Back to Journals » Patient Related Outcome Measures » Volume 6
Validity and reliability of the Patient-Reported Arthralgia Inventory: validation of a newly-developed survey instrument to measure arthralgia
Authors Castel L, Wallston K, Saville B, Alvarez J, Shields B, Feurer I, Cella D
Received 11 May 2013
Accepted for publication 18 May 2015
Published 28 July 2015 Volume 2015:6 Pages 205—214
DOI https://doi.org/10.2147/PROM.S47997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Howland
Liana D Castel,1 Kenneth A Wallston,2 Benjamin R Saville,3 JoAnn R Alvarez,3 Bradley D Shields,4 Irene D Feurer,3 David Cella5
1Meharry-Vanderbilt Alliance, Nashville, TN, USA; 2Psychology in Nursing, Vanderbilt University School of Nursing, Nashville, TN, USA; 3Surgery and Biostatistics, Vanderbilt University School of Medicine, Nashville, TN, USA; 4Medical Sciences, University of Arkansas School of Medicine, Little Rock, AR, USA; 5Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Background: There is a need for a survey instrument to measure arthralgia (joint pain) that has been psychometrically validated in the context of existing reference instruments. We developed the 16-item Patient-Reported Arthralgia Inventory (PRAI) to measure arthralgia severity in 16 joints, in the context of a longitudinal cohort study to assess aromatase inhibitor-associated arthralgia in breast cancer survivors and arthralgia in postmenopausal women without breast cancer. We sought to evaluate the reliability and validity of the PRAI instrument in these populations, as well as to examine the relationship of patient-reported morning stiffness and arthralgia.
Methods: We administered the PRAI on paper in 294 women (94 initiating aromatase inhibitor therapy and 200 postmenopausal women without breast cancer) at weeks 0, 2, 4, 6, 8, 12, 16, and 52, as well as once in 36 women who had taken but were no longer taking aromatase inhibitor therapy.
Results: Cronbach's alpha was 0.9 for internal consistency of the PRAI. Intraclass correlation coefficients of test-retest reliability were in the range of 0.87–0.96 over repeated PRAI administrations; arthralgia severity was higher in the non-cancer group at baseline than at subsequent assessments. Women with joint comorbidities tended to have higher PRAI scores than those without (estimated difference in mean scores: -0.3, 95% confidence interval [CI] -0.5, -0.2; P<0.001). The PRAI was highly correlated with the Functional Assessment of Cancer Therapy-Endocrine Subscale item “I have pain in my joints” (reference instrument; Spearman r range: 0.76–0.82). Greater arthralgia severity on the PRAI was also related to decreased physical function (r=-0.47, 95% CI -0.55, -0.37; P<0.001), higher pain interference (r=0.65, 95% CI 0.57–0.72; P<0.001), less active performance status (estimated difference in location (-0.6, 95% CI -0.9, -0.4; P<0.001), and increased morning stiffness duration (r=0.62, 95% CI 0.54–0.69; P<0.0001).
Conclusion: We conclude that the psychometric properties of the PRAI are satisfactory for measuring arthralgia severity.
Keywords: arthralgia, joint pain, pain measurement, validation studies, questionnaire design, aromatase inhibitors, postmenopause
Introduction
The Menopause Quality of Life/Breast Cancer Adjuvant Therapy longitudinal cohort study was initiated in 2009 to investigate arthralgia (defined as inflammatory or non-inflammatory joint pain) in postmenopausal women without breast cancer and in women undergoing aromatase inhibitor (AI) therapy. Since 2006, AIs have been the standard of care to prevent recurrence of hormone-sensitive early breast cancer in postmenopausal women. Arthralgia occurs more frequently with age,1,2 and is a noted secondary effect of AIs.3–6
While several validated instruments exist to measure arthritis (inflammatory joint pain),7–15 options for measuring arthralgia are limited. Because it may be non-inflammatory, arthralgia might not manifest signs that are readily measurable by a clinician. Hence, finding external and objective clinical criteria to measure arthralgia effectively poses even more of a challenge than it does for arthritis. Clinical arthritis assessments such as the Disease Activity Index for Rheumatoid Arthritis16 are made up of subscales of tender and swollen joint counts. Among rheumatoid arthritis patients, Pearson correlation coefficients for clinician–patient agreement on tender and swollen joint counts have been estimated in meta-analysis to be 0.61 (tender) and 0.44 (swollen).17 Thus, even with inflammatory joint pain, which has an externally observable component, patients’ self-assessments are often quite different from clinicians’ evaluations.
