Back to Journals » International Journal of General Medicine » Volume 17
Vaccination Shortens the Negative Nucleic Acid Conversion Time of the Older Population: A Retrospective Cohort Study of 73,456 Asymptomatic and Mild Patients with COVID-19 in Shanghai
Authors Wang C, Yang M, Zhu G, Hu Y, Shen L, Qiu J, Huang Y, Wang L
Received 23 November 2023
Accepted for publication 20 February 2024
Published 4 March 2024 Volume 2024:17 Pages 763—773
DOI https://doi.org/10.2147/IJGM.S451393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chaoqun Wang,1,* Meng Yang,2,* Guanglin Zhu,3,* Yanyan Hu,1,* Le Shen,1 Jiaona Qiu,1 Yi Huang,2 Libing Wang4
1Department of Endocrinology, the First Affiliated Hospital of Naval Medical University, Shanghai, 200433, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Naval Medical University, Shanghai, 200433, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Yangpu Hospital, School of Medicine, Tongji University, Shanghai, 200090, People’s Republic of China; 4Department of Hematology, the First Affiliated Hospital of Naval Medical University, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Libing Wang; Yi Huang, Email [email protected]; [email protected]
Purpose: To explore the influencing factors to predict the negative nucleic acid conversion time and ORF1ab gene CT value changes in patients with asymptomatic and mild COVID-19.
Patients and Methods: A total of 73,456 patients with asymptomatic and mild COVID-19 admitted to the Mobile Cabin Hospital in Shanghai from April 3 to April 23, 2022 were selected as the research objects. Epidemiological, clinical, and laboratory data were collected. Correlation analysis was performed.
Results: In patients < 18 years old and ≥ 65 years old, COVID-19 vaccine can shorten the negative nucleic acid conversion time, which is reflected in the lower median or 75% quantile (P< 0.001, P< 0.05). In patients with underlying diseases, the negative nucleic acid conversion time of booster vaccination and complete vaccination was lower than that of non-vaccinated group (P< 0.001, P< 0.05). In patients ≤ 18 years of age or > 65 years of age, patients with comorbidity and patients with symptoms, compared with patients 18– 65 years of age, patients without comorbidity and patients without symptoms, there was a greater difference in the rate of rise of CT values between vaccinated and unvaccinated patients (P< 0.05).
Conclusion: The time of nucleic acid conversion to negative in patients with asymptomatic and mild COVID-19 is affected by age, comorbidity, and first nucleic acid CT value. Vaccination could shorten the negative nucleic acid conversion time of the older population, those with complications or symptoms. The vaccination of older patients does not increase the risk of symptoms.
Keywords: COVID-19, nucleic acid detection, CT value, vaccine, older population
Introduction
It has been almost 4 years since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused severe respiratory syndrome and lethal pneumonia emerged in Wuhan, China, and the World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak a global pandemic.1 Numerous studies have unraveled the mysteries of SARS-CoV-2 pathogenesis and thus largely improved the therapeutic strategies of COVID-19.2 Although the vaccine effectiveness is not 100%, strong and consistent evidence shows that vaccines confer considerable protection to fully vaccinated individuals against SARS-CoV-2 infection. However, most studies focus on the effectiveness of vaccines against SARS-CoV-2 infection or severe COVID-19 outcomes, including hospitalization, mechanical ventilation, and death.3 Evidence on its protection against asymptomatic and mild infection is generally lacking.
From late February to late May 2022, over 600 thousand people had been laboratory confirmed SARS-CoV-2 infection in Shanghai, China. Among them, more than 550 thousand cases were asymptomatic carriers, the number of which had exceeded that in Wuhan, China, in 2020.4 During that time, the Government has implemented strict control measures and conducted citywide nucleic acid tests daily or every other day. Every patient who tested positive was sent to temporary hospitals to get treated and taken care of. Shanghai Hongqiao International Convention and Exhibition Center Mobile Cabin Hospital was one of these hospitals in Shanghai.
This study is based on patients with asymptomatic and mild COVID-19 admitted to the mobile cabin Hospital of Shanghai Hongqiao International Convention and Exhibition Center from April 3 to April 23, 2022 and aims to explore the influencing factors of patients’ CT values and the protective effect of vaccines in patients, in order to identify the population that benefits the most from vaccines and provide recommendations for vaccine administration. Clinical data were collected to analyze the variation pattern of the nucleic acid conversion time and ORF1ab gene CT values in asymptomatic and mild patients, and to explore possible influencing factors. The effectiveness of vaccines in preventing mild and asymptomatic COVID-19 infections were analyzed, providing evidence and clues for the management of COVID-19 variants in the future.
