Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Valproate Reverses Mania-Like Behavior of Clockdelta19 Mouse and Alters Monoamine Neurotransmitters Metabolism in the Hippocampus
Authors Liu S, Wei J, Ni R, Gao T, Ni P, Zhao L , Duan X, Ma X , Sham PC, Li T
Received 25 November 2020
Accepted for publication 18 January 2021
Published 11 February 2021 Volume 2021:17 Pages 471—480
DOI https://doi.org/10.2147/NDT.S293482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Shasha Liu,1 Jinxue Wei,2,3 Rongjun Ni,2,3 Tianhao Gao,1 Peiyan Ni,2,3 Liansheng Zhao,2,3 Xiyu Duan,2,3 Xiaohong Ma,2,3 Pak C Sham,4,5 Tao Li1– 3,6
1Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China; 2Psychiatric Laboratory and Mental Health Center, West China Hospital of Sichuan University, Chengdu, People’s Republic of China; 3Huaxi Brain Research Center, West China Hospital of Sichuan University, Chengdu, People’s Republic of China; 4Department of Psychiatry, The University of Hong Kong, Pokfulam, Hong Kong, People’s Republic of China; 5State Key Laboratory for Cognitive and Brain Sciences, The University of Hong Kong, Pokfulam, Hong Kong, People’s Republic of China; 6Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence, Guangzhou, People’s Republic of China
Correspondence: Tao Li
Psychiatric Laboratory of West China Hospital, Sichuan University, 1 Keyuan Si Road, Chengdu, Sichuan, 610041, People’s Republic of China
Email [email protected]
Background: Mice with a deletion at exon 19 of the circadian locomotor output cycles Kaput gene (Clockdelta19) exhibit mania-like behavior and have been one of the most common animal models for bipolar disorder (BD). The predictive validity of the Clockdelta19 was investigated via studies using lithium previously. Determination of effects of other mood stabilizers on Clockdelta19 mouse would be helpful for better understanding of the mechanism underlined.
Methods: Wildtype (WT) and Clockdelta19 mice were treated with saline (n = 10 for WT and n=10 for Clockdelta19) or valproate (VPA) (n = 10 for WT and n=10 for Clockdelta19) for 10 days. The hyperactivity, anxiety-like behaviors and depression-like behaviors were tested. The concentration of monoamine neurotransmitters and their metabolites in the hippocampus of saline or VPA treated WT and Clockdelta19 mouse (n = 8 for each) were also determined.
Results: VPA can reverse hyperactivity, lower level of anxiety-like and depression-like behaviors of the Clockdelta19 mouse. Clockdelta19 mouse exhibited lower levels of serotonin (5-HT) and dopamine (DA) in right hippocampus compared to WT mouse. Chronic VPA treatment did not affect the levels of 5-HT and DA, but can reduce the level of levodopa (L-DOPA) in the right hippocampus of Clockdelta19 mouse.
Conclusion: Our results indicated that chronic VPA treatment can reverse the mania-like behaviors of the Clockdelta19 mouse and further consolidate the validity of the Clockdelta19 mouse as a model of BD. Monoamine neurotransmitters and their metabolites in the hippocampus are partly regulated by mutation of the Clock gene or VPA treatment.
