Back to Journals » Patient Preference and Adherence » Volume 16
Risk Factors for Suboptimal Adherence Identified by Patient-Reported Outcomes Assessments in Routine HIV Care at 2 North American Clinics
Authors Short D , Wang X , Suri S, Hsu TK , Jones B, Fredericksen RJ, Crane HM, Musten A, Bacon J, Wang Y, Gough KA, Ramgopal M, Berry J, Lober WB
Received 13 June 2022
Accepted for publication 26 August 2022
Published 5 September 2022 Volume 2022:16 Pages 2461—2472
DOI https://doi.org/10.2147/PPA.S378335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Duncan Short,1 Xueqi Wang,2 Shivali Suri,3 Thomas K Hsu,2 Bryn Jones,1 Rob J Fredericksen,4 Heidi M Crane,4 Alexandra Musten,5 Jean Bacon,5 Yongwei Wang,2 Kevin A Gough,3 Moti Ramgopal,6 Jeff Berry,7 William B Lober4
1ViiV Healthcare, Brentford, UK; 2ViiV Healthcare, Durham, NC, USA; 3St Michael’s Hospital, Toronto, ON, Canada; 4Center for AIDS Research, University of Washington, Seattle, WA, USA; 5Ontario HIV Treatment Network, Toronto, ON, Canada; 6Midway Specialty Care Center, Fort Pierce, FL, USA; 7TPAN, Chicago, IL, USA
Correspondence: Duncan Short, ViiV Healthcare, 980 Great West Road, Brentford, Middlesex, TW8 9GS, UK, Tel +44 7827 282971, Email [email protected]
Purpose: Use of patient-reported outcomes assessments (PROs) can improve patient–provider communication and focus provider attention on current health issues. This analysis examines the association between suboptimal antiretroviral therapy (ART) adherence and factors obtained through PROs among people with HIV (PWH) at 2 North American outpatient clinics.
Patients and Methods: Immediately before a clinic visit, PWH completed self-administered PROs. Unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from logistic regression models to identify sociodemographic and health-related factors (satisfaction with ART, difficulty meeting housing costs, depression, intimate partner violence, risk of malnutrition, smoking status, alcohol use, and substance use) associated with suboptimal adherence (defined as self-reporting < 95% or < 80% adherence). Multiple imputation was performed to account for missing data in the multivariate analyses.
Results: Of 1632 PWH, 1239 (76%) responded to the adherence assessment; of these, 268 (22%) and 106 (9%) reported < 95% and < 80% adherence, respectively. Of 1580 PWH who responded, 354 (22%) were dissatisfied with their HIV medication. Of responding PWH, 19% reported moderate-to-severe depression, 23% indicated they were at risk of malnutrition, 34% were current smokers, and 62% reported substance use in the past 3 months. Dissatisfaction with ART was significantly associated with < 95% and < 80% adherence in the unadjusted analysis (unadjusted OR [95% CI], 3.38 [2.51– 4.56] and 4.26 [2.82– 6.42], respectively) and adjusted analysis (adjusted OR [95% CI], 2.76 [1.91– 4.00] and 3.28 [1.95– 5.52], respectively); significance remained after multiple imputation. In adjusted analyses, no risk of malnutrition was significantly associated with reduced odds of < 95% adherence after multiple imputation (adjusted OR [95% CI], 0.714 [0.511– 0.997]); no other factors were associated with < 95% or < 80% adherence.
Conclusion: These results suggest that implementation of PROs evaluating treatment satisfaction may provide value to adherence management in routine HIV care.
Keywords: patient satisfaction, antiretroviral therapy, highly active, treatment adherence, implementation science, quality of life
Introduction
Modern antiretroviral therapy (ART) regimens are highly effective at achieving virologic suppression, thereby reducing HIV-associated morbidity and mortality and increasing health-related quality of life among people with HIV (PWH).1,2 However, suboptimal adherence to ART is associated with increased rates of virologic failure.2,3 Therefore, detecting and addressing suboptimal adherence among PWH is critical in HIV clinical care.
