Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Using Neural Networks Algorithm in Ischemic Stroke Diagnosis: A Systematic Review
Authors Ruksakulpiwat S , Phianhasin L , Benjasirisan C , Schiltz NK
Received 14 May 2023
Accepted for publication 15 August 2023
Published 1 September 2023 Volume 2023:16 Pages 2593—2602
DOI https://doi.org/10.2147/JMDH.S421280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Suebsarn Ruksakulpiwat,1 Lalipat Phianhasin,1 Chitchanok Benjasirisan,1 Nicholas K Schiltz2
1Department of Medical Nursing, Faculty of Nursing, Mahidol University, Bangkok, Thailand; 2Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, OH, USA
Correspondence: Suebsarn Ruksakulpiwat, Department of Medical Nursing, Faculty of Nursing, Mahidol University, Bangkok, Thailand, Email [email protected]
Objective: To evaluate the evidence of artificial neural network (NNs) techniques in diagnosing ischemic stroke (IS) in adults.
Methods: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was utilized as a guideline for this review. PubMed, MEDLINE, Web of Science, and CINAHL Plus Full Text were searched to identify studies published between 2018 and 2022, reporting using NNs in IS diagnosis. The Critical Appraisal Checklist for Diagnostic Test Accuracy Studies was adopted to evaluate the included studies.
Results: Nine studies were included in this systematic review. Non-contrast computed tomography (NCCT) (n = 4 studies, 26.67%) and computed tomography angiography (CTA) (n = 4 studies, 26.67%) are among the most common features. Five algorithms were used in the included studies. Deep Convolutional Neural Networks (DCNNs) were commonly used for IS diagnosis (n = 3 studies, 33.33%). Other algorithms including three-dimensional convolutional neural networks (3D-CNNs) (n = 2 studies, 22.22%), two-stage deep convolutional neural networks (Two-stage DCNNs) (n = 2 studies, 22.22%), the local higher-order singular value decomposition denoising algorithm (GL-HOSVD) (n = 1 study, 11.11%), and a new deconvolution network model based on deep learning (AD-CNNnet) (n = 1 study, 11.11%) were also utilized for the diagnosis of IS.
Conclusion: The number of studies ensuring the effectiveness of NNs algorithms in IS diagnosis has increased. Still, more feasibility and cost-effectiveness evaluations are needed to support the implementation of NNs in IS diagnosis in clinical settings.
Keywords: neural networks, ischemic stroke, systematic review
Introduction
Stroke is one of the leading causes of death and a major contributor to chronic disability worldwide, with ischemic stroke accounting for 80% of cases.1 Annually, there are over 7.6 million new cases of ischemic stroke.2 Additionally, the stroke mortality rate is 42.56 per 100,000, resulting in approximately 3.3 million deaths from ischemic stroke each year.2 Based on stroke incidence, prevalence, and mortality rates, the estimated total medical stroke-related costs are projected to double from $36.7 billion to $94.3 billion between 2015 and 2035.3
Over 50% of stroke survivors are chronically disabled, affecting their quality of life, and more stroke survivors experience its long-term consequences.1,4 The quality of life among stroke survivors remains below the healthy population level, associated with higher levels of depression and anxiety.5 Likewise, one in five stroke survivors lives at least 15 years after a stroke and have poor functional, cognitive, and psychological outcomes.4 Therefore, long-term consequences management of stroke burden is necessary. However, early diagnosis is also a key to better clinical outcomes improving their quality of life.
Early detection of ischemic stroke is crucial to start thrombolytic therapy, intravenous tissue plasminogen activator (tPA). The tPA infusion, the first-line treatment for acute ischemic stroke, has a limited treatment window of 3–4.5 hours after stroke onset, as recommended by the American Heart Association/American Stroke Association.6 This is because the tPA effectiveness is highly time-dependent due to the sensitivity of brain tissue to ischemia.7 The tPA works by dissolving blood clots that block blood flow to the brain, which helps to restore blood flow to affected brain regions.8 The tPA, therefore, limits the risk of brain damage and functional impairment.8 In addition, the sooner providers can detect stroke, the lower mortality, and morbidity among patients who experience a stroke.9 In the United States, the mortality rate of stroke fell by 77% between 1969 and 2013 since the implication of the tPA to acute ischemic stroke treatment.8 Accordingly, early detection of ischemic stroke is crucial to starting the tPA infusion, effectively decreasing stroke mortality and morbidity rate.