Keywords: bipolar disorder, Clockdelta19 mouse, valproate, mania-like behavior, neurotransmitter
Introduction
Bipolar disorder (BD) is a common psychiatric disorder that affects about 1–3% of the population globally and is one of the leading causes of disability worldwide.1 As a severe psychiatric disorder, BD contributes to dominate the global burden of mental disorders associated with great morbidity and high risk of suicide.2,3 BD is characterized by suffering cycling through manic and depressive periods with euthymic or normal mood states between episodes.4,5 The manic state is associated with hyperactivity, little need for sleep, impulsivity, increased risk-taking behavior and increased reward-seeking; whereas the depressive state usually consists of sadness, increased sleep, guilt, and anhedonia. The underlying etiology of BD is unknown, both genetic and environmental factors are involved.6
Previous studies indicated that mice carrying a deletion of exon 19 of the circadian locomotor output cycles Kaput (Clock) gene (Clockdelta19) display behaviors that resemble mania in human.7,8 The Clockdelta19 mouse exhibit hyperactivity, decreased anxiety-like and decreased depression-like behavior, which are strikingly similar to patients of BD in the manic state.9–14 In addition, neurobiological and neurophysiological alterations that appeared on bipolar patients were also observed in the Clockdelta19 mouse. A series of studies have indicated that Clockdelta19 mouse are hyperdopaminergic, as demonstrated by elevated dopamine (DA) neuron activity in the ventral tegmental area (VTA) and increased DA signaling in the nucleus accumbens (NAc).10,15,16 Moreover, the manic-like behavioral traits and neurobiological and neurophysiological changes of the Clockdelta19 mouse could be ameliorated by lithium.2,11,13,14,17,18
Valproate (VPA) was first introduced as an anti-convulsant drug and has been commonly prescribed mood stabilizer in the treatment of BD.19 VPA is used as the typical mood stabilizer with a broad spectrum of anti-manic activity both in acute mania, cyclothymia, mixed state and rapid cycling in the treatment of BD.20 In addition, VPA is comparable with lithium in the treatment of acute mania, with relatively mild adverse reactions and somewhat better tolerated.21,22 The mechanism of effects of VPA on BD remains largely elusive. A series of studies suggested that the action of VPA on central nervous system neurons4,23,24 and neurotransmitters transmission25–27 may be relevant to its actions in BD, but these need to be further investigated. Application of Clockdelta19 mouse on the studies of mood-stabilizing effects of VPA would facilitate exploration of the mechanism of treatment effects of VPA that may be relevant to its actions in BD.
In this study, we examined the mood-stabilizing effect of VPA on the Clockdelta19 mouse. Moreover, we explored the association between neurotransmitters in brain regions and the effects of VPA on the mania-like behavior of the Clockdelta19 mouse.
Materials and Methods
Animals
Clockdelta19 mutant mice were created by N-ethyl-N-nitrosourea (ENU) mutagenesis and produce a dominant-negative CLOCK protein defective in transcriptional activity as previously described.28 All experiments using Clockdelta19 mutants were maintained on a BALB/cJ genetic background without disruption of circadian rhythm and purchased from the Jackson Laboratory. Clockdelta19 and wildtype (WT) littermates used in the study were 8 to12 week old adult male mice (weighing 19–35 g) and were group-housed in sets of 2–5 per cage with food and water ad libitum under standard conditions (22 ± 1°C; 12/12 light/dark cycle, ZT 0 = lights on 7:00 AM; ZT 12 = lights off 7:00 PM). WT and Clockdelta19 mouse treated with saline (n = 10 for WT and n = 10 for Clockdelta19) or VPA (n = 10 for WT and n = 10 for Clockdelta19) were used for behavioral studies and the determination of monoamines and their metabolites (n = 8 for each). All animal procedures were carried out in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals (Publication No. 85 23, revised 1985), and all animal protocols were approved by the Institutional Animal Care and Use Committee at West China Hospital, Sichuan University.
Drug Treatments
VPA used in this study was purchased from Sigma–Aldrich. VPA was dissolved in saline and administered in intraperitoneal (i.p.) injection at a volume of 400mg/kg. Animals were received one daily i.p. injection of VPA for 10 days before behavioral testing. VPA was freshly prepared before experiments. The control group was treated with the saline solution following the same schedule described to treatment groups. The doses of VPA employed in our study have been previously reported in the literature to prevent mania-like behavior in rodents.29,30 All injections were performed between ZT3 and ZT4 (10:00–11:00 AM).
Preparation of Behavioral Assays
Animals habituated to testing rooms for at least 30 minutes before the behavior tests. Testing occurred early in the light phase (ZT6-ZT10) (13:00–17:00). The open field test, elevated plus maze test and portlet forced swim paradigms test were performed as described previously.31
Open Field Test
The mouse was placed in the periphery of a novel open field environment in a dimly lit room and allowed to explore for 10 minutes. The apparatus is a 40 × 40-cm open field surrounded by 40-cm-high walls. The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision XT12, Noldus, Leesburg, VA) to determine the time in the center arena, total distance moved, and the number of entries into two areas: the periphery (15 cm from the walls) and the center (10 ×10 cm). Total distance moved in the open field is correlated with hyperactivity. The open field arenas were wiped with water-alcohol (75%) solution and allowed to dry between mice.