Adherence to ART is associated with multiple behavioral, social, and clinical factors.4–7 However, identifying and addressing all factors potentially contributing to suboptimal ART adherence in individual patients can be challenging for healthcare providers during brief clinic visits.8 Screening assessments using patient-reported outcomes assessments (PROs) in routine HIV care can improve patient–provider communication and focus provider attention on symptoms or behaviors that may not otherwise be addressed, such as mental health issues and substance use.9–14 Information obtained through PROs may also aid providers in identifying barriers to ART adherence among their patients.15 For example, previous studies have demonstrated that substance use, depression, and dissatisfaction with ART are associated with suboptimal ART adherence among PWH who completed PROs at routine HIV clinic visits.8,15
The PROgress study evaluated the implementation of PROs into routine HIV care at 2 outpatient clinics in North America and assessed the added value of PRO implementation for healthcare providers and PWH.13 In the PROgress study, both healthcare providers and PWH found that PRO administration before clinic visits was useful, facilitated the discussion of sensitive topics, and improved overall patient care. Here we examine the association between sociodemographic and health-related factors obtained through PROs and suboptimal adherence to ART among PWH enrolled in the PROgress study.
Materials and Methods
Study Design and Participants
The PROgress study was a prospective, hybrid type 3 implementation-effectiveness study conducted between August 2018 and July 2020 at 2 outpatient clinics: St Michael’s Hospital (SMH) in Toronto, Ontario, Canada, and the Midway Specialty Care Center (MSCC) in Fort Pierce, Florida, USA. Detailed methodology has been previously described.13 Eligible participants were aged ≥18 years with a diagnosis of HIV who attended a participating clinic for a routine visit during the study period and could sufficiently speak and understand English, Spanish, and/or Haitian Creole to be able to complete the PRO. Individuals with psychiatric, cognitive, or motor impairment and those visiting the clinic for a non-routine reason (ie, acute illness or injury) or to see a provider other than their primary HIV care provider were excluded.
The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki. The study was approved by the SMH Research Ethics Board and the University of Washington Institutional Review Board for MSCC. All participants provided written informed consent at the time of their visit.
Study Design and Participants
Participants completed self-administered PROs on-site immediately before a routine care visit. The PROs were administered via a touch-screen tablet using a previously developed PRO platform (http://cprohealth.org). Results of completed PROs were scored using automated algorithms, summarized, and then given to the provider immediately before the clinic visit.
Assessments contained instruments evaluating several sociodemographic and health-related domains. Adherence to ART was evaluated using a visual analog scale item asking the percentage of HIV medication taken in the last month (0–100%); suboptimal adherence was defined as self-reported adherence of either <95% or <80%.16 Satisfaction with ART was assessed using the following 2 items from the HIV/AIDS-targeted quality of life (HATQoL) instrument: in the past 4 weeks, taking my [HIV] medicine has (1) “been a burden” or (2) “made it hard to live a normal life”.17 Responses were categorized using a 5-point Likert scale; dissatisfaction with ART was defined as a response of “some of the time”, “a lot of the time”, or “all of the time” to ≥1 item and satisfaction with ART was defined as a response of “a little of the time” or “none of the time” to ≥1 item. Difficulty meeting housing costs, ie, rent or mortgage, property taxes, and utilities, was assessed with a single question. Depression was assessed using the Patient Health Questionnaire 9, with a total score of >10 defined as moderate or severe depression.18,19 Intimate partner violence was evaluated using the Intimate Partner Violence 4 Questionnaire.20 Risk of malnutrition was assessed using the Canadian Nutrition Screening Tool; individuals who reported weight loss without trying in the past 6 months and eating less than usual for more than a week were defined as high risk.21 Smoking status was assessed using a single item from the Center for AIDS Research Network in Integrated Clinical Systems Smoking Questionnaire.22 Alcohol use was evaluated using the Alcohol Use Disorders Identification Test Consumption Questionnaire;23 individuals who reported having a drink containing alcohol 2 to 3 times a week or ≥4 times a week in the past year were defined as high risk. Substance use was assessed using the modified Alcohol, Smoking, and Substance Involvement Screening Test and was defined as any non-medical use of cocaine, methamphetamine, heroin, fentanyl, narcotics, sedatives, sleeping pills, marijuana, stimulants, inhalants, hallucinogens, or anabolic steroids in the past 3 months.24
Chart reviews of medical records were performed as part of the wider PROgress study evaluation and were completed for a subset of participants to obtain information on demographic and disease characteristics.