In order to reduce the mortality and morbidity of stroke, the use of advanced technology, in particular, machine learning (ML), has been studied to aid healthcare providers in attempting to detect an ischemic stroke early in clinical settings. ML is a computing method that generally utilizes data patterns to forecast future events and facilitate probabilistic decision-making.10 In light of ischemic stroke diagnosis, multiple types of ML have shown effectiveness in detecting ischemic stroke among adults.11 One of the promising and powerful methods is neural networks, also known as artificial neural networks or simulated neural networks, which are a class of algorithms loosely modeled on connections between neurons in the brain.12 As is evident from previous studies, the use of the neural networks algorithm is shown to improve the accuracy of several medical diagnoses, including cancer diseases such as lung cancer, skin cancer, and gastric cancer, or even time-sensitive diseases, such as coronary artery disease.13–16 However, the previous review pointed out that the neural networks algorithm helped improve accuracy in ischemic stroke classification and prediction but with limited research evidence.11
To the best of our knowledge, some review articles systematically reviewed the relevant literature on the use of deep learning techniques – a subset of the neural networks algorithm – in the diagnosis of stroke.17,18 Therefore, the nonoverlapping studies between neural networks and deep learning algorithms may be missed. Also, the previous research analyzed studies from interception to 2020.17 With the rapid growth of the use of neural networks, our review included more recent studies from January 2018 to December 2022. Additionally, previous studies have not specifically discussed ischemic stroke diagnosis.17,18 In this systematic review, we aimed to evaluate the evidence of neural networks technique in making diagnoses of ischemic stroke in adults to gather the related evidence and guide future diagnosis guidelines.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)19 was applied in this review to present the flow diagram of the identification, screening, exclusion, and inclusion of the literature.
Identify Relevant Studies
In this review, four electronic databases, PubMed, MEDLINE, Web of Science, and CINAHL Plus Full Text, were systematically searched in September 2022 to identify preliminary studies published between January 2018 and December 2022, reporting using neural networks in ischemic stroke diagnosis. We combined the search terms (Ischemic Stroke* OR Ischaemic Stroke* OR CryptogenicIschemic Stroke* OR Cryptogenic Stroke* OR Cryptogenic Embolism Stroke* OR Wake up Stroke* OR AcuteIschemic Stroke* OR Embolic Stroke* OR Cardioembolic Stroke* OR Cardio-embolic Stroke* OR Thrombotic Stroke* OR Acute Thrombotic Stroke* OR Lacunar Stroke* OR Lacunar Syndrome* OR Lacunar Infarction* OR Lacunar Infarct*) AND (Computer Neural Network* OR Computer Neural Networks OR Perceptron* OR Neural Network Model* OR Connectionist Model* OR Neural Network Model* OR Neural Network* OR Computational Neural Network* OR Deep Learning OR Hierarchical Learning) AND (Diagnos* OR Diagnos* and Examination* OR Postmortem Diagnos* OR Antemortem Diagnos*) using Boolean phrases. In addition, reference lists of the included studies were manually searched to obtain relevant studies. All references identified were stored in EndNote. The detailed search strategy is shown in Supplementary Table 1.
Study Selection
Titles and abstracts of eligible studies were screened. The full text was also assessed to decide whether or not it was relevant. A third party was required to resolve disagreements when discrepancies occurred. Inclusion criteria were implemented to guarantee that only studies considered relevant to our objective were included. Similarly, exclusion criteria were used to eliminate literature not affiliated with the review (Table 1).
 |
Table 1 Study Inclusion and Exclusion Criteria |
Quality Assessment
In this systematic review, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Diagnostic Test Accuracy Studies 2020 was adopted for a quality appraisal of included studies,20,21 including (1) patient selection, and (2) an index test, composed of 10 questions. Two researchers assessed the quality of the eligible studies independently. A third researcher was required when there was any discrepancy.
Data Extraction
The summary data (Supplementary Table 2) included the following data for each study: References, publication year, country, total sample size, target population, age, gender, methodological quality, study objective, Neural Network approach/algorithm, the main features, main/optimal results, and implementation for clinical practice.