Materials and Methods
StudyDesign
A retrospective study was conducted with data from patients diagnosed with SARS-CoV-2 infection and admitted to the mobile cabin hospital in Shanghai National Exhibition and Convention Center from 3rd to 23rd April 2022. This mobile cabin hospital has 40,000 beds and serves as a designated hospital for patients with asymptomatic and mild COVID-19 in Shanghai since April 2022.
Participants
All patients hospitalized met the following criteria:
Informed consent was signed by all patients involved on admission. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Naval Medical University.
Samples Collection and Laboratory Data
Oropharyngeal samples were collected by a team of trained nurses, according to WHO guidelines.5,6 The nurses had all undergone centralized and unified sample collection training and had obtained the corresponding certificates. Specimens were stored at 4°C before nucleic acid extraction and real-time RT-PCR processing. The samples were then tested within 8 hours of collection using the China Food and Drug Administration approved commercial 2019-nCoV Nucleic Acid Detection kit (BoJie, Shanghai, China). Briefly, ORF1ab and N genes were targeted, and ORF1ab was chosen as the primary one as specific to SARS-CoV-2 viral genome and used for viral load calculation. For specimen quality assessment, the human housekeeping gene target RNAse P (RP) was also measured in each sample as human control genes. The detection limit of the ORFab1 assays was about 1 × 103 copies per milliliter. The EasyNAT-Pure20B Automatic Nucleic Acid Extraction Analyzer (Ustar Biotechnologies, Hangzhou, China) was used for PCR amplification. Samples were defined as positive when both the ORF1ab and N CT values were <35, and as negative when both CT values were ≧35. Samples with either the ORF1ab or N CT value >38.0 would be repeated. All tests were performed in accordance with the protocols available provided by the WHO and manufacturer’s instructions for use.
Clinical Data Collection
Epidemiological, clinical, and laboratory characteristics were obtained from hospital electronic medical records. The clinical data included personal characteristics, symptoms and signs, comorbidities, vaccination status, and the date of PCR positivity. Signs and symptoms were clustered according to the following 2 main groupings: fever, muscle soreness, and asthenia as inflammatory systemic involvement; cough, expectoration, sore throat, runny nose, nasal congestion, and dyspnea as respiratory involvement. Comorbidities documented included hypertension, coronary heart disease, heart failure, arrhythmia, diabetes mellitus, cerebral apoplexy, and thrombotic diseases. The duration of COVID-19 PCR positivity was defined as days from the date of PCR positivity to the first day of two consecutive PCR negativity tests.
Statistics and Data Analysis
Data analysis was performed through SPSS (version 26.0). Skewness and kurtosis tests were used to assess the normality of the distributions. Continuous variables were presented as mean ± standard deviation (SD) and analyzed using a t–test if found to follow the normal distribution, or as median ± lower and upper quartiles and analyzed by the Wilcoxon non-parametric test if found to fit the skew distribution. Dichotomous variables were compared between groups using the Chi-square test. Categorical variables were compared using the Fisher-Freeman-Halton, Chi-square, and Fisher’s exact tests. Multivariable logistic regression was used to determine the association between the duration of COVID-19 PCR positivity and clinical characteristics following standard guidelines.7 A P value of <0.05 was considered significant.