Data Analyses
Participant demographics and disease characteristics were summarized using descriptive statistics. To identify sociodemographic and health-related factors associated with suboptimal adherence of <95% and <80%, unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using univariate logistic regression models, and adjusted ORs and 95% CIs were estimated using multivariate logistic regression models. Variables that yielded P values <0.15 in unadjusted analyses were included in multivariate logistic regression analyses. Additional multivariate logistic regression models were performed using stepwise selection, with a significance level of 0.15 for a variable to enter the model and a significance level of 0.15 for a variable to stay in the model. Each multivariate logistic regression model included either the burden HATQoL item alone, the normal life HATQoL item alone, or both HATQoL items combined as variables. Current smoker and substance use (past 3 months) were not included as variables in the multivariate models due to a large number of missing values. Only participants who responded to the adherence item were included in the univariate and multivariate analyses.
To account for missing data due to non-responses in the multivariate analyses, multiple imputation was performed using the full conditional specification method with 25 imputations.25 To avoid overfitting, the number of variables was limited to less than m/10, where m is the minimum number of adherent or non-adherent participants.26 Multiple imputed results were compared with those from the full sample of participants who responded to the adherence item. P values of <0.05 were considered statistically significant for multivariate models. All analyses were performed using SAS® software version 9.4 (SAS Institute Inc, Cary, NC).
Results
Study Population
Of 1813 eligible PWH asked to participate in the study, 1632 initiated a PRO and were included in this analysis (n=600 from SMH; n=1032 from MSCC). Among 596 PWH who had data for demographic and disease characteristics available from chart reviews (n=297 from SMH; n=299 from MSCC), 69% were male at birth, 43% were Black, 28% were aged ≥60 years, and 82% had undetectable viral load (Table 1).
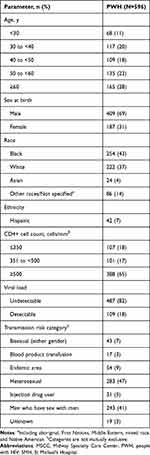 |
Table 1 Demographic and Disease Characteristics of PWH Included in Chart Reviews (SMH and MSCC) |
Characteristics of PWH Obtained from PROs
Of 1632 PWH included in this analysis, 1239 (76%) responded to the assessment relating to ART adherence; of these 268 (22%) participants reported <95% adherence and 106 (9%) reported <80% adherence (Table 2). Response rates for the other PRO instruments ranged from 61% for the substance abuse item to 99% for the risk of malnutrition and alcohol use items. Of 1580 PWH who responded to 1 or both of the HATQoL items “ … taking my [HIV] medicine has been a burden” and/or “ … taking my [HIV] medicine has made it hard to live a normal life” (response rate, 97%), 354 (22%) reported they were dissatisfied with their HIV medication. For the individual HATQoL items, dissatisfaction with their HIV medication was reported by 18% of respondents for the burden item and 16% for the normal life item. Of responding PWH, 19% reported moderate-to-severe depression, 23% indicated they were at risk of malnutrition, 34% were current smokers, and 62% reported substance use in the past 3 months. Results obtained from PROs were generally consistent across the SMH and MSCC sites, except for the proportion of PWH reporting that they were current smokers (19% at SMH vs 50% at MSCC) and the proportion reporting substance use in the past 3 months (75% at SMH vs 56% at MSCC; Supplementary Table 1).
 |
Table 2 Sociodemographic and Health-Related Characteristics of PWH Obtained from PROs (SMH and MSCC) |
Association of Suboptimal Adherence with ART Satisfaction and Characteristics of PWH
In the unadjusted analysis, dissatisfaction with ART was significantly associated with suboptimal adherence of <95% and <80% regardless of whether the burden and normal life HATQoL items were assessed individually or combined (Table 3). The significant association between ART dissatisfaction and suboptimal adherence was observed at both the SMH and MSCC sites (Supplementary Table 2). In the overall population, individuals with no risk of malnutrition and non-smokers were significantly less likely to have <95% and <80% adherence (Table 3). No difficulty meeting housing costs and mild or no depression were also significantly associated with a reduced likelihood of <95% adherence.
