferation, survival, and migration in vitro and was required for the growth and metastasis of OSCCin vivo.80 Recent research showed the expression level of YAP was higher in OSCC tissues than that in adjacent normal tissues and YAP could drive the transcription of bcl-2 and c-myc genes subsequently leading to OSCC cell proliferation and resistance to apoptosis.81 The expression of TAZ was significantly higher in tongue oral squamous cell carcinoma (TSCC) cells and specimens than those in non-cancerous cells and normal tongue mucosa.82 Increase of TAZ expression inTSCC was significantly associated with tumor size, pathological grade, clinical stage, Ki-67 expression, reduced overall, and disease-free survival.82
Cervical squamous cell carcinoma
Cervical cancer is one of the most common malignancies in the female reproductive system. It is well known that the most common histological type of cervical cancer is squamous cell carcinoma, and its onset is a continuous developmental process from cervical squamous intraepithelial neoplasia to invasive cancer.83 Liu et al reported that compared to normal tissues, YAP significantly increased in cervical SCC and was positively correlated with tumor differentiation, lymph node metastasis, and early recurrence.84
Therapeutic potential on targeting YAP/TAZ in squamous cell carcinoma
Based on the above-mentioned studies, it is clear that the YAP/TAZ, which was expressed highly in SCCs,68,73,75,80,84 has an oncogenic function. Therefore, a strategy against YAP/TAZ can prevent or at least slow the progression of SCCs. Fortunately, some inhibitors have been reported to be effective in treating SCC through direct and indirect inhibition of YAP/TAZ activation. An experimental evidence has demonstrated that verteporfin, an FDA-approved photodynamic therapy, inhibited YAP–-EAD interaction and suppressed YAP-induced cancer cells overgrowth in vivo or in vitro.85,86 In addition, a report showed verteporfin also activated the Hippo pathway and sequestered YAP in the cytoplasm by upregulating 14-3-3 .87 A number of pathways have been identified to drive YAP/TAZ activities. Some can be used as a target to treat YAP-dependent cancer, such as G-protein-coupled receptor (GPCRs).88 It was reported that viral GPCR inhibited the Hippo pathway kinases Lats1/2 through Gq/11 and G12/13, which resulted in the activation of YAP/TAZ.89 Targeting the downstream genes of YAP/TAZ such as CYR61 and CTGF is also a strategy for SCC treatment, which is required for YAP/TAZ-dependent cancer progression.90,91
.87 A number of pathways have been identified to drive YAP/TAZ activities. Some can be used as a target to treat YAP-dependent cancer, such as G-protein-coupled receptor (GPCRs).88 It was reported that viral GPCR inhibited the Hippo pathway kinases Lats1/2 through Gq/11 and G12/13, which resulted in the activation of YAP/TAZ.89 Targeting the downstream genes of YAP/TAZ such as CYR61 and CTGF is also a strategy for SCC treatment, which is required for YAP/TAZ-dependent cancer progression.90,91
Conclusion and future perspectives
The Hippo pathway is pivotal in mammalian development and organ size control. YAP and TAZ are transcriptional co-activators that are negatively regulated by the Hippo pathway. YAP/TAZ activation promotes tumor formation and progression. A subsequent study reported that YAP/TAZ was overexpressed in a significant number of human cancers, especially in SCCs derived from different organs. Therefore, targeting the inhibition of YAP/TAZ activation would be an effective approach to treat YAP/TAZ-driven cancers. There are several strategies to directly target YAP/TAZ-TEAD, including targeting upstream signaling molecules and downstream YAP/TAZ target genes. However, the side effects of these treatments remain unclear. Moreover, there are no studies indicating that YAP/TAZ inhibition can prevent the growth of metastatic cancers.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (81570344, to Ying Xin), the Norman Bethune Program of Jilin University (2015225, to Ying Xin and 2015203, to Xin Jiang), the Jilin Provincial Science and Technology Foundations (20190201200JC to Xin Jiang), and the Health and Family Planning Commission of Jilin Province Foundations (2016Q034 to Ying Xin).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914–920. doi:10.1038/ncb1050
2. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi:10.1016/j.cell.2005.06.007
3. Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. Embo J. 2000;19(24):6778–6791. doi:10.1093/emboj/19.24.6778
4. Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi:10.1101/gad.210773.112
5. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–546. doi:10.1101/gad.9.5.534
6. Kango-Singh M. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129(24):5719–5730.
7. Tapon N, Harvey KF, Bell DW, et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478.
8. Harvey KF, Pfleger CM, Hariharan IK. The drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–467.
9. Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in drosophila. Nat Cell Biol. 2003;5(10):921–927. doi:10.1038/ncb1051
10. Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with Salvador and warts. Cell. 2003;114(4):445–456.