Results
Study Population and their Clinical Characteristics
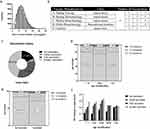
From 3rd to 23rd April 2022, a total of 73,456 patients who met the study inclusion criteria were admitted to the mobile cabin hospital in Shanghai National Exhibition and Convention Center, all of whom tested positive based on RT-PCR on admission. Patients’ clinical characteristics are summarized in Table 1. The mean age of the population was 42 years (30–53), and 40.83% (29,994/73,456) were women. About 25.7% (18,882/73,456) patients complained of at least one of the following symptoms with 5.4% (3977/73,456) having more than three symptoms on or before admission: cough, 14,917; expectoration, 9937; asthenia, 4,066; muscle soreness, 3,048; fever, 2,795; nasal congestion, 540; sore throat, 86; runny nose, 49; and dyspnea, 14. Adults among 19–65 years old were more prone to being symptomatic. About 54,574 (74.3%) people who did not complain of any symptoms on admission were defined as asymptomatic cases in this study. Medical comorbidities were present in 14.2% (10,428/73,456) of the population, the first three of which were hypertension (7,552/73,456, 10.3%), diabetes mellitus (2,410/73,456, 3.30%) and coronary heart disease (1,596/73,456, 2.2%). Generally, the duration of COVID-19 PCR positivity in all patients showed skewed distribution, with the median was 7 (IQR 5–9) days (Figure 1a). Manufacturers and classes of vaccines patients received were listed (Figure 1b).
 |
Table 1 Clinical Characteristics and Outcomes of All Patients in Mobile Cabin Hospital |
Influence of COVID-19 Vaccination on the duration of COVID-19 PCR positivity
A total of 821 patients were excluded due to unknown vaccine status. A total of 25,478 individuals (35.1%, 25,478/72,635) received full dose of COVID-19 vaccines and 32,432 (44.7%, 32,432/72,635) received booster dose, while 12,667 (17.4%, 12,667/72,635) were unvaccinated (Figure 1c). Symptoms were observed more commonly in adult patients (<65 years old) with received full or booster dose vaccines compared with these unvaccinated (Figure 1d–e). But among older patients (≥65 years old), there was no significant difference regarding their vaccine status (Figure 1f). The duration of COVID-19 PCR positivity was reduced with lower median time or third quartile time in juvenile patients (<18 years old, p<0.001) and older patients (≥65 years old, p<0.05) (Figure 2a) related to vaccine status. The duration of COVID-19 PCR positivity was not different among asymptomatic patients with diverse vaccine status, but were reduced in vaccinated symptomatic patients (Figure 2b–c). Among juvenile patients, asymptomatic ones requiring a longer duration of COVID-19 PCR positivity (p<0.05) (Figure 2d). However, the duration of COVID-19 PCR positivity in symptomatic older patients was longer (p<0.001) (Figure 2d). Similarly, the duration of COVID-19 PCR positivity was not different in patients without medical comorbidities whether they received the vaccine or not (Figure 2e). But a full or booster dose SARS-COV-2 vaccine could both shorten the duration in patients with comorbidities (p<0.001, p<0.05, respectively) (Figure 2f).
Factors Associated with the Duration of COVID-19 PCR Positivity
Multivariable logistic regression was used to determine the factors associated with the duration of COVID-19 PCR positivity, shown in Table 2. The variables that were significantly associated with the duration of COVID-19 PCR positivity were age (β=0.052, p-value <0.001), CT values on admission (β=−0.189, p-value <0.001), comorbidities (β=0.051, p-value <0.001), and vaccine status (β=0.052, p-value <0.001). Aged, unvaccinated status, comorbidities, and lower CT values would lead to prolonged hospitalization.
 |
Table 2 Multiple Linear Regression Analysis of Factors Associated with the Duration of COVID-19 PCR Positivity in Asymptomatic and Mild Patients |
Dynamics of ORFLab CT Values and Factors Associated
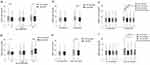
Patients were stratified into the following three groups according to ORFLab Gene Ct values detected on admission: Ct ≤ 28.0, group A; 28.0 < Ct ≤ 32.0, Group B; Ct > 32.0, Group C. The median Ct value on admission of included participants was 27.82 (IQR 21.51–40.00). Higher CT values on admission were associated with higher CT values during hospitalization and shorter duration of PCR positivity (Figure 3a). No statistical differences in CT values upon admission were found to be associated with age, symptoms, or comorbidities (p>0.05). However, the recovery speed of the Ct values seemed faster in younger patients with significance (Figure 3b). The CT values increased more steadily in patients with comorbidities, such as hypertension or diabetes mellitus, than in those without any comorbidity (p<0.05) (Figure 3d–f). Also, patients with symptoms, and those with fever or muscle soreness, showed a slower speed in CT values recovery (p<0.05) (Figure 3g–i). Data on patients with other symptoms or comorbidities are shown in Supplementary Figure 1. No statistical differences were found in patients with those comorbidities and symptoms.