Elevated Plus Maze Test
Mice were placed in the center of an elevated plus-maze (each arm is 30-cm long and 5-cm wide with two opposite arms closed by 10-cm high walls) elevated 60cm off the floor in a dimly lit room and allowed to explore for 10 minutes. The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision XT12, Noldus, Leesburg, VA) to determine time spent in the open and closed arms, time spent in the middle, as well as the number of entries into the open and closed arms. Since rodents have an innate fear of height and openness, time spent on the open arm and the number of entries in the open arm are both correlated with anxiety-like behavior. An increase in open arm activity (duration and/or entries) reflects less anxiety behavior. The apparatus was wiped with water-alcohol (75%) solution and allowed to dry between mice.
Forced Swim Test
The forced swim test was performed as described in Krishnan et al.32 Mice were placed in a 3 litters Pyrex glass beaker containing 2 liters of water at 24 ± 1°C. The mice remained in the water for 6 min and were then removed and allowed to dry in a clean dry cage before returning to their home cage. The water was changed between each subject. The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision XT12, Noldus, Leesburg, VA) to determine the immobile time in the water. The last 4 minutes of the test was scored for latency to the first immobility and total time spent immobile. Immobility was defined as no motion, except for single limb paddling to maintain flotation.
Preparation of Brain Tissue Samples
Mice were anesthetized with chloral hydrate (50 mg/kg body weight) and sacrificed by cervical dislocation. Brains were removed and rinsed in ice-cold isotonic saline. The hippocampus was quickly dissected out and stored at −80°C until use.
Extraction of Neurotransmitters
The tissue samples were weighed and ground in liquid nitrogen. 200μL of pre-cooled water and 800μL of methanol (Merck, 144,282)/acetonitrile (Merck, 1,499,230–935) (1:1, V/V/) were added to each sample and mixed by the vortex. The ultrasonic was used for 60 seconds in an ice bath. Then, the mixed samples were incubated at −20°C to precipitate the protein and centrifuged at 14,000 × g at 4°C for 20 minutes. The supernatants that contained mixture of neurotransmitters were taken and vacuum dried.
Analysis of Neurotransmitters
The samples were separated by Shimadzu Nexera X2 LC-30AD ultra-high pressure liquid chromatography (HPLC) (Shimadzu). The operating conditions for HPLC were: Mobile phase: Solution A was 5% acetonitrile with 20mM ammonium acetate (Sigma, 70,221), PH 9.45, solution B was 100% acetonitrile. The sample was placed in the automatic sampler at 4°C, the column temperature was 40°C, the flow rate was 300μL/min, the injection volume was 5μL. A quality control (QC) sample is set for a certain number of experimental samples at every interval in a sample queue to test and evaluate the stability and repeatability of the system. Mass spectrometry (MS) analysis was performed using a 5500 QTRAP mass spectrometer (AB SCIEX) in positive ion mode. During the mass spectrometric detection, acetonitrile-aqueous solutions (1:1, V/V) of different volumes (50–200μL) were added, and the same amount of the re-standard Glutamate-D3 was added.
Statistical Analyses
The data of behavioral tests and monoamines and their metabolites were analyzed by two-way analysis of variance (ANOVA) followed by Fisher’s LSD test for post hoc analysis. Bonferroni correction was employed to adjust p values of main effects and interaction in ANOVA. All statistical analyses were performed using the software Prism version 5.0 (Graphpad Software Inc, San Diego, USA). Adjusted p values less than 0.05 were considered statistically significant.
Results
VPA Reversed the Hyperactivity of Clockdelta19 Mouse
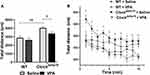
We first accessed the locomotor activity of Clockdelta19 and WT mouse treated with saline or VPA by analyzing the total distance moved in the open field test. The result showed that there is no statistically significant interaction between the effect of the Clock gene and drug treatment (F (1,36) = 0.99, p = 0.33) on total distance moved (Table S1). The main effects analysis indicated that both Clock gene (F (1, 36) = 22.63, p < 0.0001) and drug treatment (F (1,36) = 5.08, p = 0.03) had significant effects on total distance moved (Table S1). The Fisher’s LSD test for post hoc analysis revealed that when treated with saline, Clockdelta19 mouse exhibit a significant increase in the total distance moved compared to the WT mouse (p<0.0001) (Table S2, Figure 1A). VPA treatment significantly decreased the total distance moved of Clockdelta19 mouse in the open field test (p = 0.028) (Table S2, Figure 1A). There is no significant difference in the total distance moved between saline-treated WT mouse and VPA-treated Clockdelta19 mouse (p = 0.09) (Table S2, Figure 1A). We examined the total distance moved of Clockdelta19 mouse and WT mouse treated with saline or VPA for every minute over 10 minutes in the open field. The results also indicated that Clockdelta19 mouse exhibit a significant increase in the total distance moved for every minute compared to the WT mouse, VPA treatment prevents the increased total distance moved in Clockdelta19 mouse (Figure 1B). These results suggested that the hyperactivity presented in Clockdelta19 mouse can be reversed by chronic VPA treatment.