Magnetic resonance imaging may reveal disease processes of AI-associated arthralgia.18–20 However, as is often the case with the measurement of pain, no simple low-cost clinical test or biomarker for arthralgia is available for use in regular clinical practice. In addition, the role of inflammation and other biomarkers in arthralgia remains unclear; past efforts have been unsuccessful in validating patient-reported arthritis pain instruments against erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, anti-double-stranded DNA, anticyclic citrullinated peptide, rheumatoid factor, and uric acid.21,22 Therefore, the vast majority of research and clinical practice must rely on patient reports for assessment of arthralgia.
The Western Ontario and McMaster Universities Arthritis Index7 and Joint-Specific Multidimensional Assessment of Pain23 measure pain intensity and affect, but in only one joint at a time (eg, knees). The Joint-Specific Multidimensional Assessment of Pain includes ten items per joint queried. These scales do not allow one to study arthralgia in multiple joints simultaneously, yet arthralgia was observed to occur in more than one joint location in the Breast Cancer Adjuvant Therapy cohort study.5 Querying only one specific joint or bilateral joint pair could miss potential statistically or clinically significant arthralgia in other areas of the body. The Regional Pain Scale comes closer to measuring the construct of pain throughout the body, but queries pain severity in both nonarticular and articular regions. The Regional Pain Scale has only four response options (none, mild, moderate, or severe),24 while 11-point (0–10) numeric rating scales have been demonstrated in past methodological research to have a higher degree of sensitivity and validity for pain assessment.25
A reliable and valid measure of arthralgia severity is needed to assemble consistent epidemiological outcomes information about prevalence, incidence, time to onset, risk factors, severity, and duration of arthralgia, as well as to better understand the role of arthralgia in the effectiveness of AI treatment and the relationship between inflammation and AI-associated arthralgia. Such a measure is also needed to develop AI adherence interventions based on arthralgia management strategies; improving management of arthralgia has been suggested as the key to promoting AI adherence.26
In order to address the need for a valid and reliable arthralgia severity measurement instrument, we developed the Patient-Reported Arthralgia Inventory (PRAI) in the context of a longitudinal cohort study of arthralgia, health-related quality of life, and medication adherence. We adapted the PRAI from the articular regions of the Regional Pain Scale in collaboration with its author,24 revising the response options to follow best practices in patient-reported outcome measurement27 and pain measurement. The PRAI uses a 0–10 numeric rating scale to assess pain severity over a recall period of the preceding 7 days. Our objectives were to evaluate the psychometric properties of the PRAI, assessing the scale’s reliability and construct validity, and exploring how duration of morning stiffness relates to arthralgia.