Results
Search Results
An initial literature search yielded 641 articles, including 488 from PubMed and MEDLINE, 123 from Web of Science, and 30 from CINAHL Plus Full Text. No additional records were found through other sources. After deduplication, the researchers screened 639 references, of which 628 were excluded based on the inclusion and exclusion criteria following the title and abstract screening phase. This left 11 articles for full-text screening, during which two were excluded as they were irrelevant to the application of neural networks in ischemic stroke diagnosis. Nine articles were included in the final screening and quality appraisal. The retrieval process was outlined using the PRISMA guidelines and is presented in Figure 1.
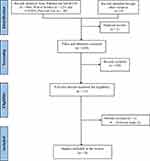 |
Figure 1 Flow chart diagram displaying the selection method of qualified studies. Notes: Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Reprint--Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269.22 |
Description of Included Studies
In each of the included studies in our review, patients’ ages were reported differently, such as mean and standard deviation, range, or median and interquartile range. Furthermore, all included studies involved both male and female participants in their analysis (Supplementary Table 2). Table 2 shows that included studies were published in 2022 (n = 3 studies, 33.33%), 2021 (n = 2 studies, 22.22%), 2020 (n = 3 studies, 33.33%), and 2019 (n = 1 study, 11.11%). The included studies were conducted in several countries, including China (n = 5 studies, 55.55%), Japan (n = 2 studies, 22.22%), the USA (n = 1 study, 11.11%), and Finland (n = 1 study, 11.11%). One study (11.11%) included a sample size range from 1 to 25. Two studies (22.22%) included participants between more than 25 to 100. Two studies (33.33%) included more than 100 to 300 and more than 300 participants each. The target population in the included studies were individuals with acute ischemic stroke (n = 7 studies, 77.77%), ischemic stroke (n = 1 study, 11.11%), and others which included Ischemic Penumbra (IP) in early Acute Cerebral Infarction (ACI) (n = 1 study, 11.11%).
 |
Table 2 The Characteristics of the Included Studies |
Assessment of Methodological Quality
According to the JBI Critical Appraisal Checklist for Diagnostic Test Accuracy Studies 2020,20,21 all included studies had 90% or more positive answers for the questions included in the appraisal tool. The only concerning methodological quality that appeared in some of the included studies was not avoiding a case-control design, thus leading to the risk of bias (Supplementary Table 3). Copyright permission was granted by JBI to adapt Supplementary Table 3.
Description of Neural Networks Algorithm in Ischemic Stroke Diagnosis
The Number of Included Studies by Features
Dataset, algorithm, and features are considered architectures of a machine learning model. The features are one of the essential combinations of a model as it demonstrates the correlation of the target variable of a model and attributes the appropriate of the model’s predictions and performance.11 Overall, in nine included studies, a total of seven main features were included. Notably, one study can report using more than one feature. Non-contrast computed tomography (NCCT) (n = 4 studies, 26.67%) and computed tomography angiography (CTA) (n = 4 studies, 26.67%) are among the most common features included in Neural Networks Algorithm. Two studies reported using computed tomography perfusion (CTP) (13.33%) and magnetic resonance imaging (MRI) (13.33%) as the features of each. One study applied computed tomography (CT) (6.67%), 8-second delay after CTA (CTA+) (6.67%), and diffusion-weighted imaging (DWI) (6.67%), each as a feature Figure 2.
The Optimal Results of Included Studies by the Algorithm
In our study, we found that five different types of neural network algorithms were particularly effective for diagnosing ischemic stroke. Table 3 displays the optimal results of the included studies, categorized by neural network algorithm. Deep Convolutional Neural Networks (DCNNs) were the most commonly used algorithm for ischemic stroke diagnosis, as they can identify patterns in images, videos, or natural language processing (n = 3 studies, 33.33%). Except for the studies by Zhang et al, Faster R-CNN, YOLOV3, and SSD algorithms were combined in the DCNNs model.23 Three-Dimensional Convolutional Neural Networks (3D-CNNs) were also used in two studies (n = 2 studies, 22.22%), as deep learning models comprising several consecutive layers of 3D convolutions are ideal for improving the identification of moving and 3D images, such as videos or medical scans. Two-stage Deep Convolutional Neural Networks (Two-stage DCNNs) were utilized in two studies (n = 2 studies, 22.22%), as they are a type of deep learning architecture designed to identify the presence and location of objects within an image or video. The local higher-order singular value decomposition denoising algorithm (GL-HOSVD) was used in one study (n = 1 study, 11.11%), which is a technique used to remove noise from multi-dimensional data such as images or videos. Lastly, a new deconvolution network model based on deep learning (AD-CNNnet) was used in one study (n = 1 study, 11.11%) specifically designed for image restoration tasks in the diagnosis of ischemic stroke.
 |
Table 3 The Optimal Results of Included Studies by the Algorithm |
Discussion
The current review summarized the Neural Networks model for ischemic stroke diagnosis. Performance observations such as accuracy, precision, sensitivity, specificity, and area under the curve (AUC) were reported. The discussion section showed how each algorithm could be implemented in the clinical setting for ischemic stroke diagnosis.