The differential kinetics of CT values on admission by vaccine status were analyzed. There was no statistical difference in CT values on admission associated with vaccine status. However, faster recovery speed of the Ct values was seen in vaccinated patients (Figure 3c). The patients were then stratified into different groups according to age, symptoms, or comorbidities. The recovery speed of the CT values was obviously slower in juvenile or older patients (Figure 4a–b) with symptoms (Figure 4c–d) or comorbidities (Figure 4e–f).
Discussion
The population density of Shanghai is very high, with about 25 million people in 6,340 square kilometers. There are a large number of older population with uncompleted vaccination status. As is reported, 5.8 million people are over 60 years old, and only 62% of them have been vaccinated, among which only 38% have received booster shots.8 At the beginning of the COVID-19 epidemic in 2022, Shanghai immediately adopted a strict quarantine policy, including daily large-scale nucleic acid and antigen screening, in order to protect vulnerable populations and prevent the spread of COVID-19. Mobile cabin hospitals, during the Wuhan outbreak in 2020, were shown to help strengthen the isolation of patients with mild cases to contain and ultimately stop the spread of COVID-19.9 Thus, most infected population in Shanghai, once tested positive for SARS-COV-2, symptomatic or asymptomatic, would be sent to Mobile cabin hospitals for early isolation and medical observation if diagnosed as asymptomatic or mild cases.10 This study analyzed the disease course characteristics of 73,456 asymptomatic and mild patients admitted to mobile cabin hospitals, where they received only symptomatic treatments, rather than antivirals or anti-inflammatory medications. We thereby were able to observe and conduct an assessment of vaccine effectiveness in both symptomatic and asymptomatic infected patients. With such a large sample size, this study can accurately reflect key features in asymptomatic and mild patients and the protective efficacy of the vaccine among these patients.
Reverse transcription polymerase chain reaction (RT-PCR)-based diagnostic tests are the gold standard for the diagnosis of SARS-CoV-2 infection.11 Although there is a possibility of inconsistent virus shedding between the upper and lower respiratory tracts, the high specificity of nucleic acid amplification tests (NAATs) allows for a diagnosis of COVID-19 based on a positive result from an upper respiratory tract sample.12 In our study, we use oropharyngeal swab for sampling. Some studies suggest that nasopharyngeal swab, or pooled nasal and throat swabs, offered the better diagnostic performance for diagnosis of SARS-CoV-2 infection.13–15 However, in mobile cabin hospital, due to the large number of patients, the limited number of nurses is not sufficient to complete nasal swab sampling. Therefore, in order to obtain reliable results, we have improved the quality of sample collection through centralized and unified training, and it is required that two consecutive NAATs with an interval of more than 24 hours must be both negative to consider it as a negative nucleic acid conversion. Cycle threshold (CT) values are semi-quantitative values, representing the number of amplification cycles required for the target gene to exceed a threshold level.16 They are inversely proportional to the viral load and were reported to be related to both disease severity and likelihood of transmission.17 ORF1ab is a relatively conserved set of coronavirus-specific gene sequences with little variability. CT values of ORF1ab can be used to evaluate the time of nucleic acid turning negative and the rate of disease improvement.18 Therefore, in this study, we use CT values to evaluate viral loads of patients.
The retrospective analysis of clinical data of patients with asymptomatic and mild COVID-19 admitted to the mobile cabin hospital of Shanghai Hongqiao International Convention and Exhibition Center showed that the average negative nucleic acid conversion time of patients with asymptomatic and mild COVID-19 was 7 (5–9) days, and the time of negative nucleic acid conversion was affected by age, admission CT value, underlying diseases, and vaccine injection. Okita Y19 also found through systematic review and meta-analysis that age, underlying diseases, disease severity, and glucocorticoid use were correlated with the duration of positive nucleic acid of COVID-19 strains in the past. In addition to the time of negative nucleic acid, the speed of recovery of the disease course also affected the physical and mental health and status of the patient. This study also found that adults, vaccinated patients, and those with no underlying disease or symptoms had a faster recovery speed in CT values, and thus a faster speed of virus clearance. In a prospective study of 121 Japanese patients, Sakano T20 concluded that CT values were higher in symptomatic patients upon admission but increased more rapidly in asymptomatic patients. Asymptomatic patients had a high virus-specific cellular immune response, which could better inhibit viral proliferation, but no significant non-specific inflammatory response.21 Finally, we could basically predict the time of negative nucleic acid conversion and the length of stay by the CT value of the first nucleic acid test on admission, the age of patients, whether they have been vaccinated or not, and whether they have underlying diseases.