Materials and methods
Study design and setting
Survey data were collected in a longitudinal prospective cohort study (ClinicalTrials.gov identifier NCT00954564), with 52 weeks of follow-up per participant. Written informed consent was obtained from each study participant. The study was approved by the Vanderbilt University institutional review board and consisted of self-administered paper questionnaires completed at weeks 0, 2, 4, 6, 8, 12, and 52. Study personnel at Vanderbilt Ingram Cancer Center, Vanderbilt Institute for Medicine and Public Health, and Vanderbilt Women’s Health Research conducted the screening, enrollment, data collection, and analysis. Data were managed using Research Electronic Data Capture (REDCap).28
Participants
We recruited participants into three groups: women initiating AIs, women who had taken AIs but were no longer on AI therapy, and postmenopausal women who had never been diagnosed with breast cancer (comparison group). We chose these three groups to serve the aims of our parent study’s program of research to understand the time course and duration of treatment-emergent arthralgia with regard to AI initiation and cessation. Because arthralgia is common in postmenopausal women irrespective of breast cancer diagnosis or endocrine therapy (affecting 45%–55% of the population),1,2 the comparison group was selected to assess the background rate of arthralgia in menopause, thus helping us quantify treatment-emergent arthralgia severity in the parent study. Participants were recruited either by their treating physician at the Vanderbilt University Medical Center or via study advertisements in the greater Nashville area between 2009 and 2013. Participants in all groups had to be female, postmenopausal (self-report of at least 12 months without a menstrual period, unrelated to surgery or medication), 35–90 years of age, and have active self-reported performance status (≤3).29
Participants in the first group had to initiate anastrozole, exemestane, or letrozole within 30 days of baseline assessment. Those in the second group had to have taken one of these three medications in the past and since discontinued for any reason. Comparison group participants had to acknowledge never having been told by a physician that they had breast cancer. Patients were ineligible if they were undergoing treatment for any other (nonbreast) cancer, were unable to provide informed consent, did not speak English, were pregnant, or had metastatic disease. Screening was done by telephone or via online self-administration. For the group of women who had stopped taking AIs, only a baseline survey was administered. Follow-up for the longitudinal AI and comparison groups was done by telephone and mail contact to assist participants in staying on schedule. Women were considered lost to follow-up if they had missed more than two surveys in a row and could not be reached after six attempts. Cohort screening and enrollment, as well as the derivation of the final analytic sample, are shown in Figure 1.
  | Figure 1 Flow diagram of cohort screening and enrollment. |
Variables
We developed and administered the PRAI for the purpose of measuring arthralgia severity in multiple joint locations. The PRAI consists of 16 items that query pain severity over the last 7 days in eight joint pair groups: bilateral (left and right as two separate sets of items) fingers, wrists, elbows, shoulders, hips, knees, ankles, and toes. The questionnaire uses a rating scale of 0–10, with 10 being greatest severity (Table S1). We scored the PRAI by first calculating the sum of responses across all joints, ranging from 0 to 160. For ease of interpretation, the average severity of pain per joint (0–10) was then calculated by dividing the total score by the number of items answered.
Data on several related constructs were collected concomitantly with the PRAI. Comorbidities were assessed using a checklist. For the analyses we categorized comorbidities as joint-related (osteoarthritis, rheumatoid arthritis, psoriatic arthritis, lupus, gout, ankylosing spondylitis, fibromyalgia, osteoporosis, osteopenia, or Sjogren’s syndrome) or not joint-related (diabetes, heart disease, hypertension, chronic obstructive pulmonary disease, Parkinson’s disease, renal failure, urinary tract problems, gastrointestinal problems, anemia, eye problems, or no other comorbidity). We assessed physical function and pain interference using the previously validated Patient-Reported Measurement Information System (PROMIS) scales.30,31 PROMIS physical function and pain interference scores were standardized according to the PROMIS instructions. We measured performance status using a patient-reported adaptation of the Eastern Cooperative Oncology Group (ECOG) performance status measure.32 The Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES) was administered to measure menopausal symptom severity.33 The last item of the FACT-ES instrument is “I have pain in my joints”, with the following response options: “None,” “A little bit,” “Somewhat,” “Quite a bit,” and “Very much.” This item about arthralgia was added to the FACT-ES as a result of the findings regarding secondary effects of the AI anastrozole in the Arimidex, Tamoxifen Alone or in Combination trial.34,35
As a patient-reported proxy for the presence of inflammation,36 we collected information regarding the duration of morning stiffness in minutes. Respondents who answered “yes” to the question “Over the past 7 days, have you had overall morning stiffness in your muscles or joints?” were asked how many minutes the morning stiffness had lasted. A check box was available to indicate if the morning stiffness had lasted more than 120 minutes. If a value for duration was entered and the “more than 120 minutes” box checked, we used the reported value.
Statistical methods
To assess the reliability of the PRAI, we calculated its internal consistency via Cronbach’s alpha for the overall score and for the score with each item removed. Additionally, we assessed test-retest reliability in the comparison group, in which minimal change would be expected, by examining the change in scores assessed at 2-week intervals (weeks 0 and 2, weeks 2 and 4, and so on). For each set of biweekly measurements, we computed a Spearman correlation coefficient with a 95% confidence interval (CI) to assess the strength of associations, and generated Bland-Altman plots to evaluate agreement. We also computed the intraclass correlation coefficient (ICC) and corresponding CIs.
