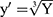Back to Journals » Journal of Inflammation Research » Volume 16
Umbilical Cord Stem Cell Lysate: A New Biologic Injectate for the Putative Treatment of Acute Temporomandibular Joint Inflammation
Authors Ward CK , Gill RG, Liddell RS, Davies JE
Received 30 May 2023
Accepted for publication 4 August 2023
Published 27 September 2023 Volume 2023:16 Pages 4287—4300
DOI https://doi.org/10.2147/JIR.S420741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Christopher K Ward,1,* Rita G Gill,2,* Robert S Liddell,2 John E Davies1,2
1Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada; 2Institute of Biomedical Engineering (BME), University of Toronto, Toronto, Ontario, Canada
*These authors contributed equally to this work
Correspondence: John E Davies, Institute of Biomedical Engineering (BME), University of Toronto, 164 College Street, Toronto, Ontario, M5S 3G9, Canada, Tel +1 416 414 0908, Email [email protected]
Objective: To compare in vivo, the acute anti-inflammatory effects of a lysate derived from human umbilical perivascular mesenchymal cells with the cells themselves in both an established hind-paw model of carrageenan-induced inflammation and also in the inflamed temporomandibular joint.
Study Design: Human umbilical cord perivascular cells were harvested and cultured in xeno- and serum-free conditions to P3. In addition, P3 cells were used to prepare a proprietary 0.22 micron filtered lysate. First, CD1 immunocompetent mice underwent unilateral hind-paw injections of carrageenan for induction of inflammation, followed immediately by treatment with saline (negative control), 1% cell lysate, or viable cells. The contralateral paw remained un-injected with carrageenan. Paw circumference was measured prior to injections and 48 hr later and myeloperoxidase and TNF-alpha concentrations were measured post-sacrifice in excised tissue. Second, immunocompetent Male Wistar rats underwent unilateral intra-articular temporomandibular (TMJ) injections from the same treatment groups and were sacrificed at 4 and 48 hr post-injection. The contralateral TMJ remained un-injected with carrageenan. Articular tissue and synovial aspirates, from the treated TMJ were obtained for histologic and leukocyte infiltration analyses.
Results: The lysate and cell-treated hind-paw demonstrated reduced tissue edema, and significantly lower concentrations of myeloperoxidase and TNF-alpha at 48 hr compared to untreated controls. Treated TMJs demonstrated lower concentrations of leukocytes in the synovium compared to controls and histologic evidence, in the peri-articular tissue, of reduced inflammation.
Conclusion: In this preliminary study, both the human umbilical perivascular cells and a highly diluted lysate produced therefrom were anti-inflammatory.
Keywords: TMJ-OA, mesenchymal cell, cell lysate, anti-inflammatory, carrageenan model, mouse and rat
Introduction
Degenerative diseases that affect the temporomandibular joint (TMJ) are characterized by loss of articular fibrocartilage, bone remodeling, and synovitis.1 Although the etiopathogenesis of TMJ osteoarthritis (TMJ-OA) is not fully understood, pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-alpha) have been associated with progression and severity of TMJ degeneration.2 Myeloperoxidase (MPO), an enzyme stored in polymorphonuclear leukocytes (PMNs) and used to assess neutrophil infiltration and inflammation, is also elevated in the synovial fluid of patients with progressive TMJ-OA.3
To alleviate symptoms associated with TMJ-OA, corticosteroids, hyaluronic acid (HA), and recently, platelet-rich plasma (PRP) injections have been used as intra-articular therapies.4 Corticosteroid injections have traditionally been the preferred therapeutic intervention for TMJ intra-articular supplementation; a practice adopted from its long-standing use in managing arthritis in various joints for more than 50 years, originally employed to alleviate symptoms in patients with rheumatoid arthritis.5 However, the administration of corticosteroids into joints can expedite the advancement of degenerative arthritis through the suppression of synthesis and deposition of chondroitin sulfate in cartilage, leading to chondrocyte degeneration,5 and by triggering chondrocyte apoptosis.6 In response, some practitioners have shifted towards using hyaluronic acid (HA) as an alternative intra-articular therapeutic to restore lost viscoelastic properties such as cushioning and lubrication in progressive TMJ degeneration.7 However, the off-label use of HA remains controversial due to conflicting evidence and a lack of consistent randomized clinical trials demonstrating these benefits.8 A recent meta-analysis has shown that adjuvant TMJ PRP injections following arthroscopy or arthrocentesis significantly reduce pain levels in