Chinese Control and Decision Conference (CCDC) had reported that the SARS-CoV-2 viral genomes in Shanghai were clustered into the SARS-CoV-2 BA.2.2, a sub-lineage of the omicron variant of SARS-CoV-2 (B.1.1.529).8 Studies have found that the Omicron variant had a shorter average incubation period, stronger transmission capacity, and faster transmission rate than other previous strains of COVID-19. Compared with the Delta variant, systemic symptoms, the risk of hospitalization and duration of symptoms were reduced while respiratory symptoms were increased during the Omicron pandemic.22 Moreover, the injection of COVID-19 vaccines showed partially protection against the Omicron variant.23 Although studies have confirmed that the Omicron variant is less virulent than previous COVID-19 strains, the rate of severe illness and death in unvaccinated people, especially the older population, is still significantly higher than in the general population.24 According to the CCDC, in Omicron infected cases, fully vaccinated patients over 60 years old had a 72% lower risk of developing pneumonia than those who were not vaccinated.25 Although the protection of the vaccine against the new variant strains of Omicron decreased, it was still higher than that of unvaccinated individuals and even higher than that of previously infected individuals who had been infected with the Omicron sub-type BA.1.26 Although natural immunity induced by SARS-CoV-2 infection also protects against reinfection, study showed that the vaccinated had significantly lower rates of all-cause ED visits, hospitalizations, and mortality than the previously infected population.27 There is also evidence that supports SARS-CoV-2 vaccination help in the prevention of long COVID.28 All these suggest that vaccines are still needed. However, few studies have focused on its efficacy in mild infection. We found that for asymptomatic and mild patients, vaccination can improve the rate of virus clearance and shorten the time of nucleic acid turning negative. There are greater differences in the rate of rise of CT values between vaccinated and unvaccinated people aged ≤18 or >65 years, with comorbidities, and with symptoms, compared with 18–65 years, without comorbidities, and without symptoms, respectively. This suggests that the benefit of vaccination was greater in people aged ≤18 years or >65 years, with comorbidities and post-infection symptoms. Tsang NNY et al also confirmed the effectiveness of booster doses to protect against mild and asymptomatic infection, which supported our findings. In brief, vaccination is still a very effective means for infected but non-severe patients. Interestingly, for adolescent and young patients, vaccination increased the incidence of symptoms, the mechanism of which needs to be further explored.
Although this study had a large sample size, it still had some limitations. This was a single-center retrospective study, only asymptomatic and mild patients experiencing their initial infection were included in this study, which cannot fully reflect the influencing factors of disease course and vaccine efficacy of infected people with different disease severity. Symptom indicators were not fully included, and duration was not measured. The date patients got vaccinated and types of vaccines were not recorded and analyzed, which may also have an influence on its efficacy. Besides, despite employing multiple methods to improve sample quality, it must be acknowledged that the false-positive rate for throat swabs is higher than that for nasal swabs. Also, we did not perform live virus culture, and the evaluation of CT value on viral load may be affected by various factors, which will need to be further explored and studied.
Conclusion
In conclusion, this study found that, for patients with asymptomatic and mild COVID-19, age, underlying diseases, vaccination status, and CT value of the onset of the disease all affected the length of the disease course and the speed of recovery of the disease course. And vaccines could promote the recovery of the disease for patients, especially minors and older patients. In the older population, those with comorbidities and those with symptoms after infection still benefited from vaccination, and there was no increased risk of symptoms in older patients receiving the vaccine. In view of the above, early vaccination or even booster shots for people with no contraindications is still of great significance.
Data Sharing Statement
The raw data of this article will be made available by the authors upon reasonable request. Requests may be sent to [email protected].
Ethics Approval and Consent to Participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of the First Affiliated Hospital of Naval Medical University. Written informed consent was obtained from individual or guardian participants.