In our study, two included studies applied 3D-CNNs and provided optimal results for ischemic stroke diagnosis.24,27 The study by Oman et al proposed investigating the feasibility of ischemic stroke detection from CTA-SI using 3D-CNNs. The result shows that 3D-CNNs have improved ischemic stroke detection (sensitivity = 0.93, specificity = 0.82, AUC = 0.93).24 Similarly, Wang et al demonstrated that when 3D-CNNs were utilized to identify acute ischemic stroke from NCCT and CTA features, the ischemic stroke diagnostic decisions showed high accuracy (0.90±0.04).27 Comparatively, a previous study found that when used 3D-CNNs to other chronic diseases diagnoses such as Alzheimer’s disease (AD), the model demonstrated promising classification performances with an accuracy of 87.15% and AUC of 92.26% for AD classification.32 Our findings suggested that 3D-CNNs have a high potential in ischemic stroke diagnosis. Moreover, it could further implement in the clinical section to assist healthcare providers (eg, radiologists and clinicians) in disease diagnosis, especially in patients with stroke, which can lead to a more accurate treatment that can promote complication prevention.
Two-stage deep convolutional neural networks or two-stage DCNNs models were used in two of our included studies.25,31 The research from Nishio et al developed and evaluated an automatic acute ischemic stroke (AIS) detection system utilizing CT and MRI image features.25 The result showed that a board-certified radiologist combined with two-stage DCNNs models had improved AIS detection (sensitivity = 41.3%, precision = 62%, false positive per one case = 0.388).25 The study suggested that the detection system involving two-stage DCNNs could significantly improve radiologists’ sensitivity in detecting AIS, similar to a study by Lu et al, who found that using NCCT features in two-stage DCNNs to identify AIS shows high diagnostic value with AUC of 83.61%, sensitivity = 68.99%, specificity = 98.22%, and accuracy = 89.87%.31
Our results are consistent with previous studies that used two-stage DCNNs models for lung nodule detection33 and breast cancer classification.34 The result showed high accuracy and competitiveness compared to existing traditional methods. Based on this evidence, it is suggested that two-stage DCNNs models are generalizable and could help the healthcare team, especially radiologists and clinicians, screen diseases early (eg, AIS, breast cancer, lung nodule) and provide more effective guidance in making patients’ treatment plans in the clinic. The researcher, however, recommends that future studies with more diverse sample sizes and feasibility studies are needed before use in the clinical field.
Three of our included studies utilized the DCNNs model to diagnose ischemic stroke and show optimal results.23,28,29 For example, Stib & Vasquez (2020) developed DCNNs to detect large vessel occlusion (LVO) at multiphase CTA. The results showed that DCNNs trained to detect LVOs at multiphase CTA achieved an AUC of 0.89 and a sensitivity of 100%.28 The research suggested that DCNNs have the potential to implement to triage LVOs in the emergency setting and can potentially shorten the time to LVO detection with ultimate improvements in patient outcomes.28
Furthermore, Shinohara et al developed an interactive deep learning-assisted identification of the hyperdense middle cerebral artery sign (HMCAS) on NCCT among 22 patients with AIS.29 The result showed that the diagnostic performance of DCNN for HMCAS shows sensitivity = 82.9%, specificity = 89.7%, accuracy = 86.5%, and AUC = 0.947.29 The researcher suggested that DCNNs appear potentially beneficial for identifying HMCAS on NCCT in patients with AIS. However, further studies, including larger sample sizes with various populations, are recommended before testing for feasibility in the clinical setting. Previous studies utilized DCNNs to diagnose COVID-19,35,36 pulmonary disease,37 and thoracic abnormality38 show that DCNNs have high diagnosis precision. Results suggested that DCNNs are generalizable and useful for the efficient diagnosis and prognosis of diseases. Future research comparing the effectiveness of the traditional diagnosis approach and DCNNs should be conducted before applying the model routinely in the clinical setting.