 |
Table 3 Unadjusted Odds Ratios for Association with <95% and <80% Adherence to ART Among PWH (N=1239; SMH and MSCC) |
In multivariate logistic regression models that included either the burden HATQoL item alone, the normal life HATQoL item alone, or both HATQoL items combined, dissatisfaction with ART was significantly associated with <95% adherence (adjusted OR [95% CI], 3.36 [2.26–4.98], 2.29 [1.49–3.52], and 2.76 [1.91–4.00], respectively; P<0.05; Table 4) and <80% adherence (adjusted OR [95% CI], 3.83 [2.25–6.53], 3.12 [1.76–5.52], and 3.28 [1.95–5.52], respectively; P<0.0001; Table 5). After multiple imputation, dissatisfaction with ART remained significantly associated with suboptimal adherence of <95% and <80% across all 3 models. After multiple imputation, no risk of malnutrition was significantly associated with reduced odds of <95% adherence in the model that included the normal life HATQoL item alone or both HATQoL items combined but not in the model that included the burden HATQoL item alone (Table 4). The other participant characteristics included in the multivariate logistic regression models were not associated with <80% adherence in any model (Table 5).
 |
Table 4 Adjusted Odds Ratios from Multivariate Logistic Regression Models for Association with <95% Adherence to ART Among PWH (SMH and MSCC) |
 |
Table 5 Adjusted Odds Ratios from Multivariate Logistic Regression Models for Association with <80% Adherence to ART Among PWH (SMH and MSCC) |
At the SMH site, dissatisfaction with ART was significantly associated with <95% adherence both before and after multiple imputation in multivariate logistic regression models including the burden HATQoL item alone or both HATQoL items combined and after multiple imputation in the model including the normal life HATQoL item alone (Supplementary Table 3). At the MSCC site, a significant association between ART dissatisfaction and <95% adherence was observed in each model both before and after multiple imputation. After multiple imputation in all 3 models, significantly reduced odds of <95% adherence were observed among individuals with no difficulty meeting housing costs at SMH but not at MSCC. At SMH, <80% adherence was significantly associated with ART dissatisfaction before and after multiple imputation in the burden HATQoL item model and after multiple imputation in the other 2 models (Supplementary Table 4); no results from multivariate logistic regression models were available for <80% adherence at MSCC because HATQoL factors were the only variables with unadjusted P values <0.15.
In multivariate stepwise selection models that included either the burden HATQoL item alone, the normal life HATQoL item alone, or both HATQoL items combined, dissatisfaction with ART was significantly associated with <95% adherence (adjusted OR [95% CI], 4.06 [2.94–5.60], 2.41 [1.70–3.42], and 3.18 [2.35–4.30], respectively; P<0.0001), with each association remaining significant after multiple imputation across all 3 models (Table 6). Before and after multiple imputation in all stepwise selection models, PWH with no risk of malnutrition were significantly less likely to have <95% adherence. No results from stepwise multivariate analyses were available for <80% adherence because the only variables remaining after stepwise selection were HATQoL factors.
 |
Table 6 Adjusted Odds Ratios from Multivariate Stepwise Selection Logistic Regression Models for Association with <95% Adherence to ART Among PWH (SMH and MSCC) |
At the SMH site, a significant association between ART dissatisfaction and <95% adherence was observed by each stepwise selection model both before and after multiple imputation (Supplementary Table 5); results from stepwise multivariate analyses at the MSCC site were not available because HATQoL factors were the only variables remaining after stepwise selection. Before and after multiple imputation at the SMH site, significantly reduced odds of <95% adherence were observed among PWH with no difficulty meeting housing costs in all 3 models and those with no risk of malnutrition in the normal life HATQoL item model.
