Back to Journals » Patient Preference and Adherence » Volume 16
Virological Outcomes After Switching to Abacavir/Lamivudine/Dolutegravir Combined with Adherence Support in People Living with HIV with Poor Adherence: A Phase IV, Multicentre Randomized Prospective Open Label Study (TriiADD-CTN 286)
Authors Klein MB , Young J , Ortiz-Paredes D , Wang S, Walmsley S, Wong A, Martel-Laferrière V, Pick N , Conway B, Angel J, Baril JG, Fraser C, Lebouché B, Tan DH , Sandre R, Trottier S, Peiris H, Jayaraman J, Singer J
Received 18 June 2022
Accepted for publication 12 October 2022
Published 13 December 2022 Volume 2022:16 Pages 3267—3281
DOI https://doi.org/10.2147/PPA.S379065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Marina B Klein,1– 4 Jim Young,2 David Ortiz-Paredes,3 Shouao Wang,3 Sharon Walmsley,4,5 Alexander Wong,6 Valérie Martel-Laferrière,7 Neora Pick,8 Brian Conway,9 Jonathan Angel,10 Jean-Guy Baril,11 Chris Fraser,12 Bertrand Lebouché,3,13 Darrell HS Tan,14 Roger Sandre,15 Sylvie Trottier,16 Hansi Peiris,3 Jayamarx Jayaraman,4 Joel Singer4 On behalf of the CTN 286 Study Investigators
1Division of Infectious Diseases and Chronic Viral Illness Service, Department of Medicine, McGill University Health Centre, Montreal, Canada; 2Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada; 3Centre for Outcomes Research and Evaluation, Research Institute of the McGill University Health Centre, Montreal, Canada; 4Canadian Institutes of Health Research, Canadian HIV Trials Network, Vancouver, Canada; 5University Health Network, University of Toronto, Toronto, Canada; 6Department of Medicine, University of Saskatchewan, Regina, Canada; 7Department of Microbiology and Infectious Diseases, Centre de recherche du Centre hospitalier de l’Université de Montréal, Montreal, Canada; 8Department of Medicine, Division of Infectious Diseases, University of British Columbia, Vancouver, Canada; 9Vancouver Infectious Diseases Centre, Vancouver, Canada; 10Ottawa Hospital Research Institute, Ottawa, Canada; 11Clinique de Médecine Urbaine du Quartier Latin, Montreal, Canada; 12Cool Aid Community Health Centre, Victoria, Canada; 13Department of Family Medicine, McGill University, Montreal, Canada; 14Toronto General Hospital Research Institute, University Health Network, Toronto, Canada; 15HAVEN Program, Health Sciences North, Sudbury, Canada; 16Centre de Recherche du CHU de Québec, Department of Microbiology, Infectiology and Immunology, Université Laval, Quebec, Canada
Correspondence: Marina B Klein, Division of Infectious Diseases and Chronic Viral Illness Service, McGill University Health Centre, 1001 Decarie Boulevard, D02.4110, Montréal, H4A 3J1, Canada, Tel +1-514-843-2090, Fax +1-514-843-2092, Email [email protected]
Background: Many people living with HIV struggle to consistently adhere to antiretroviral therapy, fail to achieve long-term virologic control and remain at risk for HIV-related disease progression, development of resistance and may transmit HIV infection to others.
Objective: To determine if switching from current multi-tablet (curART) to single-tablet antiretroviral therapy (abacavir/lamivudine/dolutegravir; ABC/3TC/DTG), both combined with individualized adherence support, would improve HIV suppression in non-adherent vulnerable populations.
Methods: TriiADD was an investigator-initiated randomized, multicentre, open label study. HIV+ adults with documented non-adherence on curART were randomized in a 1:1 ratio to immediately switch to ABC/3TC/DTG or to continue curART. Both arms received adherence support. The primary outcome was the proportion of participants in each arm with HIV RNA < 50 copies/mL 24 weeks after randomization.
Results: In total, 50 people were screened and 27 randomized from 11 sites across Canada before the trial was stopped early due to slow recruitment. Participants were predominantly from ethnocultural communities, Indigenous people and/or had a history of injection drug use. The proportion achieving HIV RNA < 50 copies/mL at week 24 was 4/12 (33%) in the curART arm vs 7/13 (54%) in the ABC/3TC/DTG arm; median Bayesian risk difference, 5% (95% CrI, − 17 to 28%) higher for those randomized to ABC/3TC/DTG. We encountered difficulties with recruitment of participants without prior drug resistance, retention despite intensive support, reliably measuring adherence and in overcoming entrenched adherence barriers.
Conclusion: Results of our trial are consistent with a slight improvement in viral suppression in a vulnerable population when a single tablet regimen is combined with patient-level adherence support. Beyond treatment simplicity and tolerability, tailored interventions addressing stigma and social determinants of health are still needed. The numerous challenges we encountered illustrate how randomised trials may not be the best approach for assessing adherence interventions in vulnerable populations.
Keywords: adherence interventions, human immunodeficiency virus, HIV, antiretroviral therapy, single tablet regimen, vulnerable populations
Plain Language Summary
Many people living with HIV struggle to take their antiretroviral medication consistently. As a result, they may experience poor control of their infection and are at increased risk of developing resistance to treatment, getting sick and transmitting HIV infection to others. Vulnerable people face many barriers to taking medications regularly such as substance use, side effects, stigma, and financial and food insecurity. The aim of this study was to determine if simplifying treatment to a single tablet per day along with providing individualized adherence support could improve adherence and HIV control. Participants were randomly assigned to switch their current multiple tablet treatments to a single tablet of abacavir/lamivudine/dolutegravir or continue with their current multiple tablet treatment. Both groups received individualized adherence support to address their specific adherence challenges, reminders and financial support for co-payments. While switching to the single tablet appeared to improve control of HIV, the trial encountered many challenges and was stopped early due to slow recruitment. It was difficult to find participants without prior drug resistance. Many could not adhere or stay in the trial despite intensive support. Overcoming entrenched adherence barriers was difficult. Our trial illustrates that beyond improving treatment simplicity and tolerability, tailored interventions that address stigma and social determinants of health are still needed to ensure all people can benefit from HIV therapy. Alternatives to trials should be considered for assessing what interventions may work best to improve adherence in vulnerable populations.