To examine the construct validity of the PRAI, we assessed both known-groups validity and convergent validity. Known-groups validity was addressed by: comparing PRAI scores of AI patients with those of comparison group subjects at baseline and over time; and comparing PRAI scores of patients with joint comorbidities with those without joint comorbidities at baseline. Wilcoxon rank-sum tests and boxplots were used to assess differences in groups for each of the above comparisons. We assessed concurrent validity by comparing PRAI scores at baseline and at follow-up with scores from the single FACT-ES question reporting pain in joints. To assess convergent validity, we examined whether PRAI scores correlated with other validated measures, including the PROMIS physical function, PROMIS pain interference, and ECOG performance status. Spearman correlation coefficients and CIs were used to evaluate these associations.
To better understand the relationship between morning stiffness and arthralgia, we examined the association of PRAI scores with patient-reported duration of morning stiffness using Spearman correlation coefficients, Bland-Altman plots, and ICCs. CIs for Spearman correlation coefficients were obtained using percentiles of bootstrap samples.
Results
Data from 330 women were included in the final analysis (Figure 1). A detailed breakdown of population characteristics, univariate descriptive characteristics, and univariate comparisons by group can be found in Castel et al.5 The individual PRAI items were all highly positively skewed; the median value for all items was zero. Respondents reported the lowest arthralgia severity in the elbows and higher arthralgia severity in the knees, hips, and shoulders. Cronbach’s alpha for the PRAI pain severity items was estimated to be 0.90. None of the alpha values based on individual item removal were greater than the total alpha, indicating that no item was negatively affecting the scale’s internal consistency reliability.
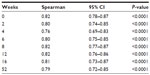
Test-retest reliability is reported in the form of ICC coefficients, which were calculated within subjects in the comparison group using PRAI assessments collected 2 weeks apart (Table 1). ICC values ranged from 0.87 to 0.96 for the repeated assessments. The corresponding Spearman correlation coefficient estimates also indicate fairly strong associations (range: 0.71–0.86). The Bland-Altman plots in Figure 2 show levels of agreement between assessments taken 2 weeks apart. There is strong overall agreement, with less agreement between weeks 0 and 2 than at subsequent time points.
  | Figure 2 Test-retest reliability. Bland-Altman plot of agreement between Patient-Reported Arthralgia Inventory assessments taken 2 weeks apart, with smoothed curve. |
With regard to validity, Figure 3 shows boxplots of baseline PRAI scores for those participants with and without joint comorbidities. The estimated difference in mean scores between groups was −0.3 (CI −0.5, −0.2; P<0.001). As expected, women with joint comorbidities tended to have higher arthralgia severity scores than those without joint comorbidities. The Wilcoxon rank-sum test of the null hypothesis that both groups had the same distribution of scores was highly significant (P<0.001), suggesting known-groups validity with respect to joint comorbidity.
  | Figure 3 Baseline mean Patient-Reported Arthralgia Inventory scores by presence of joint comorbidity. |
Figure 4 shows boxplots of the PRAI scores in relation to different levels of response to the FACT-ES item “I have pain in my joints” separately by week. The PRAI scores increase with higher FACT-ES ratings, further indicating known-groups validity. Table 2 lists the Spearman correlation estimates between the two measures over time, which show high associations (range: 0.76–0.82). This supports the concurrent validity of the PRAI.
We assessed convergent validity by examining the relationship between the mean PRAI scores and each of the PROMIS physical function T scores, PROMIS pain interference T scores, and ECOG performance status. PRAI scores tended to be lower among those with better physical function scores (estimated Spearman correlation coefficient: −0.47, 95% CI −0.55, −0.37; P<0.001). PRAI scores were higher among those with higher PROMIS pain interference (estimated Spearman correlation coefficient: 0.65, 95% CI 0.57–0.72; P<0.001). ECOG performance status that was not fully active was associated with higher PRAI scores (estimated difference in location scores: −0.6, 95% CI −0.9, −0.4; P<0.001).