TMJ-OA patients compared to HA or saline.9 However, the efficacy of PRP TMJ injections versus other treatments such as HA remains inconclusive due to variations in preparation methods, clinical outcomes, administration procedures, and follow-up periods across studies.10 Measuring the effectiveness of PRP is also challenging since its therapeutic advantages depend on the release of endogenous growth factors, which is influenced by platelet concentration and activation methods. Currently, different preparation protocols, patient sex, and natural biologic variation all affect the final platelet concentration in PRP, making it difficult to establish conclusive evidence regarding its superiority over other treatment modalities for TMJ-OA.10
Mesenchymal stromal/stem cell (MSC) therapy has been utilized for treatment of pro-inflammatory conditions such Crohn’s disease, graft-versus-host disease, and post-cardiac infarction,11 and has also shown to be promising in the treatment of OA due to the multipotent differentiation capacity and immune modulatory phenotype of the cells,12 and their lack of negative side-effects even after relatively long 5–137 months (mean 75 months) follow-up in the knee joints of patients.13
TMJ-OA has also been the target of MSC therapy,2 although the majority of studies have focused on the regeneration of TMJ tissues, especially cartilage and sub-chondral bone, and how tissue source and cell culture conditions can affect regenerative potential.14 In pre-clinical studies, human stem cells have been employed in both immune competent rodents,2,14,15 and lagomorphs,16,17 although they have not yet been reported in human clinical trials.18 However, as has been known for intravenous delivery of MSCs, which become trapped in the lung microvasculature within minutes and cannot be found days later,19 the dwell time of MSC in OA joints is also thought to be transient.20 This has led to the belief that paracrine effects, or phagocytosis of released cell particulates by host resident macrophages, result in MSC administration being an indirect therapy termed a “hit-and-run” effect.21 Indeed, various derivatives of MSCs, such as micro-particles, extracellular matrix vesicles, and exosomes, may be equally immunotherapeutic as live cells, in some circumstances, and have resulted in the coining of the term “the necrobiology of mesenchymal stromal cells.”22 Such findings prompted the promising work of Kim et al23 who, while exploring the mechanism of action of human MSC therapy in the TMJ of immune competent rabbits, showed that lysates of their human umbilical cord cells contained interleukin-1 receptor antagonist, which has been associated with exosome secretion.24 Exosomes have been a therapeutic target for both inflammatory diseases in general,25 and TMJ-OA in particular,26 Thus, exploring the immunotherapeutic properties of MSCs, and their derivatives, in the setting of TMJ-OA could yield novel therapies.
Human MSCs have been isolated from bone marrow, adipose tissue, and placenta, but human umbilical cord mesenchymal cells can be harvested easily from an otherwise discarded tissue, producing high cell yields while having potent immunomodulatory capabilities compared to other MSC sources.27–29 However, in contrast to the approach of Kim et al who reported the anti-inflammatory effect of MSCs as comparable to 0.5mg/mL of dexamethasone,23 we wished to test the hypothesis that a mesenchymal cell lysate (CL) would be equally effective as a viable cell population in abrogating acute TMJ inflammation and could thus provide a putative novel biologic therapeutic. To address our hypothesis, we first produced a CL by micronizing a human umbilical cord perivascular (HUCPVC) mesenchymal cell population in saline. The result is a complex mixture of molecules in solution, cell membrane particles <220 nm in size, and includes exosomes and other small membrane-bound bodies. By comparing the CFU-frequency of whole cord30 compared to the perivascular region,31 the latter represents a richer source of such MSCs than that employed by Kim et al23 as discussed in more detail elsewhere.29,32
In this preliminary study, to demonstrate that a CL would reflect the anti-inflammatory properties of the cells themselves, we chose to test both cells and lysate in the classical hind-paw edema model described by DiRosa and Willoughby,33 which has been employed to test anti-inflammatory drug for decades,34 and also in the TMJ.35 This allowed us to reasonably associate potential acute changes in the TMJ with the demonstrated anti-inflammatory properties of this novel MSC-derived CL. Thus, the aim of our study was to compare the acute (over 48 hr) anti-inflammatory properties of CL-derived from HUCPVCs with the viable cell population itself in abrogating carrageenan-induced inflammation in the rat TMJ.