Introduction
With the improved safety and efficacy of modern combination antiretroviral therapy (ART) and a growing recognition that prolonged viral suppression reduces both HIV and non-HIV morbidity and mortality and transmission, ART is now recommended for all HIV infected person regardless of CD4 cell count.1 Despite the benefits of modern ART, an important sub-group (as many as 30%2–4) of HIV-infected persons is unable to maintain adherence to treatment.5 Consequently, they fail to achieve long-term virologic control and remain at risk for HIV-related disease progression, development of resistance and may transmit HIV infection to others.6 In Canada and elsewhere, vulnerable populations, such as people who inject drugs (PWID) and people from Indigenous and ethno-cultural communities are at higher risk of non-adherence.7–10 A variety of factors contribute including stigma, financial and food insecurity, problematic substance use, mental illness, side effects and lack of perceived benefits of treatment.10,11 Ongoing transmission in these at-risk populations, because of unsuppressed HIV, is an important driver of the epidemic12,13 and has contributed to failing to meet UNAIDS 2020 targets for ending AIDS.14
For vulnerable populations, an ideal regimen should be simple, tolerable, combine well with treatment for hepatitis C virus (HCV) and other comorbidities, in addition to being easy to integrate into complex lives where barriers to treatment include financial and food insecurity. Because of poor adherence, clinicians are also concerned about past and future HIV resistance when recommending treatment. However, many regimens with high barriers to resistance (eg, boosted protease inhibitors) are inherently difficult to adhere to with multiple tablets or side effects.15 Once daily ART and single tablet regimens (STR) have been associated with improved adherence and HIV suppression,6,16,17 reduced hospitalizations and lower health-care costs compared multiple tablet regimens (MTR).6,18,19 These previous studies however have been limited by their retrospective design and uncontrolled allocation of treatment – temporal changes and confounding may have biased results. The availability of the integrase strand inhibitor-based ART, abacavir/lamivudine/dolutegravir (ABC/3TC/DTG; TriumeqTM), represented an opportunity to offer an STR with many features that could enhance adherence in vulnerable populations: a high barrier to resistance, no food restrictions, few drug–drug interactions and a good side effect profile.20
The aim of TriiADD was to determine if switching from current ART (curART) to ABC/3TC/DTG combined with adherence support would improve the rate of HIV suppression in vulnerable populations non-adherent to their curART. This was to be the first randomized controlled trial to directly compare MTR and STR regimens in populations most in need of, and most likely to benefit from, enhanced adherence interventions. The trial was stopped early due to slow recruitment. This report describes the trial design and results and evaluates challenges and lessons learned from conducting research on adherence in vulnerable populations in order to inform future trials and interventions.
Materials and Methods
Trial Design
TriiADD was an investigator-initiated randomized, multicentre, open label study (Canadian Institutes of Health Research (CIHR)-Canadian HIV Trials Network (CTN) 286; Clinical trials.gov NCT02354053). Participants were randomized in a 1:1 ratio to immediately switch to ABC/3TC/DTG or to continue with their currently prescribed curART regimen. Both arms received adherence support (see below). Those randomized to maintain curART were permitted to switch to ABC/3TC/DTG after week 24 if they chose to (Figure 1). The funders had no role in the design, management, data collection, analysis, interpretation, reporting, or decision to publish results.
 |
Figure 1 Study flow through 72 weeks. Additional study exclusion criteria were: women who were pregnant or breastfeeding, planning pregnancy or who did not use contraception if able to conceive; active Centers for Disease and Prevention Control (CDC) Category C disease53 (except cutaneous Kaposi’s sarcoma not requiring systemic therapy); moderate to severe hepatic impairment (Child-Pugh classification Class B or C); alanine aminotransferase (ALT) greater than 5 times the upper limit of normal, or ALT greater than or equal to 3 times the upper limit of normal and bilirubin greater than or equal to 1.5 times the upper limit of normal (with greater than 35% direct bilirubin); creatinine clearance of less than 50 mL/min via Cockroft-Gault method; and those taking dofetilide or immunosuppressants. None of the screened patients were excluded for these reasons. Abbreviations: ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; curART, current antiretroviral therapy. |
Participants
Patients recruited into the trial were adults aged over 18 years old with documented HIV infection, with negative HLA-B5701 testing and without chronic Hepatitis B infection (HBsAg surface antigen negative) and had to have been receiving ART for at least one year. Participants were required to have evidence of non-adherence to their curART regimen defined as: HIV RNA ≥400 copies/mL at least once in last 12 months (not explained by normal viral decay after initiating curART), or if HIV RNA <400 copies/mL, to have a clinical history of non-adherence by patient self-report or pharmacy refill data.
Given patients may have had virologic failure at the time of enrollment, they were excluded if they had evidence of resistance to any component of their current regimen or to ABC/3TC/DTG based on the presence of primary resistance-associated mutations with these drugs according to the Stanford HIV drug resistance database21,22 on any available historical resistance test or on screening genotype for patients with HIV RNA ≥400 copies/mL. Additional exclusion criteria can be found in Figure 1 (footnotes) – however, no patient was excluded for these reasons.
The study was reviewed and approved by the ethics committee of each participating centre and by the Community Advisory Committee of the CTN. Written informed consent was obtained prior to screening.