VPA Reversed the Level of Anxiety-Like Behavior of Clockdelta19 Mouse
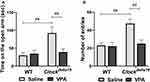
We then examined anxiety-like behavior in Clockdelta19 and WT mouse treated with saline or VPA by analyzing time in open arms and the number of entries in open arms in the elevated plus-maze test. There is a statistically significant interaction between the effect of the Clock gene and drug treatment (F (1, 34) = 6.84, p = 0.013) on time in open arms (Table S1). The main effects analysis indicated that both Clock gene (F (1, 34) = 9.20, p = 0.005) and drug treatment (F (1,34) = 4.86, p = 0.034) had significant effects on time in the open arms (Table S1). The Fisher’s LSD test for post hoc analysis revealed that when treated with saline, Clock delta19 mouse exhibited a significant increase in the time in open arms compared to the WT mouse (p<0.0001) (Table S2, Figure 2A). In Clock delta19 mouse, VPA treatment significantly decreased the time in the open arms (p = 0.002) (Table S2, Figure 2A). There is no significant difference in the time in the open arms between saline-treated WT mouse and VPA-treated Clockdelta19 mouse (p = 0.55) (Table S2, Figure 2A). There is a statistically significant interaction between the effect of the Clock gene and drug treatment (F (1, 34) = 7.01, p = 0.012) on the number of entries in the open arms (Table S1). The main effects analysis indicated that both Clock gene (F (1, 34) = 11.12, p = 0.002) and drug treatment (F (1, 34) = 8.09, p = 0.007) had significant effects on number of entries in the open arms (Table S1). The Fisher’s LSD test for post hoc analysis revealed that when treated with saline, Clockdelta19 mouse exhibit a significant increase in the number of entries in the open arms compared to the WT mouse (p < 0.0001) (Table S2, Figure 2B). In Clockdelta19 mouse, VPA treatment significantly decreased the number of entries in the open arms (p = 0.001) (Table S2, Figure 2B). There is no significant difference in the number of entries between saline-treated WT mouse and VPA-treated Clockdelta19 mouse (p = 0.72) (Table S2, Figure 2B). These results suggested that decreased anxiety-like behavior in Clockdelta19 mouse can be reversed by chronic VPA treatment.
VPA Reversed the Level of Depression-Like Behavior of Clockdelta19 Mouse
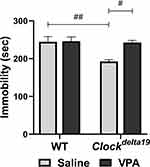
We examined the depression-like behavior of Clockdelta19 and WT mouse treated with saline or VPA by analyzing immobile time in the forced swim test. The result showed that there is a statistically significant interaction between the effect of the Clock gene and drug treatment (F (1,30) = 5.67, p = 0.024) on immobile time (Table S1). The main effects analysis indicated that both Clock gene (F (1, 30) = 7.42, p = 0.011) and drug treatment (F (1, 30) = 6.38, p = 0.017) had significant effects on immobile time (Table S1). The Fisher’s LSD test for post hoc analysis revealed that when treated with saline, Clock delta19 mouse exhibit a significant decrease in immobile time compared to the WT mouse (p = 0.001) (Table S2, Figure 3). In Clockdelta19 mouse, VPA treatment significantly increased the immobile time (p = 0.002) (Table S2, Figure 3). There is no significant difference in immobile time between saline-treated WT mouse and VPA-treated Clockdelta19 mouse (p = 0.90) (Table S2, Figure 3). These results suggested decrease depression-like behavior in Clockdelta19 mouse can be reversed by chronic VPA treatment.