The study by Sheng et al investigated the effect of GL-HOSVD in diffusion-weighted imaging (DWI) images and evaluated the effect in examining IP of 210 patients with early ACI.26 The results showed that the GL-HOSVD algorithm had high specificity, accuracy, and consistency (81.25%, 87.62%, and 0.52, respectively) in IP detection.26 The study suggested that novel GL-HOSVD algorithms are worthy of clinical application and promotion. Moreover, this study reflects that the deep learning algorithm has a good development prospect in the field of imaging, and its clinical auxiliary effect can be expected in the future.
Another included study discussed the application values of a deep learning algorithm (AD-CNNnet) based CTP imaging combined with head and neck CTA in diagnosing early AIS.30 The results found that the peak signal-to-noise ratio and feature similarity of the AD-CNNnet method were significantly higher than those of traditional methods. At the same time, the normalized mean square error was significantly lower than that of traditional algorithms (P < 0.05). The sensitivity of the AD-CNNnet method was 93.66%, and the specificity was 96.18%. This study provides a reference for the combined application of AD-CNNnet and clinical imaging. Accordingly, it is suggested that before implementation in the clinical setting, more patient sample data will be collected in the later study to explore further the application value of CTP combined with CTA based on deep learning algorithm in the prognosis of patients with AIS.
Although neural networks have the potential to utilize in ischemic stroke diagnosis, it has some limitations. Like other machine-learning methods, neural networks require a huge amount of data from instrumental and clinical analysis of diseases to facilitate diagnoses of great relevance.39 Neural networks also require a training period before the modeling process to allow computations on large data sets to avoid over and under-learning.40 In particular, ischemic stroke diagnosis, the fact of health disparities in access to healthcare affects the number of necessary resources utilized in neural networks, like brain imaging, that may not be readily available.41 To illustrate, rural areas are prone to limited access to brain imaging, which is one of the essential combinations of neural networks to bring about an appropriate diagnosis. While neural networks hope to bridge the gap of rapid response in ischemic stroke diagnosis, health disparities hinder the accuracy of neural network development.
In addition, ethical issues, such as autonomy and informed consent of patients, may be raised when using neural networks, as they provide outputs without explaining the underlying logic or reasoning. This may raise questions about how to communicate the diagnosis and treatment options to patients and how to respect their preferences and values.42 Another ethical issue is the responsibility and liability of using neural networks in clinical practice. Neural networks are complex and dynamic systems that may evolve over time, which may pose challenges for quality control and regulation.43 Therefore, it is essential to clarify each stakeholder’s legal and professional obligations and rights and establish mechanisms for monitoring, evaluation, and feedback to support neural networks in the future.
There are limitations in the systematic review to note. First, the sample sizes of some included studies were significantly small and hence, cannot effectively represent the ischemic stroke population. Moreover, small sample size can cause variability, which in turn causes bias. In addition, the English language requirement in the inclusion criteria was a limiting factor. Because of this, qualified studies reported in other languages that also aimed to apply the Neural Networks algorithm to diagnose patients with ischemic stroke may have needed to be included are omitted. According to the methodological quality assessment, all included studies had 90% or more positive answers for the questions included in the appraisal tool. However, there concerning methodological quality was the fact that numerous included studies were not avoided in a case-control design, thus leading to the risk of bias. As each of the included studies featured a different sample size, target population, study design, machine learning algorithm, and features, these may be potential sources of heterogeneity that cause variation in study outcomes.
Conclusion
This systematic review included nine studies with sample sizes ranging from 22 to 986. DCNNs, 3D-CNNs, Two-stage DCNNs, GL-HOSVD, and AD-CNNnet were identified as the optimal algorithms for diagnosing ischemic stroke. Larger multi-center studies comparing neural network algorithms are needed for increased generalizability and standardized diagnostic methods. Additionally, further evaluations on feasibility, cost-effectiveness, and specialized training for utilizing imaging data are crucial for implementing neural networks in stroke diagnosis in clinical settings.