Discussion
In this analysis, dissatisfaction with ART was significantly associated with suboptimal adherence for both the <95% and <80% adherence thresholds in multiple multivariate logistic regression models. Individuals who felt that their HIV medicines were a burden and/or made living a normal life difficult were 2.2 to 5.0 times more likely to have suboptimal adherence than those who were satisfied with their ART medications. Similarly, a cross-sectional study in Brazil demonstrated that PWH who self-reported having low or insufficient adherence had lower medication satisfaction as measured by the HATQoL instrument compared with those who self-reported having strict adherence.27 Consistent with these results, dissatisfaction with interference with daily routine, efficacy, and simplicity of ART was significantly associated with unstable or poor adherence among PWH enrolled in a cross-sectional study in Germany.28 Overall, these results indicate that dissatisfaction with ART likely contributes to suboptimal adherence in many PWH. Therefore, screening for treatment satisfaction among PWH via use of PROs may be of value in routine HIV care.
High rates of adherence were self-reported by this self-selecting sample of PWH in the PROgress study, with 78% of participants reporting ≥95% adherence and 91% reporting ≥80% adherence. These adherence rates were higher than those reported in real-world observational studies or claims database studies conducted in Canada, which showed that 56% to 67% of PWH had ≥95% adherence using either refill compliance or proportion of days covered to measure adherence.29–31 Using proportion of days covered, one US claims database study reported that 52% to 64% of PWH had ≥95% adherence, while another reported that 58% had ≥80% adherence.32,33 By contrast, other Canadian and US claims database studies reported 86% to 93% of PWH had ≥80% adherence using proportion of days covered, similar to the ≥80% adherence rates observed in the present study.31,32 Thus, adherence rates reported across studies using different adherence measurements vary widely, and comparisons between such studies should be interpreted with caution.
Because the minimum adherence level required to maintain durable virologic suppression is unclear, thresholds for optimal adherence to ART are not well defined.2 The widely used adherence level of ≥95% is primarily based on a 2000 study of PWH treated with unboosted protease inhibitors (N=81), which showed that PWH with ≥95% adherence had lower rates of virologic failure compared with those with <95% adherence.3,34 A 2019 systematic review found that >90% and >95% adherence were consistently associated with virologic suppression, with inconsistent findings observed when thresholds of <90% were used.2 A 2016 meta-analysis of 43 studies found that the odds of virologic failure were not significantly different between studies using optimal adherence thresholds of 98% to 100%, ≥95%, and 80% to 90%.3 A 2019 real-world database analysis found similar results, demonstrating no significant differences in the odds of virologic suppression for PWH with adherence levels of 80% to <85%, 85% to <90%, and ≥90%.35 Overall, these recent analyses suggest that the improved efficacy and durability of modern antiretroviral agents may allow for some dose forgiveness, with acceptable levels of virologic suppression occurring with adherence levels as low as 80% for daily oral therapy.
This analysis has some limitations. The PROgress study included a self-selecting sample from 2 clinics in North America, which may limit the generalizability of these findings. Analyses could not be controlled by demographics and disease characteristics because these data were only available for a portion of the study sample. Adherence was self-reported and can at times be overestimated and influenced by recall or reporting bias.36 In addition, adherence was assessed at a single time point and does not reflect changes in adherence over time. Dissatisfaction with ART was not assessed in the context of specific regimens in this study; as more data become available from PROs, the association between suboptimal adherence and dissatisfaction with individual ART regimens can be evaluated.
Conclusion
Use of PROs can provide important information about a patient’s adherence and related risk factors to healthcare providers in real-time. In these 2 North American HIV clinics, dissatisfaction with ART was significantly associated with suboptimal adherence among PWH, indicating the potential value of implementing PROs that evaluate treatment satisfaction in routine HIV care.
Abbreviations
ART, antiretroviral therapy; CI, confidence interval; HATQoL, HIV/AIDS-targeted quality of life; MSCC, Midway Specialty Care Center; PRO, patient-report outcomes assessment; OR, odds ratio; PWH, people with HIV; SMH, St Michael’s Hospital.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
The study was conducted in full compliance with International Conference on Harmonization Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki, and the laws and regulations of the countries in which the research was conducted. The study was approved by institutional review boards for the participating clinics. Data handling within the PRO system was in accordance with current US privacy and security regulations governing the storage and transmission of protected health information (http://tiny.cc/cirgHIPAApolicies). All study participants provided written informed consent.