Monoamines and Their Metabolites in the Hippocampus
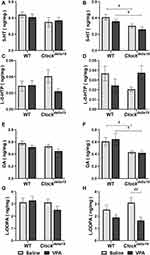
To explore the potential roles of neurotransmitters in the effect of VPA on mania-like behavior of Clockdelta19 mouse, we determined levels of dopamine (DA), serotonin (5-HT), levodopa (L-DOPA) and 5-hydroxy-L-tryptophan (L-5-HTP) in the hippocampus of Clockdelta19 and WT mouse treated with saline or VPA. There were no statistically significant interactions between the effect of the Clock gene and drug treatment on levels of the four neurotransmitters tested in this study (Table S3). The main effects analysis indicated Clock gene had significant effects on the levels of 5-HT (F = 13.02, p = 0.001) and DA (F = 13.35, p = 0.001) in right hippocampus (Table S3), drug treatment had significant effects on the levels of L-DOPA in right hippocampus (F = 10.32, p = 0.003) (Table S3). The Fisher’s LSD test for post hoc analysis revealed that Clockdelta19 mouse exhibit a significant decrease in levels of 5-HT (p = 0.013 when treated with saline; p = 0.02 when treated with VPA) and DA (p = 0.023 when treated with saline; p = 0.011 when treated with VPA) in right hippocampus compared to the WT mouse (Table S4, Figure 4B and F). There was no significant difference in the level of 5-HT and DA in left hippocampus between Clockdelta19 mouse and WT mouse treated with saline or VPA (Table S4, Figure 4A and E). VPA treatment significantly decreased levels of L-DOPA (p = 0.004) in right hippocampus in Clockdelta19 mouse (Table S4, Figure 4H). In left hippocampus, no significant difference in the level of L-DOPA was observed between Clockdelta19 mouse and WT treated with saline or VPA (Table S4, Figure 4G). There was no significant difference in L-5-HTP level in the hippocampus between Clockdelta19 mouse and WT treated with saline or VPA (Table S4, Figure 4C and D).
Discussion
In this study, we examined the effects of VPA on the mania-like behaviors in Clockdelta19 mouse. We also explored the potential roles of VPA on DA and 5-HT and their metabolites in brain regions. We found that VPA can reverse the mania-like behavior observed in the Clockdelta19 mouse. Our results also showed that there were significant differences in the level of monoamines and their metabolites in the left and right hippocampus of Clockdelta19 mouse compared with WT mouse. Administration of VPA partially altered the level of neurotransmitters in the right hippocampus of Clockdelta19 mouse. These results suggest that the mania-like behaviors of Clockdelta19 mouse may be related to the changed level of DA and 5-HT systems and the treatment effects of VPA on Clockdelta19 mouse may be related to DA and 5-HT metabolism.
Clockdelta19 mouse have been proved to be an animal model for BD with substantial face and predictive validity. Clockdelta19 mouse exhibited hyperactivity, decreased anxiety-related behavior, and decreased depression-related behavior that similar to the manic state of patients with BD.9–11,14,15,17,33–36 Chronic lithium treatment could reverse behavioral abnormalities9,11,15,17,18 and neurobiological abnormalities observed in the Clockdelta19 mouse.3,15,18,37 In this study, we indicated that chronic VPA treatment can reverse the mania-like behavior of the Clockdelta19 mouse. This result further proved the well predictive validity of the Clockdelta19 mouse as the model of BD underly the previous studies. It would be interesting in the future to examine the effect of other medication treatments frequently used in BD on the Clockdelta19 mouse, such as lamotrigine, carbamazepine, oxcarbazepine, antipsychotics, and electroconvulsive therapy. In addition, our results identified that WT mouse are not altered behaviorally by administrated with VPA. This can against the possibility that VPA is causing the Clockdelta19 mouse to not feel well and thus simply lowers their activity and behavior on all measures, which further consolidates the credibility of our study results.