11. Lai ZC, Wei X, Shimizu T, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120(5):675–685. doi:10.1016/j.cell.2004.12.036
12. Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14(3):377–387. doi:10.1016/j.devcel.2008.01.006
13. Guo T, Lu Y, Li P, et al. A novel partner of scalloped regulates Hippo signaling via antagonizing scalloped-yorkie activity. Cell Res. 2013;23(10):1201–1214. doi:10.1038/cr.2013.120
14. Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in drosophila. Cell. 2006;126(4):767–774. doi:10.1016/j.cell.2006.07.013
15. Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9(8):2145–2152.
16. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi:10.1016/j.cell.2015.10.044
17. Fu V, Plouffe SW, Guan KL. The Hippo pathway in organ development, homeostasis, and regeneration. Curr Opin Cell Biol. 2017;49:99–107. doi:10.1016/j.ceb.2017.12.012
18. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi:10.1152/physrev.00005.2014
19. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi:10.1038/ncb2303
20. Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13(6):324–337. doi:10.1038/nrgastro.2016.59
21. Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24(12):2076–2086. doi:10.1038/sj.onc.1208445
22. Hwang E, Ryu KS, Paakkonen K, et al. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc Natl Acad Sci U S A. 2007;104(22):9236–9241. doi:10.1073/pnas.0610716104
23. Ni L, Zheng Y, Hara M, Pan D, Luo X. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev. 2015;29(13):1416–1431. doi:10.1101/gad.264929.115
24. Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–5509. doi:10.1074/jbc.M709037200
25. Schumacher B, Skwarczynska M, Rose R, Ottmann C. Structure of a 14-3-3sigma-YAP phosphopeptide complex at 1.15 A resolution. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(Pt 9):978–984. doi:10.1107/S1744309110025479
26. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24(1):72–85. doi:10.1101/gad.1843810
27. Guo L, Teng L. YAP/TAZ for cancer therapy: opportunities and challenges (review). Int J Oncol. 2015;46(4):1444–1452. doi:10.3892/ijo.2015.2877
28. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi:10.1101/gad.1664408
29. Lei QY, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–2436. doi:10.1128/MCB.01874-07
30. Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109(37):E2441–50. doi:10.1073/pnas.1212021109
31. Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278(35):33334–33341. doi:10.1074/jbc.M305597200
32. Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc Natl Acad Sci U S A. 2005;102(50):18034–18039. doi:10.1073/pnas.0509109102
33. Manderfield LJ, Engleka KA, Aghajanian H, et al. Pax3 and hippo signaling coordinate melanocyte gene expression in neural crest. Cell Rep. 2014;9(5):1885–1895. doi:10.1016/j.celrep.2014.10.061
34. Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. doi:10.1242/dev.045500
35. Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24(9):862–874. doi:10.1101/gad.1909210
36. Kim M, Kim T, Johnson RL, Lim DS. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11(2):270–282. doi:10.1016/j.celrep.2015.03.015
37. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi:10.1038/nrc3458
38. Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi:10.1016/j.cell.2012.06.037
39. Webb C, Upadhyay A, Giuntini F, et al. Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry. 2011;50(16):3300–3309. doi:10.1021/bi2001888
40. Ernst A, Appleton BA, Ivarsson Y, et al. A structural portrait of the PDZ domain family. J Mol Biol. 2014;426(21):3509–3519. doi:10.1016/j.jmb.2014.08.012
41. Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi:10.1016/j.cell.2011.02.031
42. Xu CM, Liu WW, Liu CJ, Wen C, Lu HF, Wan FS. Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo. Cancer Gene Ther. 2013;20(8):453–460. doi:10.1038/cgt.2013.40
43. Bai H, Zhang N, Xu Y, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56(3):1097–1107. doi:10.1002/hep.25769
44. Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5(4):1060–1069. doi:10.1016/j.celrep.2013.10.030