Trial Management
An adjudication committee comprised of 3 persons (not including the principal investigator) reviewed all cases where non-adherence or the resistance test results were not clear and determined eligibility for the trial. The trial was reviewed every 6 months for safety and any ongoing procedural concerns by the CIHR CTN Data Safety Monitoring Committee (DSMC).
Randomization
A computer-generated randomization list was prepared prior to study onset by a statistician at the CIHR CTN unassociated with the study. Site coordinators accessed the allocation codes by interacting with a password-protected webpage. Randomization was stratified by study centre and whether HIV RNA was >400 copies/mL at screening using variable permuted blocks of size 2 and 4.
Interventions
Antiretroviral Therapy
Prescribed curART included any recommended or alternative regimen in the guideline current at the time22 which the treating physician considered appropriate for their patient (except those containing dolutegravir) taken for at least 6 months. Eligible participants were randomized to continue curART or immediately switch to abacavir 600 mg/lamivudine 300 mg/dolutegravir 50 mg once daily administered as fixed dose combination tablet (ABC/3TC/DTG; TruimeqTM supplied by ViiV Healthcare, Laval, Canada).
Adherence Support
As we recruited patients with documented non-adherence and poor virologic control, it was ethical to offer adherence counselling and support to all enrolled. However, based on prior literature, we anticipated that the effect of such counselling would be modest (at best a 20% increase in adherence and virologic control from baseline).16 Patients in both arms received an intervention made up of the same components: clinic-based assessment and follow-up specific to the individual’s identified challenges with adherence to assess if they had been adequately addressed, telephone/electronic reminders and dosette boxes. The clinic-based adherence intervention was inspired by the “Treatment Manual for Managed Problem-Solving (MAPS)” which has been specifically designed for health-care professionals working with HIV-infected populations to improve ART adherence.23 The intent was that everyone received an individualized adherence support program equal in approach and structure, but which could vary in intensity. In addition, co-pay coverage (where needed) was provided so there was no financial burden for participants randomized to the curART arm as ABC/3TC/DTG was supplied.
Outcomes
The primary outcome was the proportion of participants in each arm with HIV RNA < 50 copies/mL at week 24 after randomization. The secondary outcome was the median adherence score over time in the two arms at week 24 post randomization. The adherence score was determined from pill counts by calculating the proportion of ART medications used (dispensed minus returned) divided by the amount dispensed per month. Pill count was corroborated with self-reported adherence using the Visual Analogue Scale (VAS last week and/or last month) which measures item ratings by participants in percentile terms from 0 to 100%.24 Adherence among all patients was also determined at weeks 48 and 72 post-randomization to assess durability of the intervention. Additional planned secondary outcomes were proportion of participants in each arm with HIV RNA < 50 copies/mL at weeks 48 and 72; changes in HIV viral load and CD4 cell counts through week 72; and the proportion of participants in each arm developing new drug resistance mutations.
Sample Size
We planned for a sample size of 100 patients (50 per arm) to provide 80% power to detect a difference between 85% and 60% in virologic suppression rates between the two arms at week 24. While this difference is large, for the population we were targeting, we considered that an improvement in virologic suppression rates of at least this amount would be required to be clinically meaningful.
Statistical Analyses
Planned Analyses
Planned analyses were based on intention-to-treat (ITT). Dropouts and crossovers were counted as treatment failures. For the primary outcome, the proportion of participants with HIV RNA < 50 copies/mL at week 24 was defined by the FDA snapshot analysis.25 For the secondary outcome, mean adherence scores were compared between arms at week 24. Dropouts were considered to have 0% adherence. The effect of treatment on outcome was assessed using odds ratios estimated in logistic regression models with HIV RNA >400 copies/mL at screening as a covariate. This covariate was added to all regression models to reflect the stratified randomization. The logistic regression model for the secondary outcome was fitted using quasi-likelihood because the adherence score is a fractional response.26
Supplemental Analyses
Given the small sample size recruited prior to study termination, and the resultant lack of precision in estimates, we conducted Bayesian analyses to obtain more plausible estimates for the treatment effect from these data. We used a weakly informative prior for the effect of treatment, assuming that its effect should lie within the range from 0.25 to 4.0 with 95% confidence.27 We fitted Bayesian logistic and beta regression models for the primary and secondary outcomes, respectively. In addition, adherence scores at week 24 were compared in the per-protocol population. This per-protocol analysis was added because it is not clear whether zero adherence ought to be attributed to those patients that withdraw, so results of this additional analysis represent the effect of treatment on adherence to study medication for those able to remain in the trial.
All estimates are reported with 95% confidence (CI) or credible (CrI) intervals. All models were fitted in SAS 9.4 (TS Level 1M5, procedures LOGISTIC, GLIMMIX and MCMC).
Qualitative Analysis
We used data collected in the standardized format of the MAPS adherence tool to conduct a post hoc qualitative content analysis of barriers and solutions used to overcome them for adherence support.28,29 Barriers were inductively categorized in themes representing similar obstacles. Solutions were initially inductively organized in themes and then deductively categorized in the three domains of the Information-Motivation-Behavioural skill model (IMB), a framework to understand and promote HIV preventive behaviours.30 DOP conducted the initial analysis using NVivo 12® for Mac and met with MBK in debriefing meetings to ensure reliability of the resulting themes.
Results
The trial opened for enrollment in December 2015 and closed in December 2018 before reaching the target sample size due to slow enrollment. In total, 50 patients were screened and 27 were randomized from 11 sites across Canada. Two participants, one in each arm, were determined after randomization to have had baseline resistance and then withdrawn leaving 25 enrolled; 13 were randomized to immediate switch to ABC/3TC/DTG and 12 to remain on curART.
Among patients who failed screening, the principal reasons were: the presence of baseline resistance mutations (n = 10), failure to come to baseline visit (n = 5) and insufficient evidence of non-adherence (n = 5). Eight patients were referred to the trial adjudication committee of whom 4 were determined not to be eligible (2 for resistance and 2 for insufficient evidence of non-adherence). The study flow through week 72 is shown in Figure 1.