VPA was first introduced as an anticonvulsant and has been widely used as a mood stabilizer in the treatment of BD for many years.38 VPA is effective for the treatment of acute mania and the maintenance therapy for mania prevention.39,40 Our study identified that chronic VPA treatment can reverse the mania-like behavior of the Clockdelta19 mouse. This is consistent with the clinical treatment effect of VPA on BD patients. However, some important aspects of VPA known from human studies remain to be investigated. Studies suggesting that VPA causes users tremors, which may be related to its anticonvulsant effect. To further consolidate the predictive validity of the Clockdelta19 mouse as an animal model for BD, it would be of interest to investigate whether VPA also causes to develop tremors in this mouse strain. Similarly, cosmetic side effects such as hair loss, color changes and curling of hair have also been reported in VPA-treated patients.41 To strengthen the predictive validity of the Clockdelta19 mouse as an animal model for BD, future studies should examine whether the Clockdelta19 mouse also emerges cosmetic side effects following treatment with VPA. In addition, the mechanisms of action of VPA are still not fully understood. The application of the Clockdelta19 mouse on the studies of mood-stabilizing effects of VPA would facilitate exploration of the pathophysiology underlying.
Previous studies have indicated that dopaminergic abnormalities were observed in several brain regions of the Clockdelta19 mouse, such as the ventral tegmental area (VTA),11 striatum16 and nucleus accumbens (NAC).15 In this study, we found that the Clockdelta19 mouse exhibited a reduction of DA compared with the WT mouse in the right hippocampus. Hippocampus is a critical brain region that has been associated with depression and anxiety. The expression of the Clock gene in the hippocampus is important in regulating mood-related behaviors. The antidepressant treatment has been shown to increase Clock expression in the hippocampus.42 Clockdelta19 mouse display mania-like behavior and exhibit a lower level of DA compared with the WT mouse in the right hippocampus in our study. It can be suspected that there may be underline relationship between mania-like behavior phenotype and the alteration on level of DA. However, the lower level of DA was observed in right hippocampus of Clockdelta19 mouse in our study, which is inconsistent with the elevated dopaminergic activity in VTA of the Clockdelta19 mouse.15 It is maybe relevant to the expression of the Clock gene in the hippocampus in the Clockdelta19 mouse. Study had indicated that the Clock gene has the potential to regulate the second group of genes including the genes related to the dopamine system.43 Thus, we could suspect that the discrepancy on the level of DA in the Clockdelta19 mouse may be relevant to the regulation of the Clock gene in the hippocampus. It is also likely that the Clock gene in other brain regions is important in regulating mood-related behaviors. The ventral and orbital part of the medial prefrontal cortex (mPFC) has been suggested to contribute to the emotional regulation impairment in bipolar disorder.44 It is a limitation that the present study did not explore the function of the Clock gene in mPFC.
Simultaneously, we found that VPA could reduce the level of L-DOPA in the right hippocampus of the Clockdelta19 mouse. As the major metabolites of DA, the level of L-DOPA in the brain indicates the turnover of DA. The effect of VPA on L-DOPA in right hippocampus of the Clockdelta19 mouse is consistent with the effect of lithium on DA and its metabolisms in VTA and NAC of Clockdelta19 mouse.15,18 Thus, the effects of VPA on the mania-like behavior of the Clockdelta19 mouse may be related to the dopaminergic activity in the hippocampus. Further experiments are needed to implement and to explore the underlying mechanism in the future.
The central 5-HT system is known to be involved in emotion, learning, and memory.45 The pathophysiology of anxiety and depression involves dysfunctions in serotonergic activity.46 Our results showed that there is a lower level of 5-HT in right hippocampus of the Clockdelta19 mouse compared with WT mouse. Studies explicated that deficiency of 5-HT is thought to cause depression and anxiety.47,48 Yun-Fang Jia et al49 showed that the anxiety-like behaviors of the central 5-HT-deficient mouse were decreased and the depression-like behaviors were similar to the control mouse. We supposed that the alteration of anxiety and depression-like behavior in the Clockdelta19 mouse may be related to the serotonergic activity in the hippocampus. Besides, we could not exclude the possibility that the Clock gene influenced the behavioral performances of the Clockdelta19 mouse by possible regulatory mechanisms that are associated with the 5-HT transmitter or serotonergic neurons. Our current investigation provides suggestive research data on the relationship between serotonergic and psychiatric disorders.
In conclusion, our study indicated that chronic VPA treatment can reverse the mania-like behavior of the Clockdelta19 mouse, which further consolidated the predictive validity of the Clockdelta19 mouse as an animal model for BD. Monoamine neurotransmitters and their metabolites in the hippocampus are partly regulated by mutation of the Clock gene or VPA treatment.