45. Zhang W, Cohen SM. The Hippo pathway acts via p53 and microRNAs to control proliferation and proapoptotic gene expression during tissue growth. Biol Open. 2013;2(8):822–828. doi:10.1242/bio.20134317
46. Bai H, Gayyed MF, Lam-Himlin DM, et al. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Hum Pathol. 2012;43(9):1376–1385. doi:10.1016/j.humpath.2011.12.001
47. Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493(7430):106–110. doi:10.1038/nature11693
48. Fujii M, Toyoda T, Nakanishi H, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209(3):479–494. doi:10.1084/jem.20111653
49. Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108(49):E1312–E1320. doi:10.1073/pnas.1110428108
50. Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–2060. doi:10.1016/j.cub.2007.10.039
51. Heinemann A, Cullinane C, De Paoli-Iseppi R, et al. HDAC inhibitors synergistically induces apoptosis of melanoma and suppresses AKT and YAP signaling. Oncotarget. 2015;6(25):21507–21521. doi:10.18632/oncotarget.4242
52. Lin Z, Zhou P, von Gise A, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116(1):35–45. doi:10.1161/CIRCRESAHA.115.304457
53. Lau AN, Curtis SJ, Fillmore CM, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. Embo J. 2014;33(5):468–481. doi:10.1002/embj.201386082
54. Perra A, Kowalik MA, Ghiso E, et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61(5):1088–1096. doi:10.1016/j.jhep.2014.06.033
55. Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. doi:10.1371/journal.pone.0091770
56. Wu Q, Li J, Sun S, et al. YAP/TAZ-mediated activation of serine metabolism and methylation regulation is critical for LKB1-deficient breast cancer progression. Biosci Rep. 2017;37(5):BSR20171072. doi:10.1042/BSR20171072
57. Zhou GX, Li XY, Zhang Q, et al. Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013;14(9):5199–5205.
58. Wang Y, Xie C, Li Q, Xu K, Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol. 2013;34(4):2169–2174. doi:10.1007/s13277-013-0751-x
59. Debaugnies M, Sanchez-Danes A, Rorive S, et al. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018;19:7. doi:10.15252/embr.201845809
60. Maglic D, Schlegelmilch K, Dost AF, et al. YAP-TEAD signaling promotes basal cell carcinoma development via a c-JUN/AP1 axis. Embo J. 2018;37:17. doi:10.15252/embj.201798642
61. Akladios B, Mendoza Reinoso V, Cain JE, et al. Positive regulatory interactions between YAP and Hedgehog signalling in skin homeostasis and BCC development in mouse skin in vivo. PLoS One. 2017;12(8):e0183178. doi:10.1371/journal.pone.0183178
62. Quan T, Xu Y, Qin Z, et al. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am J Pathol. 2014;184(4):937–943. doi:10.1016/j.ajpath.2013.12.017
63. Guo P, Kang S, Zhao F. High expression of TAZ/YAP promotes the progression of malignant melanoma and affects the postoperative survival of patients. Pharmazie. 2018;73(11):662–665. doi:10.1691/ph.2018.8499
64. Xiong H, Yu Q, Gong Y, et al. Yes-Associated Protein (YAP) promotes tumorigenesis in melanoma cells through stimulation of low-density Lipoprotein Receptor-Related Protein 1 (LRP1). Sci Rep. 2017;7(1):15528. doi:10.1038/s41598-017-14764-4
65. Kim MH, Kim CG, Kim SK, et al. YAP-induced PD-L1 expression drives immune evasion in BRAFi-resistant melanoma. Cancer Immunol Res. 2018. doi:10.1158/2326-6066.CIR-17-0320
66. Sambandam SAT, Kasetti RB, Xue L, Dean DC, Lu Q, Li Q. 14-3-3sigma regulates keratinocyte proliferation and differentiation by modulating Yap1 cellular localization. J Invest Dermatol. 2015;135(6):1621–1628. doi:10.1038/jid.2015.42
67. Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143(10):1674–1687. doi:10.1242/dev.133728
68. Jia J, Li C, Luo S, et al. Yes-associated protein contributes to the development of human cutaneous squamous cell carcinoma via activation of RAS. J Invest Dermatol. 2016;136(6):1267–1277. doi:10.1016/j.jid.2016.02.005
69. Li Y, Kong F, Wang J, et al. S100A7 induction is repressed by YAP via the Hippo pathway in A431 cells. Oncotarget. 2016;7(25):38133–38142. doi:10.18632/oncotarget.9477
70. Li Y, Kong F, Shao Q, et al. Activity are suppressed by S100A7 via p65/NFkappaB-mediated repression of DeltaNp63. Mol Cancer Res. 2017;15(12):1752–1763. doi:10.1158/1541-7786.MCR-17-0349
71. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
72. Yamana H. [Molecular biology for esophageal squamous cell carcinoma]. Nihon Rinsho. 2011;69(Suppl 6):51–56.
73. Muramatsu T, Imoto I, Matsui T, et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32(3):389–398. doi:10.1093/carcin/bgq254
74. Yeo MK, Kim SH, Kim JM, et al. Correlation of expression of phosphorylated and non-phosphorylated Yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012;32(9):3835–3840.
75. Ge L, Smail M, Meng W, et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS One. 2011;6(11):e27529. doi:10.1371/journal.pone.0027529
76. Saladi SV, Ross K, Karaayvaz M, et al. ACTL6A is co-amplified with p63 in squamous cell carcinoma to drive YAP activation, regenerative proliferation, and poor prognosis. Cancer Cell. 2017;31(1):35–49. doi:10.1016/j.ccell.2016.12.001
77. Wang Y, Gersten A, Moleirinho S, Gunn-Moore FJ, Reynolds PA, Prystowsky MB. Fibroblasts in head and neck squamous cell carcinoma associated with perineural invasion have high-level nuclear Yes-Associated Protein (YAP) Expression. Acad Pathol. 2015;2(4):2374289515616972. doi:10.1177/2374289515616972
78. Verduci L, Ferraiuolo M, Sacconi A, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. 2017;18(1):237. doi:10.1186/s13059-017-1368-y
79. Snijders AM, Schmidt BL, Fridlyand J, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24(26):4232–4242. doi:10.1038/sj.onc.1208601
80. Hiemer SE, Zhang L, Kartha VK, et al. A YAP/TAZ-regulated molecular signature is associated with oral squamous cell carcinoma. Mol Cancer Res. 2015;13(6):957–968. doi:10.1158/1541-7786.MCR-14-0580
81. Chen X, Gu W, Wang Q, et al. C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC. Oncotarget. 2018;9(1):668–679. doi:10.18632/oncotarget.23089
82. Wei Z, Wang Y, Li Z, et al. Overexpression of Hippo pathway effector TAZ in tongue squamous cell carcinoma: correlation with clinicopathological features and patients’ prognosis. Int J Stomatol Occlusion Med. 2013;42(10):747–754. doi:10.1111/jop.12062
83. Silverberg SG, Ioffe OB. Pathology of cervical cancer. Cancer J. 2003;9(5):335–347.
84. Liu T, Liu Y, Gao H, Meng F, Yang S, Lou G. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer. 2013;23(4):735–742. doi:10.1097/IGC.0b013e31828c8619
85. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi:10.1101/gad.192856.112
86. Brodowska K, Al-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res. 2014;124:67–73. doi:10.1016/j.exer.2014.04.011
87. Wang C, Zhu X, Feng W, et al. Verteporfin inhibits YAP function through up-regulating 14-3-3sigma sequestering YAP in the cytoplasm. Am J Cancer Res. 2016;6(1):27–37.
88. Zhou X, Wang Z, Huang W, Lei QY. G protein-coupled receptors: bridging the gap from the extracellular signals to the Hippo pathway. Acta Biochim Biophys Sin (Shanghai). 2015;47(1):10–15. doi:10.1093/abbs/gmu108
89. Liu G, Yu FX, Kim YC, et al. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34(27):3536–3546. doi:10.1038/onc.2014.281
90. Hsu YL, Hung JY, Chou SH, et al. Angiomotin decreases lung cancer progression by sequestering oncogenic YAP/TAZ and decreasing Cyr61 expression. Oncogene. 2015;34(31):4056–4068. doi:10.1038/onc.2014.333
91. Kang W, Huang T, Zhou Y, et al. miR-375 is involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in gastric carcinogenesis. Cell Death Dis. 2018;9(2):92. doi:10.1038/s41419-017-0134-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.