Acknowledgments
We thank the patients, providers, and research staff from the 2 PROgress study clinics, the Midway Specialty Care Center in Fort Pierce, Florida, USA, and St Michael’s Hospital in Toronto, Ontario, Canada, for their commitment to this work. This study was funded by ViiV Healthcare. Editorial assistance was provided under the direction of the authors by Megan Schmidt, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by ViiV Healthcare. The study sponsor had a role in the study design; the collection, analysis and interpretation of data; and the writing of the manuscript. The decision to submit the manuscript for publication was made by the authors.
Disclosure
DS, XW, TKH, BJ, and YW are employees of ViiV Healthcare and may own stock in GSK. SS has received grants from the Ontario HIV Treatment Network. HMC has received grants from ViiV Healthcare. MR has received personal fees from Gilead, ViiV Healthcare, Janssen, and Merck for speaking engagements and/or consultancies. JBe has received personal fees from Bristol Myers Squibb, Tibotec, Merck, ViiV Healthcare, and Gilead for advisory board participation. RF, AM, JBa, KAG, and WBL have nothing to disclose.
References
1. Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161. doi:10.1101/cshperspect.a007161
2. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490. doi:10.2147/PPA.S192735
3. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine. 2016;95(15):e3361. doi:10.1097/MD.0000000000003361
4. Billoro BB, Mamo G, Jarso H. Adherence to antiretroviral therapy and associated factors among HIV infected patients in Nigist Eleni Mohammed Memorial General Hospital, Hossana, Southern Ethiopia. J AIDS Clin Res. 2018;9(8):774.
5. Joseph B, Kerr T, Puskas CM, Montaner J, Wood E, Milloy M-J. Factors linked to transitions in adherence to antiretroviral therapy among HIV-infected illicit drug users in a Canadian setting. AIDS Care. 2015;27(9):1128–1136. doi:10.1080/09540121.2015.1032205
6. Lee WK, Milloy MJS, Nosova E, Walsh J, Kerr T. Predictors of antiretroviral adherence self-efficacy among people living with HIV/AIDS in a Canadian setting. J Acquir Immune Defic Syndr. 2019;80(1):103–109. doi:10.1097/QAI.0000000000001878
7. Carvalho PP, Barroso SM, Coelho HC, Rodrigues de Oliveira Penaforte F. Factors associated with antiretroviral therapy adherence in adults: an integrative review of literature. Cien Saude Colet. 2019;24(7):2543–2555. doi:10.1590/1413-81232018247.22312017
8. Suri S, Yoong D, Short D, et al. Feasibility of implementing a same-day electronic screening tool for clinical assessment to measure patient-reported outcomes for eliciting actionable information on adherence to HIV medication and related factors in a busy Canadian urban HIV clinic. Int J STD AIDS. 2021;2021:9564624211032796.
9. Crane HM, Crane PK, Tufano JT, et al. HIV provider documentation and actions following patient reports of at-risk behaviors and conditions when identified by a web-based point-of-care assessment. AIDS Behav. 2017;21(11):3111–3121. doi:10.1007/s10461-017-1718-5
10. Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res. 2007;5(1):109–118. doi:10.2174/157016207779316369
11. Fredericksen RJ, Tufano J, Ralston J, et al. Provider perceptions of the value of same-day, electronic patient-reported measures for use in clinical HIV care. AIDS Care. 2016;28(11):1428–1433. doi:10.1080/09540121.2016.1189501
12. Høgh Kølbæk Kjær AS, Rasmussen TA, Hjollund NH, Rodkjaer LO, Storgaard M. Patient-reported outcomes in daily clinical practise in HIV outpatient care. Int J Infect Dis. 2018;69:108–114. doi:10.1016/j.ijid.2018.02.015
13. Short D, Fredericksen RJ, Crane HM, et al. Utility and impact of the implementation of same-day, self-administered electronic patient-reported outcomes assessments in routine HIV care in two North American clinics. AIDS Behav. 2022;26(7):2409–2424. doi:10.1007/s10461-022-03585-w
14. Sinha N, Yang A, Pradeep A, et al. Feasibility and acceptability of a psychosocial and adherence electronic patient reported outcomes (PROs) system at an HIV care center in southern India. AIDS Care. 2020;32(5):630–636. doi:10.1080/09540121.2019.1668532
15. Kozak MS, Mugavero MJ, Ye J, et al. Patient reported outcomes in routine care: advancing data capture for HIV cohort research. Clin Infect Dis. 2012;54(1):141–147. doi:10.1093/cid/cir727
16. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277. doi:10.1097/00002030-200201250-00017
17. Holmes WC, Shea JA. A new HIV/AIDS-targeted quality of life (HAT-QoL) instrument: development, reliability, and validity. Med Care. 1998;36(2):138–154. doi:10.1097/00005650-199802000-00004
18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
19. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737–1744. doi:10.1001/jama.282.18.1737
20. Fitzsimmons E, Loo S, Dougherty S, et al. Development and content validation of the IPV-4, a brief patient-reported measure of intimate partner violence for use in HIV care.