Figure 5 shows a plot of the duration of morning stiffness in minutes and PRAI scores at baseline. The Spearman correlation between these measures was 0.62 (95% CI 0.54–0.69; P<0.0001), indicating greater morning stiffness among those women with higher PRAI scores.
  | Figure 5 Duration of morning stiffness in minutes and PRAI. |
Discussion
Since the discovery of arthralgia as a secondary effect of AIs, there has been a need for a validated multi-item arthralgia measurement instrument. Our overall objective was to understand the psychometric properties of the PRAI. Our findings can be interpreted in the context of established thresholds for strength of association. For Cronbach’s alpha,37 a threshold value of 0.70 indicates good internal consistency;38 we observed very high internal consistency reliability (0.90) among the PRAI pain severity items. Similarly, 0.70 is a criterion for adequate test-retest reliability.39 The PRAI’s high ICC values (range: 0.87–0.96) indicate that the variation in scores at each 2-week interval is almost exclusively due to differences between patients, rather than variability within patients.
We observed that despite the PRAI’s strong overall test-retest reliability, with all coefficients exceeding 0.70, arthralgia severity was reported higher at baseline than at any subsequent assessment. This resulted in less agreement between scores at weeks 0 and 2 than those at later weeks among the women without breast cancer. Mishra and Kuh similarly observed greater patient-reported menopausal symptom severity upon first assessment as compared with subsequent assessments in a general population, excluding the baseline data from their results for this reason.2 In the absence of a feasible biological explanation for why symptom severity might be greater at baseline than at later assessments, future research should examine a potential pattern of greater severity the first time a symptom severity question is asked.
Our findings confirmed expectations based on past research that greater arthralgia severity as reflected by higher PRAI scores was associated with increased severity/impact of other symptoms of post menopause, aging, and illness in general as reflected by other patient-reported outcomes measures. Specifically, we observed concomitant decline in physical function, increased interference of pain with activities of daily living, reduced performance status, and increased morning stiffness among the patients with greater PRAI-measured arthralgia severity. Our finding regarding increased morning stiffness as a proxy for inflammation suggests that patients experiencing more severe arthralgia were more likely to present with patient-reported symptoms of inflammation. Further research should explore, perhaps by administering the PRAI concurrently with biomarker collection and/or rheumatology examination (including range of motion assessment), the role of arthralgia in inflammation and vice versa, so that diagnostic methods, treatments, and/or symptom-dependent decisions regarding AI therapy may be more informatively developed for and discussed with patients.
The PRAI fulfills an important clinical research need for a multi-item scale, the benefits of which for measurement are described by Nunnally and Bernstein.38 Because the PRAI was developed in collaboration with patients and with experts in rheumatology, it also has good face and content validity. Our previous work with this scale also lends evidence to support its construct validity. The model-based trajectory of PRAI scores in women initiating AI therapy at baseline diverged at week 6 from the trajectory of postmenopausal women without cancer (P<0.01). The trajectories also showed an increase in arthralgia severity among those taking AI therapy over the 52 weeks of per-participant observation.5
Our findings indicate that this instrument has satisfactory reliability and validity for use in assessing the severity of arthralgia in clinical and nonclinical/general populations. Our key results indicate strong internal consistency and test-retest reliability of the PRAI, and provide evidence of its construct validity using a multi-method approach.
Acknowledgments
We gratefully acknowledge members of the study team, ie, Tonya Brown, Jessica Islam, Danielle LaMorte, Ashley Pasquariello, Angel Sherrill, and Angela Zito. We are also grateful for programming support from Joseph Burden, Gregory Todd Salter, Mikhail Zemmel, and Joseph Schneider, and contributions to screening provided by the Vanderbilt Cancer Trials Information Program. Dr Castel would like to thank her mentor Dr Katherine Hartmann. We thank two rheumatologists: Dr Fred Wolfe of the National Data Bank for Rheumatic Diseases, and Dr Chad Boomershine of Vanderbilt University Medical Center, who provided expert input and helped develop the measure. This study was funded by the American Cancer Society (119475-MRSG-10-169-01-PCSM, Dr Liana Castel, principal investigator [PI]), Vanderbilt Institute for Clinical and Translational Research (UL1RR024975-01 and UL1TR000011 from the National Institutes of Health, Dr Liana Castel, PI), and 5K12HD43483-10 (Dr Katherine Hartmann, PI) from the National Institutes of Health Building Interdisciplinary Careers in Women’s Health Research.