Enrolled patients were diverse and had many sociodemographic characteristics that suggested vulnerabilities including history of mental illness and substance use (Table 1). HCV co-infection was common (n = 11; 44%). Participants in both arms were predominantly from ethnocultural communities (n = 11; 44%) or Indigenous people (n = 7; 28%). While injection drug use was the most common risk factor for HIV acquisition overall (n = 11; 44%), a large proportion of participants acquired HIV heterosexually in Canada (n = 10; 40%) or were immigrants from HIV endemic countries (n = 5; 20%). There were only 2 participants who identified as being men who have sex with men. Allowing for the small numbers of patients enrolled, there were no major differences between study arms (Table 1). Participants generally had lived for long time with HIV infection (median 12.5 years; interquartile range (IQR), 8.8, 15.3 years) and had many years of experience with ART (9.9 years; IQR, 6.4, 14.5 years). Baseline CD4 cell counts were relatively high and most had low level viremia (<500 copies/mL) or were undetectable at time of enrolment. curART regimens were similar in both arms with almost all participants receiving three or four tablets per day (two in each arm were receiving an STR). More participants had completely interrupted ART prior to enrollment in the ABC/3TC/DTG arm (n = 5 vs 0).
 |
Table 1 Baseline Characteristics of Study Participants at Enrollment |
Virologic Suppression
The proportion of participants achieving HIV RNA < 50 copies/mL at week 24 was 4/12 (33%) in the curART arm vs 7/13 (54%) in the ABC/3TC/DTG arm. At week 24, the odds ratio (OR) for HIV RNA <50 copies/mL was 3.1 (95% CI, 0.57 to 21) for those randomized to ABC/3TC/DTG. With our weakly informative prior, the median Bayesian OR for HIV RNA <50 copies/mL was 1.2 (CrI, 0.46 to 3.3). Fitting a Bayesian logistic regression via Markov Chain Monte Carlo allowed us to calculate other statistics within the chain and sample these: the median Bayesian risk difference for the effect of treatment was 5% (95% CrI, −17 to 28%) higher for those randomized to ABC/3TC/DTG.
All seven participants achieving HIV RNA < 50 copies/mL at week 24 in the ABC/3TC/DTG continued in study and maintained viral suppression through week 72 (Figure 2). Of six remaining participants in curART arm who transitioned to ABC/3TC/DTG at week 24, 3 achieved HIV RNA < 50 copies/mL at weeks 48 and 72.
Adherence
All six participants who dropped out or stopped treatment (4 randomised to curART and 2 to ABC/3TC/DTG) were assigned an adherence of 0%. Reasons for dropouts are shown in Figure 1. The median reported adherence score was 90% (IQR 0% to 100%) for those randomised to curART (n = 12) and 90% (IQR 87% to 100%) for those to ABC/3TC/DTG (n = 13). The OR for mean adherence score at week 24 was 2.4 (95% CI, 0.42 to 14) for those randomised to ABC/3TC/DTG.
Reported adherence was high in both study arms for those remaining in the trial through to week 24 (n = 19). In this per-protocol population, the median reported adherence score was 100% (IQR 90% to 100%) and 90% (IQR 87% to 100%) in curART (n = 8) and ABC/3TC/DTG (n = 11) arms, respectively. The median Bayesian OR was 1.1 (CrI, 0.48 to 2.5) for those randomised to ABC/3TC/DTG. The median Bayesian risk difference for the effect of treatment was 1% (95% CrI, −9 to 10%) higher for those randomized to ABC/3TC/DTG.
Barriers to Adherence and Solutions Employed
Participants identified 119 barriers to adherence in total, which were grouped into six core themes (Table 2; Figure 3): forgetfulness, competing demands, substance use, negative treatment experiences, economic barriers/lack of insurance and insufficient support. Of these, forgetfulness was the most frequently reported (88% overall). This barrier was particularly important for PWID. Negative treatment experiences and competing demands, also commonly interfered with adherence.
 |
Table 2 Barriers to Adherence Reported by Study Participants at Enrollment and Specific Solutions Employed to Improve Adherence |
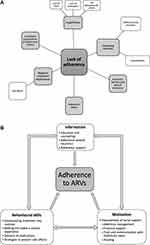 |
Figure 3 Adherence barriers experienced and solutions employed by participants. (A) The six core themes of barriers to antiretroviral adherence (in light grey) reported by participants and their contributing factors (in white). (B) Proposed solutions developed with participants grouped according to the Information-Motivation-Behavioral skill (IMB) model.30 |
In total, participants employed 113 different solutions which were grouped in the three categories (Table 2). Information-based solutions were used by 71% of participants. Behavioural skills were also commonly employed (67%), particularly by PWID. Both information and behavioural solutions aimed to improve participants’ “abilities and sense of self-efficacy concerning performance of a given health-related behaviour”30 and thus principally targeted forgetfulness. Motivation-related solutions, especially those that sought to improve participants’ social support, were used by 54%.
Discussion
Meeting the needs of vulnerable patients living with HIV remains a challenge and priority for reaching the enhanced UNAIDS 95-95-95 targets for ending AIDS worldwide by 2025.31 In particular, ensuring 95% of those on ART maintain undetectable HIV RNA (“the last 95”) is seen as critical to halting AIDS and onward HIV transmission.31 Our trial was meant to address a specific knowledge gap at the time of its design: could an STR provide sufficient improvement in adherence to permit people with history of non-adherence to achieve viral suppression? The trial was stopped early due to slow enrollment. We can therefore only provide weak evidence that switching to ABC/3TC/DTG might improve rates of virologic suppression over 24 weeks compared to remaining on current ART when combined with enhanced adherence support. Viral suppression on ABC/3TC/DTG, once achieved, was durable through 72 weeks. However, despite providing personalised adherence support, almost 50% of those randomised to ABC/3TC/DTG did not achieve HIV RNA < 50 copies/mL, which was more than we expected and a reflection of the high risk, real-world population enrolled in this study. Many of these patients experienced low grade viremia, and while no resistance mutations were detected over the short term (data not shown), it is clear that solutions to improve adherence are still needed.