Funding
No funding was received for this review. The article processing charge (APC) was supported by Mahidol University, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Donkor ES. Stroke in the 21 st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:1–10. doi:10.1155/2018/3238165
2. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917
3. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Supplement 2):S6–S16. doi:10.1212/WNL.0000000000012781
4. Crichton SL, Bray BD, McKevitt C, Rudd AG, Wolfe CD. Patient outcomes up to 15 years after stroke: survival, disability, quality of life, cognition and mental health. J Neurol Neurosurg Psychiatry. 2016;87(10):1091–1098. doi:10.1136/jnnp-2016-313361
5. De Wit L, Theuns P, Dejaeger E, et al. Long-term impact of stroke on patients’ health-related quality of life. Disabil Rehabil. 2017;39(14):1435–1440. doi:10.1080/09638288.2016.1200676
6. Chugh C. Acute ischemic stroke: management approach. Indian J Crit Care Med. 2019;23(Suppl 2):S140.
7. Brodoehl S, Günther A, Witte OW, Klingner CM. How to manage thrombolysis interruptions in acute stroke? Clin Neuropharmacol. 2015;38(3):85–88. doi:10.1097/WNF.0000000000000081
8. NINDS. Tissue plasminogen activator for acute ischemic stroke (Alteplase, Activase®); 2022. Available from: https://www.ninds.nih.gov/about-ninds/impact/ninds-contributions-approved-therapies/tissue-plasminogen-activator-acute-ischemic-stroke-alteplase-activaser.
9. Sajjadi M, Karami M, Amirfattahi R, Bateni V, Ahamadzadeh MR, Ebrahimi B. A promising method of enhancement for early detection of ischemic stroke. J Res Med Sci. 2012;17(9):843.
10. Sarker IH. Machine learning: algorithms, real-world applications and research directions. SN Comput Sci.2021;2(3):160. doi:10.1007/s42979-021-00592-x
11. Ruksakulpiwat S, Thongking W, Zhou W, et al. Machine learning-based patient classification system for adults with stroke: a systematic review. Chronic Illn. 2023:19(1)26–39.
12. Brown N, Cambruzzi J, Cox PJ, et al. Chapter Five - Big Data in Drug Discovery. In: Witty DR, Cox B, eds. Progress in Medicinal Chemistry. Elsevier; 2018:277–356.
13. Alzubi JA, Bharathikannan B, Tanwar S, Manikandan R, Khanna A, Thaventhiran C. Boosted neural network ensemble classification for lung cancer disease diagnosis. Appl Soft Comput. 2019;80:579–591. doi:10.1016/j.asoc.2019.04.031
14. Zhang N, Cai Y-X, Wang Y-Y, Tian Y-T, Wang X-L, Badami B. Skin cancer diagnosis based on optimized convolutional neural network. Artif Intell Med. 2020;102:101756. doi:10.1016/j.artmed.2019.101756
15. Li L, Chen Y, Shen Z, et al. Convolutional neural network for the diagnosis of early gastric cancer based on magnifying narrow band imaging. Gastric Cancer. 2020;23(1):126–132. doi:10.1007/s10120-019-00992-2
16. Alizadehsani R, Abdar M, Roshanzamir M, et al. Machine learning-based coronary artery disease diagnosis: a comprehensive review. Comput Biol Med. 2019;111:103346. doi:10.1016/j.compbiomed.2019.103346
17. Karthik R, Menaka R, Johnson A, Anand S. Neuroimaging and deep learning for brain stroke detection - A review of recent advancements and future prospects. Comput Methods Programs Biomed. 2020;197:105728. doi:10.1016/j.cmpb.2020.105728
18. Zhang S, Zhang M, Ma S, et al. Research progress of deep learning in the diagnosis and prevention of stroke. Biomed Res Int. 2021;2021. doi:10.1155/2021/5213550
19. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. doi:10.1093/ptj/89.9.873
20. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi:10.7326/0003-4819-155-8-201110180-00009
21. Campbell JM, Klugar M, Ding S, et al. Diagnostic test accuracy: methods for systematic review and meta-analysis. JBI Evid Implement. 2015;13(3):154–162.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; Prisma Group. Reprint--Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi:10.7326/0003-4819-151-4-200908180-00135
23. Zhang SJ, Xu SH, Tan LW, Wang HY, Meng JL. Stroke lesion detection and analysis in MRI images based on deep learning. J Healthc Eng. 2021;2021:5524769. doi:10.1155/2021/5524769