21. Laporte M, Keller HH, Payette H, et al. Validity and reliability of the new Canadian Nutrition Screening Tool in the ‘real-world’ hospital setting. Eur J Clin Nutr. 2015;69(5):558–564. doi:10.1038/ejcn.2014.270
22. Cropsey KL, Willig JH, Mugavero MJ, et al. Cigarette smokers are less likely to have undetectable viral loads: results from four HIV clinics. J Addict Med. 2016;10(1):13–19. doi:10.1097/ADM.0000000000000172
23. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–1795. doi:10.1001/archinte.158.16.1789
24. Newcombe DAL, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–226. doi:10.1080/09595230500170266
25. van Buuren S. Flexible Imputation of Missing Data.
26. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
27. Primeira MR, Santos WM, Paula CC, Padoin SM. Quality of life, adherence and clinical indicators among people living with HIV. Acta Paul Enferm. 2020;33:eAPE20190141. doi:10.37689/acta-ape/2020AO0141
28. Boretzki J, Wolf E, Wiese C, et al. Highly specific reasons for nonadherence to antiretroviral therapy: results from the German adherence study. Patient Prefer Adherence. 2017;11:1897–1906. doi:10.2147/PPA.S141762
29. Koehn K, McLinden T, Collins AB, et al. Assessing the impact of food insecurity on HIV medication adherence in the context of an integrated care programme for people living with HIV in Vancouver, Canada. Public Health Nutr. 2020;23(4):683–690. doi:10.1017/S1368980019002532
30. O’Neil CR, Palmer AK, Coulter S, et al. Factors associated with antiretroviral medication adherence among HIV-positive adults accessing highly active antiretroviral therapy (HAART) in British Columbia, Canada. J Int Assoc Physicians AIDS Care. 2012;11(2):134–141. doi:10.1177/1545109711423976
31. University of Calgary: Centre for Health Informatics Alberta Real-World Evidence Consortium [homepage on the Internet]. Retrospective database analysis to estimate adherence rates in PLHIV in Canada; 2020. Available from: https://cumming.ucalgary.ca/sites/default/files/teams/30/publications/Parexel%20_Canada_HIVAdherence_Report_Final_05DEC2020.pdf.
32. Farr AM, Johnston SS, Ritchings C, Brouillette M, Rosenblatt L. Persistence, adherence, and all-cause healthcare costs in atazanavir- and darunavir-treated patients with human immunodeficiency virus in a real-world setting. J Med Econ. 2016;19(4):386–396. doi:10.3111/13696998.2015.1128942
33. McComsey GA, Lingohr-Smith M, Rogers R, Lin J, Donga P. Real-world adherence to antiretroviral therapy among HIV-1 patients across the United States. Adv Ther. 2021;38(9):4961–4974. doi:10.1007/s12325-021-01883-8
34. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi:10.7326/0003-4819-133-1-200007040-00004
35. Byrd KK, Hou JG, Hazen R, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr. 2019;82(3):245–251. doi:10.1097/QAI.0000000000002142
36. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122. doi:10.15386/mpr-1201
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