Since this study was designed, several large observational studies have shown that STRs are related to better adherence in real-world settings.19 A recent meta-analysis of 8 studies and 30,470 patients led to an OR of 1.63 (95% CI, 1.52–1.74) when comparing STRs to MTRs with respect to optimal adherence (≥95%).32 However, of five studies included that assessed the association between STR and virologic outcomes, the only one to include integrase inhibitor-based ART, from the Women’s interagency HIV study, found that STRs were associated with greater virologic suppression (RR 1.06, 95% CI 1.01–1.11).33 A 5% difference in the rate of virologic suppression associated with ABC/3TC/DTG is a plausible estimate of the treatment effect in our study where patients had prior non-adherence. By comparison, in an observational study of treatment-naive patients, the estimated risk difference for virologic failure was 3.4% when taking a single tablet rather than a three-pill efavirenz-based regimen for one year.34 In randomised trials in naïve patients or those simplifying ART, it has been difficult to show any advantage of STR over MTR with respect to virologic suppression. Since virologic failure rates are so low with all modern ART regimens, any differences are generally driven by tolerability of trial regimens.19 There have been no such randomized trials conducted specifically in non-adherent populations. Thus, while STR are associated with improved adherence, it remains unclear to what extent this benefit translates into improved virologic control, particularly in vulnerable, treatment experienced patients such as those included in our trial.
The very nature of non-adherence makes it difficult to study. Patients frequently under-report non-adherence and often re-start ART prior to clinic visits or blood testing due to social desirability bias.35 This can result in difficulties with defining and capturing non-adherence in study inclusion criteria. Having a detectable viral load while receiving treatment, while easier to document, may not capture many people with suboptimal or intermittent adherence who could benefit from interventions. On the other hand, including many people with undetectable viral loads at baseline, despite clearly documented past non-adherence, can make it difficult to observe differences in virologic suppression between intervention and control arms over the short term. We chose to use broad inclusion criteria that would permit enrolment under a variety of scenarios – criteria which reflected the heterogeneity of the target population - and then stratified by HIV RNA at baseline. However, the resulting heterogeneity then made it impossible to draw conclusions about whether certain subpopulations might have benefited more from an STR than others.
There is also a need to ensure safety in trials. Finding non-adherent patients without major resistance was challenging. Indeed, the majority of screening failures (56%) were for this reason. The use of an adjudication committee was helpful in allowing us to assess patients for inclusion into the study given it was not always easy to apply simple inclusion and exclusion criteria. Given past histories of non-adherence, archived drug resistance was likely present in most enrolled participants. The impact of common nucleoside reverse transcriptase inhibitor mutations, such as the M184V/I, on the virologic success of triple regimens containing 3TC (ie, effectively resulting in dual therapy) was not clear early in the era of second-generation integrase inhibitors. While dual therapies using dolutegravir have now been shown to be effective,36 at the time of our study’s design, there was insufficient evidence to support enrolling patients with such mutations—the very patients for whom adherence interventions are most needed.
As we recruited non-adherent patients, it was appropriate to provide support to those randomised to the control arm and therefore all participants were offered adherence support. Combined approaches using patient education, behavioural change strategies and reminders are considered to be most effective for improving adherence.37,38 However, their impact is at best modest (eg, 10–20% improvement)16 in part because they fail to address structural and systemic barriers to treatment such as stigma,39 racism and economic disparities.40 We employed a number of measures in an attempt to address the numerous barriers faced by our study population. These measures included personalised adherence interventions, frequent study contact via telephone or text messaging and financial assistance with co-payments and with transportation to attend visits. Despite these labour intensive and costly measures, we were unable to recruit and retain sufficient participants to complete the trial. Indeed, a substantial proportion of eligible patients failed to return after screening for their randomization visit. The measures employed should have addressed forgetfulness, the barrier most commonly identified by participants. That the measures failed to substantially improve outcomes also suggests that participants themselves do not, or cannot, acknowledge the real reasons they struggle to adhere. Our study further underscores the importance of specifically addressing mental health and addictions which were among most common barriers we encountered.