Ethics Approval and Consent to Participate
The experiments had approval by the Institute for Experimental Animals of West China Hospital, Sichuan University.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81920108018); Special Foundation for Brain Research from Science and Technology Program of Guangdong (2018B030334001); Key R & D projects of Science and Technology Department of Sichuan Province (2019YFS0535, 2019YFS0039); 1.3.5 Project for disciplines of excellence, West China Hospital of Sichuan University (ZY2016103, ZY2016203, and ZYGD20004).
Disclosure
All authors declare no personal or financial conflicts of interest in this work.
References
1. Merikangas K, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi:10.1016/j.jaac.2010.05.017
2. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet. 2002;359(9302):241–247. doi:10.1016/s0140-6736(02)07450-0
3. Bauer M, Altshuler L, Evans D, Beresford T, Williford W, Hauger R. Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Disord. 2005;85(3):301–315. doi:10.1016/j.jad.2004.11.009
4. Miklowitz D, Johnson S. The psychopathology and treatment of bipolar disorder. Ann Rev Clin Psych. 2006;2:199–235. doi:10.1146/annurev.clinpsy.2.022305.095332
5. Belmaker R, Bersudsky Y. Bipolar disorder: mania and depression. Discov Med. 2004;4(23):239–245.
6. Marangoni C, Hernandez M, Faedda G. The role of environmental exposures as risk factors for bipolar disorder: a systematic review of longitudinal studies. J Affect Disord. 2016;193:165–174. doi:10.1016/j.jad.2015.12.055
7. King D, Zhao Y, Sangoram A, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89(4):641–653. doi:10.1016/s0092-8674(00)80245-7
8. Gekakis N, Staknis D, Nguyen H, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280(5369):1564–1569. doi:10.1126/science.280.5369.1564
9. Easton A, Arbuzova J, Turek F. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2(1):11–19. doi:10.1034/j.1601-183x.2003.00002.x
10. McClung C, Sidiropoulou K, Vitaterna M, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102(26):9377–9381. doi:10.1073/pnas.0503584102
11. Roybal K, Theobold D, Graham A, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. doi:10.1073/pnas.0609625104
12. Ozburn A, Larson E, Self D, McClung C. Cocaine self-administration behaviors in ClockΔ19 mice. Psychopharmacology. 2012;223(2):169–177. doi:10.1007/s00213-012-2704-2
13. Ozburn A, Falcon E, Mukherjee S, et al. The role of clock in ethanol-related behaviors. Neuropsychopharmacology. 2013;38(12):2393–2400. doi:10.1038/npp.2013.138
14. van Enkhuizen J, Minassian A, Young J. Further evidence for ClockΔ19 mice as a model for bipolar disorder mania using cross-species tests of exploration and sensorimotor gating. Behav Brain Res. 2013;249:44–54. doi:10.1016/j.bbr.2013.04.023
15. Coque L, Mukherjee S, Cao J, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology. 2011;36(7):1478–1488. doi:10.1038/npp.2011.33
16. Spencer S, Torres-Altoro M, Falcon E, et al. A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem. 2012;123(1):124–134. doi:10.1111/j.1471-4159.2012.07857.x
17. Arey R, McClung C. An inhibitor of casein kinase 1 ε/δ partially normalizes the manic-like behaviors of the ClockΔ19 mouse. Behav Pharmacol. 2012;23(4):392–396. doi:10.1097/FBP.0b013e32835651fd
18. Dzirasa K, Coque L, Sidor M, et al. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30(48):16314–16323. doi:10.1523/jneurosci.4289-10.2010
19. Goodwin G, Haddad P, Ferrier I, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495–553. doi:10.1177/0269881116636545
20. Kusumakar V, Yatham L, Haslam D, et al. Treatment of mania, mixed state, and rapid cycling. Can J Psychiatry. 1997;42 Suppl 2:79S–86S.
21. Vining E, Mellitis E, Dorsen M, et al. Psychologic and behavioral effects of antiepileptic drugs in children: a double-blind comparison between phenobarbital and valproic acid. Pediatrics. 1987;80(2):165–174.