Disclosure
The authors report no conflicts of interest in this work.
References
Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74:574–579. | |
Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ. 2012; 344:e402. | |
Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223–234. | |
Buzdar AU; ATAC Trialists Group. Clinical features of joint symptoms observed in the ‘Arimidex’, Tamoxifen, Alone or in Combination (ATAC) trial. Available from: http://meeting.ascopubs.org/cgi/content/short/24/18_suppl/551. Accessed May 20, 2015. | |
Castel LD, Hartmann KE, Mayer IA, et al. Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer. 2013;119:2375–2382. | |
Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. | |
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. | |
Bremander AB, Petersson IF, Roos EM. Validation of the Rheumatoid and Arthritis Outcome Score (RAOS) for the lower extremity. Health Qual Life Outcomes. 2003;1:55. | |
Doyle DV, Dieppe PA, Scott J, Huskisson EC. An articular index for the assessment of osteoarthritis. Ann Rheum Dis. 1981;40:75–78. | |
Hawker GA, Davis AM, French MR, et al. Development and preliminary psychometric testing of a new OA pain measure – an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–414. | |
Mason JH, Anderson JJ, Meenan RF, Haralson KM, Lewis-Stevens D, Kaine JL. The Rapid Assessment of Disease Activity in Rheumatology (RADAR) questionnaire. Validity and sensitivity to change of a patient self-report measure of joint count and clinical status. Arthritis Rheum. 1992;35:156–162. | |
Ren XS, Kazis L, Meenan RF. Short-form Arthritis Impact Measurement Scales 2: tests of reliability and validity among patients with osteoarthritis. Arthritis Care Res. 1999;12:163–171. | |
Ritchie DM, Boyle JA, McInnes JM, et al. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968;37:393–406. | |
Stucki G, Liang MH, Stucki S, Bruhlmann P, Michel BA. A self-administered Rheumatoid Arthritis Disease Activity Index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995;38:795–798. | |
Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–2017. | |
van der Heijde DM, van ‘t HM, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–581. | |
Barton JL, Criswell LA, Kaiser R, Chen YH, Schillinger D. Systematic review and metaanalysis of patient self-report versus trained assessor joint counts in rheumatoid arthritis. J Rheumatol. 2009;36:2635–2641. | |
Morales L, Pans S, Paridaens R, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat. 2007;104:87–91. | |
Morales L, Pans S, Verschueren K, et al. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–3152. | |
Savnik A, Malmskov H, Thomsen HS, et al. Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol. 2001;28:2193–2200. | |
Dizdar O, Ozcakar L, Malas FU, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia. J Clin Oncol. 2009;27:4955–4960. | |
Taal E, Abdel-Nasser AM, Rasker JJ, Wiegman O. A self-report Thompson articular index: what does it measure? Clin Rheumatol. 1998;17:125–129. | |
O’Malley KJ, Suarez-Almazor M, Aniol J, et al. Joint-specific multidimensional assessment of pain (J-MAP): factor structure, reliability, validity, and responsiveness in patients with knee osteoarthritis. J Rheumatol. 2003;30:534–543. | |
Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol. 2003;30:369–378. | |
Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. | |
Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. | |
US Department of Health and Human Services FDA Center for Drug Evaluation and Research; US Department of Health and Human Services FDA Center for Biologics Evaluation and Research; US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. | |
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. | |
Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23:3552–3561. | |
Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. | |
Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2008;61:17–33. | |
Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25:5374–5380. | |
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55:189–199. | |
Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–1810. | |
Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A. Quality of life of postmenopausal women in the ATAC (“Arimidex”, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100:273–284. | |
Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54:569–578. | |
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. | |
Nunnally JC, Bernstein IH. Psychometric Theory. 3rd ed. New York, NY, USA: McGraw-Hill; 1994. | |
Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. |
Supplementary materials
  | Table S1 Patient-Reported Arthralgia Inventory |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.