Comparing the effect of a single versus multiple tablet regimens requires an open label trial design by necessity – increasing pill count to allow for blinding defeats the purpose of simplifying treatment. Indeed, it is not possible to blind adherence interventions in general. The lack of blinding is problematic in adherence trials as participants may intentionally or unintentionally change their actual and/or self-reported behavior to appear more adherent.41 Regular study visits in a trial and added follow-up measures further create an artificial environment so that estimates may not reflect real-world effectiveness. Indeed, in our trial, more than 50% of participants had detectable viral loads during follow-up despite very high levels of reported adherence (>90%) in both study arms – this apparent disconnect suggests that participants over-reported adherence or failed to return pills. Virologic endpoints therefore provided an objective unbiased measure of actual adherence, at least over the short term. Alternate approaches for objectively measuring adherence over the long term could be introduced into adherence studies for example, electronic drug monitoring. Measurement of antiretroviral concentrations in short hair samples has also been shown to strongly predict HIV treatment outcomes.42,43
While randomised trials are considered the gold standard for assessing efficacy of interventions, as we experienced, they are not well suited to assessing the effectiveness of adherence interventions, especially in small populations of refractory non-adherent patients.44 Alternative trial designs where the level of intervention is not the individual patient, such as cluster randomisation or step wedge designs with clinics, providers or health systems delivering the intervention could avoid issues related to individual patient recruitment and changing behaviours due to trial participation, but may be too complex and costly given the small number of patients who might benefit.45 A trial nested within an existing cohort, with randomisation of non-adherent patients to an intervention desired by patients, might alleviate slow recruitment.46 Alternatively, well-designed observational studies using statistical methods to mimic randomised trials47,48 can provide estimates of effectiveness (rather than efficacy).49
At the time the trial was designed, there were few STR options available and gaining access to ABC/3TC/DTG through the trial was an incentive for patients and their providers to participate. During the study, however, multiple STR (including ABC/3TC/DTG) were approved and are now the mainstay of HIV care, both in high and low-and-middle-income countries which likely impacted trial recruitment. Participating in research, when other alternatives exist, can be difficult in populations that inherently mistrust research due to negative historical experiences (eg, Indigenous and ethnocultural communities).50 The challenges we faced in recruiting and retaining participants and the small benefit of STR we observed demonstrates that despite the simplicity of STR, gains in adherence may only be possible using completely new approaches for some patients. Long-acting and injectable therapies provide promise to overcome issues of forgetfulness and to reduce stigma and negative feelings around HIV treatment that represent major barriers to adherence.51 Unfortunately, long half-lives that permit long dosing intervals then mean that adherence is even more critical; otherwise patients are exposed to sub-therapeutic drug levels and the associated risk of developing resistance. In addition, prior drug resistance to integrase inhibitors or rilpivirine is a contraindication to the only approved long-acting treatment, further putting this option out of reach for many of the patients who need it most.52
Conclusions
Our trial, along with existing literature, is consistent with a slight improvement in viral suppression in a vulnerable population when an STR is combined with patient-level adherence support. However, even this comprehensive approach cannot be relied upon to reach the “last 95”. Beyond treatment simplicity and tolerability, tailored interventions addressing stigma, substance use, financial and social determinants of health are still urgently needed for equitable treatment outcomes across all HIV infected populations. Our trial illustrates how randomised trials may not be the best approach for assessing adherence interventions given the heterogeneity of populations in adherence difficulties, treatment histories and the entrenched barriers they face.
Data Sharing Statement
De-identified participant data from the trial can be made available upon request by contacting MBK. Data will be available for 15 years following completion of the trial (July 2019). The study protocol is available through clinicaltrials.gov (NCT02354053).
Ethics Statement
The study has been approved by research ethics boards at each of the participating institutions as follows: community advisory committee of the Canadian Institutes of Health Research Canadian HIV Trials Network, the Research Ethics Board of the McGill University Health Centre (2016-1285), the Comité d’éthique de la recherche du CHUM (2016-5951), the Comité d’éthique de la recherche du CHU de Québec-Université Laval (2016-2730), the Veritas Independent Review Board (16030-11:28:5825-02-2016), the Ottawa Health Science Network Research Ethics Board (20160084-01H), the Research Ethics Board of Health Sciences North (15-039), the University Health Network Research Ethics Board (15-9172-B), the UBC-Providence Health Care Research Ethics Board, the Institutional Review Board Services of the Regina Qu’Appelle Health Region Research Ethics Board (REB-15-93), St Michael’s Hospital Research Ethics Board (15-283). The study was conducted according to the Declaration of Helsinki. All participants provided written informed consent prior to being screened for the study.
Consent for Publication
All authors of this study have read the manuscript, accepted responsibility for the content of manuscript, consented for publication.
Acknowledgments
We also acknowledge Hansi Peiris (trial coordination), Jayamarx Jayaraman (CIHR CTN project coordination); Erica Jaaf (CIHR CTN data management); Bertrand Lebouche (medical monitor); Stephen Shafran and members of the CIHR CTN DSMC; Terry Lee (DSMC reports) and Joel Singer (CIHR CTN methodology).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Additional Contributions
We thank all study participants for their involvement in this study. We acknowledge the CTN286 investigators: Jonathan Angel (Ottawa Hospital, Ottawa, ON), Jean Guy Baril (Clinique Médicale du Quartier Latin, Montreal, QC), Brian Conway (Downtown Infectious Diseases Clinic, Vancouver, BC); Chris Fraser (Cool Aid Community Health Centre, Victoria, BC), Valerie Martel-Laferrière (Centre Hospitalier de l’Université de Montréal, Montreal, Qc), Neora Pick (Oak Tree Clinic, BC Women’s Hospital & Health Centre, Vancouver, BC), Sharon Walmsley (Toronto General Hospital, Toronto, ON), Alex Wong (Regina General Hospital, Regina, SK), Sylvie Trottier (Centre hospitalier de l’Université Laval, Quebec, QC), Darrell H.S. Tan (St. Michael’s Hospital, Toronto, ON), Roger Sandre (Sudbury Outpatient Centre, Sudbury, ON).
Funding
Funding for this investigator-initiated trial was provided by ViiV Healthcare and the CIHR Canadian HIV Trials Network (CTN286). ABC/3TC/DTG (TriumeqTM) was supplied by ViiV Healthcare Canada, Laval, Canada. MBK is supported by a Tier I Canada Research Chair. VML is supported by the Clinical Research Scholar – Junior 1 award from the FRQ-S. BL is supported by a career award, LE 250, from the Quebec’s Ministry of Health for researchers in Family Medicine. DHST is supported by a Tier 2 Canada Research Chair in HIV Prevention and STI Research.