22. Bowden C, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57(5):481–489. doi:10.1001/archpsyc.57.5.481
23. Einat H, Yuan P, Gould T, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23(19):7311–7316. doi:10.1523/jneurosci.23-19-07311.2003
24. Kim A, Shi Y, Austin R, Werstuck G. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci. 2005;118(1):89–99. doi:10.1242/jcs.01562
25. Dixon J, Hokin L. The antibipolar drug valproate mimics lithium in stimulating glutamate release and inositol 1,4,5-trisphosphate accumulation in brain cortex slices but not accumulation of inositol monophosphates and bisphosphates. Proc Natl Acad Sci U S A. 1997;94(9):4757–4760. doi:10.1073/pnas.94.9.4757
26. Freeman M, Freeman S, McElroy S. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology, and treatment issues. J Affect Disord. 2002;68(1):1–23. doi:10.1016/s0165-0327(00)00299-8
27. Li X, Ketter T, Frye M. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord. 2002;69:1–14. doi:10.1016/s0165-0327(00)00361-x
28. King D, Vitaterna M, Chang A, et al. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics. 1997;146(3):1049–1060.
29. Barbosa F, Hesse B, de Almeida R, Baretta I, Boerngen-Lacerda R, Andreatini R. Magnesium sulfate and sodium valproate block methylphenidate-induced hyperlocomotion, an animal model of mania. Pharmacol Rep. 2011;63(1):64–70. doi:10.1016/s1734-1140(11)70399-1
30. Souza L, Silva E, Santos W, et al. Lithium and valproate prevent methylphenidate-induced mania-like behaviors in the hole board test. Neurosci Lett. 2016;629:143–148. doi:10.1016/j.neulet.2016.06.044
31. Mukherjee S, Coque L, Cao J, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68(6):503–511. doi:10.1016/j.biopsych.2010.04.031
32. Krishnan V, Han M, Graham D, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi:10.1016/j.cell.2007.09.018
33. Dzirasa K, McGarity D, Bhattacharya A, et al. Impaired limbic gamma oscillatory synchrony during anxiety-related behavior in a genetic mouse model of bipolar mania. J Neurosci. 2011;31(17):6449–6456. doi:10.1523/jneurosci.6144-10.2011
34. Bernardi R, Spanagel R. The Clock 19 mutation in mice fails to alter the primary and secondary reinforcing properties of nicotine. Drug Alcohol Depend. 2013;133(2):733–739. doi:10.1016/j.drugalcdep.2013.08.024
35. Bernardi R, Spanagel R. Enhanced extinction of contextual fear conditioning in ClockΔ19 mutant mice. Behav Neurosci. 2014;128(4):468–473. doi:10.1037/a0037020
36. Sidor M, Spencer S, Dzirasa K, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry. 2015;20(11):1406–1419. doi:10.1038/mp.2014.167
37. Arey R, Enwright J, Spencer S, et al. An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Mol Psychiatry. 2014;19(3):342–350. doi:10.1038/mp.2013.12
38. Emrich H, von Zerssen D, Kissling W, Möller H. Therapeutic effect of valproate in mania. Am J Psychiatry. 1981;138(2):256.
39. Besag F. Behavioural effects of the newer antiepileptic drugs: an update. Expert Opin Drug Saf. 2004;3(1):1–8. doi:10.1517/14740338.3.1.1
40. Findling R, McNamara N, Youngstrom E, et al. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(5):409–417. doi:10.1097/01.chi.0000155981.83865.ea
41. Kakunje A, Prabhu A, Sindhu Priya E, et al. Valproate: it’s effects on hair. Int J Trichology. 2018;10(4):150–153. doi:10.4103/ijt.ijt_10_18
42. Uz T, Ahmed R, Akhisaroglu M, et al. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134(4):1309–1316. doi:10.1016/j.neuroscience.2005.05.003
43. Oishi K. [Clock genes and clock-controlled genes in mammals]. Nihon Rinsho. 2012;70(7):1109–1114.
44. Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829,833–857. doi:10.1038/mp.2008.65
45. Duman R, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi:10.1016/j.tins.2011.11.004
46. Naughton M, Mulrooney J, Leonard B. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15(6):397–415.
47. Jacobsen J, Medvedev I, The CM. 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367(1601):2444–2459. doi:10.1098/rstb.2012.0109
48. Albert P, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi:10.3389/fnbeh.2014.00199
49. Jia Y, Song N, Mao R, et al. Abnormal anxiety- and depression-like behaviors in mice lacking both central serotonergic neurons and pancreatic islet cells. Front Behav Neurosci. 2014;8:325. doi:10.3389/fnbeh.2014.00325
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.