Funding
The publication was supported by Shanghai Sailing Program (21YF1458800), the Youth Fund Project of the First Affiliated Hospital of Navy Medical University (2020QNB15) and Changhai Hospital Funding Program (COVID-TS-004). The funders had no role in the design of the study, in the analyses of data, or in the decision to publish the results.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi:10.23750/abm.v91i1.9397
2. Murakami N, Hayden R, Hills T, et al. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2023;19(1):38–52. doi:10.1038/s41581-022-00642-4
3. Kirsebom FCM, Andrews N, Stowe J, et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022;22(7):931–933. doi:10.1016/S1473-3099(22)00309-7
4. Information Office of Shanghai Municipality. Shanghai reports on the prevention and control of COVID-19; 2022. https://www.shio.gov.cn/TrueCMS/shxwbgs/2022n_6y/2022n_6y.html.
5. World Helath Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance; 2020.
6. Biohazards/Standards CO. Interim guidelines for collection, processing and transport of clinical specimens from patients under investigation for Middle East Respiratory Syndrome (MERS). Contain Bio Stand. 2024; 24:1.
7. Lee SW. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3. doi:10.54724/lc.2022.e3
8. Zhang X, Zhang W, Chen S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399(10340):2011–2012. doi:10.1016/S0140-6736(22)00838-8
9. Li J, Yuan P, Heffernan J, et al. Fangcang shelter hospitals during the COVID-19 epidemic, Wuhan, China. Bull World Health Organ. 2020;98(12):830–841D. doi:10.2471/BLT.20.258152
10. Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386(7):e14. doi:10.1056/NEJMp2119682
11. Bhimraj A. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health (US); 2021.
12. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi:10.1001/jama.2020.3786
13. Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Dkm I. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(9):1233–1245. doi:10.1016/S1473-3099(21)00146-8
14. Wang X, Tan L, Wang X, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–109. doi:10.1016/j.ijid.2020.04.023
15. Mohammadi A, Esmaeilzadeh E, Li Y, Bosch RJ, Li JZ. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EBioMedicine. 2020;59:102903. doi:10.1016/j.ebiom.2020.102903
16. Tom MR, Mina MJ. To Interpret the SARS-CoV-2 test, consider the cycle threshold Value. Clin Infect Dis. 2020;71(16):2252–2254. doi:10.1093/cid/ciaa619
17. Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold Values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573–586. doi:10.1007/s40121-020-00324-3
18. Badua CLDC, Baldo KAT, Medina PMB. Genomic and proteomic mutation landscapes of SARS-CoV-2. J Med Virol. 2021;93(3):1702–1721. doi:10.1002/jmv.26548
19. Okita Y, Morita T, Kumanogoh A. Duration of SARS-CoV-2 RNA positivity from various specimens and clinical characteristics in patients with COVID-19: a systematic review and meta-analysis. Inflamm Regen. 2022;42(1):16. doi:10.1186/s41232-022-00205-x
20. Sakano T, Urashima M, Takao H, Takeshita K, Kobashi H, Fujiwara T. Differential kinetics of cycle threshold values during admission by symptoms among patients with mild COVID-19: a prospective cohort study. Int J Environ Res Public Health. 2021;18(15):8181. doi:10.3390/ijerph18158181
21. Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5):doi:10.1084/jem.20202617
22. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi:10.1016/S0140-6736(22)00327-0
23. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532–1546. doi:10.1056/NEJMoa2119451
24. Cheung P-H-H, Chan C-P, Jin D-Y. Lessons learned from the fifth wave of COVID-19 in Hong Kong in early 2022. Emerg Microbes Infect. 2022;11(1):1072–1078. doi:10.1080/22221751.2022.2060137
25. Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 mortality and vaccine coverage - hong kong special administrative region, China. MMWR Morb Mortal Wkly Rep. 2022;71(15):545–548. doi:10.15585/mmwr.mm7115e1
26. Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28(9):1785–1790. doi:10.1038/s41591-022-01911-2
27. Tu W, Zhang P, Roberts A, et al. SARS-CoV-2 Infection, hospitalization, and death in vaccinated and infected individuals by age groups in Indiana, 2021‒2022. Am J Public Health. 2023;113(1):96–104. doi:10.2105/AJPH.2022.307112
28. Ceban F, Kulzhabayeva D, Rodrigues NB, et al. COVID-19 vaccination for the prevention and treatment of long COVID: a systematic review and meta-analysis. Brain Behav Immun. 2023;111:211–229. doi:10.1016/j.bbi.2023.03.022
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.