Disclosure
M.B.K. has received research support and consulting fees from ViiV Healthcare, AbbVie and Gilead. S.W. has received grants, consulting fees, lecture fees, nonfinancial support, and fees for the development of educational presentations from ViiV Health Care, GSK, Merck, Janssen, and Gilead Sciences. A.W. received consulting fees and honoraria from Merck, Gilead, ViiV Healthcare, and AbbVie. V.M.-L. has received research support from Gilead and Merck and consulting fees from AbbVie. N.P. has received honoraria from Gilead and ViiV Healthcare. B.C. is a board member, consultant, and has received grants and payment for lectures from AbbVie, Gilead, and Merck, and payment for educational presentations from AbbVie. J.A. serves on Advisory Boards for Gilead and ViiV and has performed contract research and other work for Gilead, ViiV and Merck. J.-G.B. has received consulting fees from Gilead Sciences, Merck, ViiV Healthcare, grants from Gilead Sciences, Merck, ViiV Healthcare, and payment for lectures from Gilead Sciences, Merck, ViiV Healthcare. C.F. has received grants from AbbVie, Gilead Sciences, Merck, and ViiV Healthcare. B.L. has received for investigator-initiated studies from ViiV Healthcare, Merck, and Gilead; consulting fees from ViiV Healthcare, Merck, and Gilead. He is the holder of a Canadian Institutes for Health Research, Strategy for Patient-Oriented Research Mentorship Chair in Innovative Clinical Trials for HIV Care. D.H.S.T. has received investigator-initiated research grants from AbbVie, Gilead Sciences inc. and ViiV Healthcare. D.H.S.T. is a Site Principal Investigator for clinical trials sponsored by Glaxo Smith Kline. Also, D.H.S.T. reports grants, personal fees from Canadian Institutes of Health Research, grants from Canada Research Chairs, grants from CIHR Canadian HIV Trials Network, during the conduct of the study. S.T. reports grants from GSK, outside the submitted work. The authors report no other conflicts of interest to declare.
References
1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf.
2. Godin G, Côté J, Naccache H, Lambert LD, Trottier S. Prediction of adherence to antiretroviral therapy: a one-year longitudinal study. AIDS Care. 2005;17(4):493–504. doi:10.1080/09540120412331291715
3. Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–774. doi:10.1097/00002030-200203290-00012
4. Tesoriero J, French T, Weiss L, Waters M, Finkelstein R, Agins B. Stability of adherence to highly active antiretroviral therapy over time among clients enrolled in the treatment adherence demonstration project. J Acquir Immune Defic Syndr. 2003;33(4):484–493. doi:10.1097/00126334-200308010-00009
5. McComsey GA, Lingohr-Smith M, Rogers R, Lin J, Donga P. Real-world adherence to antiretroviral therapy among HIV-1 patients across the United States. Adv Ther. 2021;38(9):4961–4974. doi:10.1007/s12325-021-01883-8
6. Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US Medicaid population with HIV. BMJ Open. 2013;3(8):e003028. doi:10.1136/bmjopen-2013-003028
7. Werb D, Milloy MJ, Kerr T, Zhang R, Montaner J, Wood E. Injection drug use and HIV antiretroviral therapy discontinuation in a Canadian setting. AIDS Behav. 2013;17(1):68–73. doi:10.1007/s10461-012-0136-y
8. Martin LJ, Houston S, Yasui Y, Wild TC, Saunders LD. Rates of initial virological suppression and subsequent virological failure after initiating highly active antiretroviral therapy: the impact of aboriginal ethnicity and injection drug use. Curr HIV Res. 2010;8(8):649–658. doi:10.2174/157016210794088227
9. Miller CL, Spittal PM, Wood E, et al. Inadequacies in antiretroviral therapy use among Aboriginal and other Canadian populations. AIDS Care. 2006;18(8):968–976. doi:10.1080/09540120500481480
10. Sumari-de Boer IM, Sprangers MA, Prins JM, Nieuwkerk PT. HIV stigma and depressive symptoms are related to adherence and virological response to antiretroviral treatment among immigrant and indigenous HIV infected patients. AIDS Behav. 2012;16(6):1681–1689. doi:10.1007/s10461-011-0112-y
11. Logie C, James L, Tharao W, Loutfy M. Associations between HIV-related stigma, racial discrimination, gender discrimination, and depression among HIV-positive African, Caribbean, and Black women in Ontario, Canada. AIDS Patient Care STDS. 2013;27(2):114–122. doi:10.1089/apc.2012.0296
12. Remien RH, Exner TM, Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: implications for transmission of resistant virus. AIDS Behav. 2007;11(5):663–675. doi:10.1007/s10461-006-9201-8
13. Magidson JF, Li X, Mimiaga MJ, et al. Antiretroviral medication adherence and amplified HIV transmission risk among sexually active HIV-infected individuals in three diverse international settings. AIDS Behav. 2016;20(4):699–709. doi:10.1007/s10461-015-1142-7
14. UNAIDS. 90-90-90: treatment for all. Available from: https://www.unaids.org/en/resources/909090.
15. Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23(9):1035–1046. doi:10.1097/QAD.0b013e32832ba8ec
16. Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48(4):484–488. doi:10.1086/596482
17. Raboud J, Li M, Walmsley S, et al. Once daily dosing improves adherence to antiretroviral therapy. AIDS Behav. 2011;15(7):1397–1409. doi:10.1007/s10461-010-9818-5
18. Young J, Wong S, Janjua NZ, Klein MB. Comparing direct acting antivirals for hepatitis C using observational data - why and how? Pharmacol Res Perspect. 2020;8(5):e00650. doi:10.1002/prp2.650
19. Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17. doi:10.1186/s12981-018-0204-0
20. ViiV Healthcare ULC Product monograph: prTRIUMEQ. dolutegravir, abacavir, and lamivudine tablets; 2020. Available from: https://viivhealthcare.com/content/dam/cf-viiv/viiv-healthcare/en_CA/triumeq.pdf.
21. Stanford University. HIV drug resistance database. HIVdb program: mutations analysis. Available from: https://hivdb.stanford.edu/hivdb/by-patterns/.
22. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–1618. doi:10.1086/503914
23. Gross R, Bellamy SL, Chapman J, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173(4):300–306. doi:10.1001/jamainternmed.2013.2152
24. Belzer ME, Naar-King S, Olson J, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–696. doi:10.1007/s10461-013-0661-3
25. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment. Guidance for Industry; 2015. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-immunodeficiency-virus-1-infection-developing-antiretroviral-drugs-treatment.
26. Papke LE, Wooldridge JM. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. J Appl Econ. 1996;11(6):619–632. doi:10.1002/(SICI)1099-1255(199611)11:6<619::AID-JAE418>3.0.CO;2-1
27. Greenland S. Bayesian perspectives for epidemiological research: i. Foundations and basic methods. Int J Epidemiol. 2006;35(3):765–775. doi:10.1093/ije/dyi312
28. Mayring P. Qualitative content analysis. Forum Qual Soc Res. 2000;1:159–176.
29. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi:10.1177/1049732305276687
30. Fisher WA, Fisher JD, Harman J. The information-motivation-behavioral skills model: a general social psychological approach to understanding and promoting health behavior. Social Psychol Found Health Illness. 2003;22:82–106.
31. UNAIDS. 2025 AIDS targets 2021 [2022-06-15]. Available from: https://aidstargets2025.unaids.org/.
32. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490. doi:10.2147/PPA.S192735
33. Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(5):587–596. doi:10.1097/QAI.0000000000000082
34. Young J, Smith C, Teira R, et al. Antiretroviral pill count and clinical outcomes in treatment-naïve patients with HIV infection. HIV Med. 2018;19(2):132–142. doi:10.1111/hiv.12562
35. Nieuwkerk PT, de Boer-van der Kolk IM, Prins JM, Locadia M, Sprangers MA. Self-reported adherence is more predictive of virological treatment response among patients with a lower tendency towards socially desirable responding. Antivir Ther. 2010;15(6):913–916. doi:10.3851/IMP1644
36. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis. 2020;71(8):1920–1929. doi:10.1093/cid/ciz1243
37. Kanters S, Park JJ, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–e40. doi:10.1016/S2352-3018(16)30206-5
38. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. Aids. 2014;28(Suppl 2):S187–S204. doi:10.1097/QAD.0000000000000252
39. Rueda S, Mitra S, Chen S, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7):e011453. doi:10.1136/bmjopen-2016-011453
40. Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Longitudinal relationships between antiretroviral treatment adherence and discrimination due to HIV-serostatus, race, and sexual orientation among African-American men with HIV. Ann Behav Med. 2010;40(2):184–190. doi:10.1007/s12160-010-9200-x
41. Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43(4):1272–1283. doi:10.1093/ije/dyu115
42. Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–1275. doi:10.1093/cid/cir131
43. Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23(4):471–478. doi:10.1097/QAD.0b013e328325a4a9
44. Haynes RB, Ra J, Keepanasseril A, Nl W, Navarro-Ruan T, Team P. Methods for trials of interventions to enhance patient adherence to medication prescriptions, based on a systematic review of recent randomized trials. Clin Res Trials. 2015;1:20–25.
45. Dron L, Taljaard M, Cheung YB, et al. The role and challenges of cluster randomised trials for global health. Lancet Glob Health. 2021;9(5):e701–e10. doi:10.1016/S2214-109X(20)30541-6
46. Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ. 2010;340(mar19 1):c1066. doi:10.1136/bmj.c1066
47. Rubin DB. For objective causal inference, design trumps analysis. Ann Appl Stat. 2008;2(3):808–840. doi:10.1214/08-AOAS187
48. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi:10.1093/aje/kwv254
49. Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312(7040):1215–1218. doi:10.1136/bmj.312.7040.1215
50. Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol. 2013;68(4):225–236. doi:10.1037/a0032705
51. Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12(1):142. doi:10.1186/s12916-014-0142-1
52. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services; 2021. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
53. Ward M, Buehler MJ, Jaffe MH, Berkelman RL. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep Recomm Rep. 1992;41(RR–17):1–19.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Experiences of Antiretroviral Therapy Initiation Among HIV-Positive Adults in Ethiopia: A Descriptive Phenomenological Design
Tefera E, Mavhandu-Mudzusi AH
HIV/AIDS - Research and Palliative Care 2022, 14:243-254
Published Date: 24 May 2022
High Prevalence of Doravirine Resistance in HIV-1-Infected Patients with Virological Failure to an NNRTI-Based Single-Tablet Regimen
Tsai HC, Chen IT, Chang HM, Lee SSJ, Chen YS
Infection and Drug Resistance 2022, 15:3857-3869
Published Date: 20 July 2022
Risk Factors for Suboptimal Adherence Identified by Patient-Reported Outcomes Assessments in Routine HIV Care at 2 North American Clinics
Short D, Wang X, Suri S, Hsu TK, Jones B, Fredericksen RJ, Crane HM, Musten A, Bacon J, Wang Y, Gough KA, Ramgopal M, Berry J, Lober WB
Patient Preference and Adherence 2022, 16:2461-2472
Published Date: 5 September 2022
Describing Engagement in the HIV Care Cascade: A Methodological Study
Jhuti D, Zakaryan G, El-Kechen H, Rehman N, Youssef M, Garcia C, Arora V, Zani B, Leenus A, Wu M, Makanjuola O, Mbuagbaw L
HIV/AIDS - Research and Palliative Care 2023, 15:257-265
Published Date: 25 May 2023
Prevalence and Patterns of Adverse Drug Events Among Adult Patients with Human Immune Virus Infection on Dolutegravir-Based Antiretroviral Drug Regimens in Amhara Comprehensive Specialized Hospitals, Northwest Ethiopia: A Multicenter Retrospective Follow-Up Study
Zemariam AB, Tadesse YB, Kassaw AT
HIV/AIDS - Research and Palliative Care 2023, 15:271-278
Published Date: 1 June 2023