24. Oman O, Makela T, Salli E, Savolainen S, Kangasniemi M. 3D convolutional neural networks applied to CT angiography in the detection of acute ischemic stroke. Eur Radiol Exp. 2019;3(1)8. doi:10.1186/s41747-019-0085-6
25. Nishio M, Koyasu S, Noguchi S, et al. Automatic detection of acute ischemic stroke using non-contrast computed tomography and two-stage deep learning model. Comput Methods Programs Biomed. 2020;196:105711. doi:10.1016/j.cmpb.2020.105711
26. Sheng H, Wang XL, Jiang MP, Zhang ZS. Deep learning-based diffusion-weighted magnetic resonance imaging in the diagnosis of ischemic penumbra in early cerebral infarction. Contrast Media Mol Imaging. 2022;2022:6270700. doi:10.1155/2022/6270700
27. Wang CY, Shi Z, Yang M, et al. Deep learning-based identification of acute ischemic core and deficit from non-contrast CT and CTA. J Cereb Blood Flow Metab. 2021;41(11):3028–3038. doi:10.1177/0271678x211023660
28. Stib MT, Vasquez J. Detecting large vessel occlusion at multiphase CT angiography by using a deep convolutional neural network. Radiology 2020;297(3):640–649. doi:10.1148/radiol.2020200334
29. Shinohara Y, Takahashi N, Lee Y, Ohmura T, Kinoshita T. Development of a deep learning model to identify hyperdense MCA sign in patients with acute ischemic stroke. Jpn J Radiol. 2020;38(2):112–117. doi:10.1007/s11604-019-00894-4
30. Yang Y, Yang JJ, Feng J, Wang Y. Early diagnosis of acute ischemic stroke by brain computed tomography perfusion imaging combined with head and neck computed tomography angiography on deep learning algorithm. Contrast Media Mol Imaging. 2022;2022:5373585. doi:10.1155/2022/5373585
31. Lu J, Zhou YR, Lv WZ, et al. Identification of early invisible acute ischemic stroke in non-contrast computed tomography using two-stage deep-learning model. Theranostics. 2022;12(12):5564–5573. doi:10.7150/thno.74125
32. Cheng D, Liu M, Fu J, Wang Y. Classification of MR brain images by combination of multi-CNNs for AD diagnosis. SPIE. 2017;10420:875–879.
33. Cao H, Liu H, Song E, et al. A two-stage convolutional neural networks for lung nodule detection. IEEE J Biomed Health Informat. 2020;24(7):2006–2015. doi:10.1109/JBHI.2019.2963720
34. Nazeri K, Aminpour A, Ebrahimi M. Two-Stage Convolutional Neural Network for Breast Cancer Histology Image Classification. Springer; 2018:717–726.
35. Salama AA, Darwish SH, Abdel-Mageed SM, Meshref RA, Mohamed EI. Deep convolutional neural networks for accurate diagnosis of COVID-19 patients using chest X-ray image databases from Italy, Canada, and the USA. Univ Louisville J Respir Infect. 2021;5(1):34.
36. Kugunavar S, Prabhakar C. Convolutional neural networks for the diagnosis and prognosis of the coronavirus disease pandemic. Visual Comput Indus Biomed Art. 2021;4(1):1–14. doi:10.1186/s42492-021-00078-w
37. Hosseini M, Ren H, Rashid H-A, Mazumder AN, Prakash B, Mohsenin T. Neural networks for pulmonary disease diagnosis using auditory and demographic information. arXiv preprint arXiv:201113194; Preprint. 2020
38. Gakhar M, Aggarwal A. ThoraciNet: thoracic abnormality detection and disease classification using fusion DCNNs. Phys Eng Sci Med. 2022;45(3):961–970. doi:10.1007/s13246-022-01137-z
39. Amato F, López A, Peña-Méndez ME, Vaňhara P, Hampl A, Havel J. Artificial neural networks in medical diagnosis. journal article. J Appl Biomed. 2013;11(2):47–58. doi:10.2478/v10136-012-0031-x
40. Abedi V, Goyal N, Tsivgoulis G, et al. Novel screening tool for stroke using artificial neural network. Stroke. 2017;48(6):1678–1681. doi:10.1161/STROKEAHA.117.017033
41. Kamal H, Lopez V, Sheth SA. Machine Learning in Acute Ischemic Stroke Neuroimaging. Mini Review. Front Neurol. 2018;9. doi:10.3389/fneur.2018.00945
42. Gerke S, Minssen T, Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare. In: Artificial Intelligence in Healthcare. Elsevier; 2020:295–336.
43. Zhang J, Zhang ZM. Ethics and governance of trustworthy medical artificial intelligence. BMC Med Inform Decis Mak. 2023;23(1):7. doi:10.1186/s12911-023-02103-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