Materials and Methods
HUCPVC Source
Human umbilical cord perivascular cells were isolated and expanded as described previously.36 Human umbilical cords were collected, with informed written consent, under a protocol approved by both the Health Sciences Research Ethics Board of the University of Toronto and the Research Ethics Board of Mount Sinai Hospital, Toronto (#s 28546 and 13-0066-E respectively). Human umbilical cord perivascular cells (HUCPVCs) were isolated using a proprietary serum and xeno-free process of Tissue Regeneration Therapeutics, Inc., Toronto, Canada. In brief, the primary cell harvest was seeded at a density of 2000 cells/cm2 and incubated at 37°C in a humidified 5% CO2 atmosphere. Cells were expanded to passage 3 (P3) using RoosterNourish-MSC (RoosterBio, Cat. KT-001) complete medium and enzymatically dissociated from the flask at 90% confluency using TrypLE Select CTS (Invitrogen Cat. A12859-01). These HUCPVC populations were employed for the mouse and rat experiments detailed herein under protocols approved by the Local Animal Care Committee of the Faculty of Medicine, University of Toronto (#20012308 and #20012455 respectively).
Lysate Production
The P3 cells derived as described above were grown to 90% confluency, the spent medium was aspirated, and the cells were rinsed twice with PBS (Life Technologies Cat. 14190) then incubated for 24 hr using RoosterBasal-MSC basal medium. To produce the lysate, cells were manually detached, by scraping, and the cell suspension, together with the PBS, was collected in a 50 mL polypropylene tube (BD, Cat. 352070) for micronization using the Mini-Beadbeater-16 (Biospec, Cat. 607) homogenizer for a total of 1.5 min in 30-s intervals. This solution was centrifuged at 290g at 4°C for 15 min and the resulting supernatant volume was adjusted using 0.9% saline to provide 1 million cells/mL (50,000 cells in 50 µL injection volume). The final cell lysate solution was filtered through a 0.22 micron syringe filter, and aliquots of dilutions of 10x (10%) and 100x (1%) of the original concentration (containing ~1 million cells/mL) were then prepared and stored at −80°C until further use. Information regarding the composition of the lysate can be found in Table S1.
Animal Experimentation
Animal experiments were performed in accordance with Canadian Council on Animal Care and the University of Toronto Animal Care Committee under protocol numbers 20012308 and 20012455. For the hind-paw study, 38 ten-week-old female CD1 mice were acclimatized for 1 week prior to animal studies. Mice were divided at random into the five experimental groups (Table 1) with a sample size of n=8 per experimental group and n=6 for controls. For the TMJ-OA study, 64 six-week-old male Wistar rats (180–200 g) were housed for 7 days prior to experiments for acclimatization. Rats were divided at random into eight experimental groups with a sample size of n=8 per group (Table 2). Both mice and rats were fed standard chow and had access to water ad-libitum during acclimatization and during the study. No animal deaths or animals were excluded from this study from adverse experimental effects.
 |
Table 1 Outline of Experimental Group Allocation for the Mouse Hindpaw Experiments. All Treatments Were Administered Prior to Injection of 50 µL of 1% w/v Carrageenan to Induce Inflammation |
 |
Table 2 Outline of Experimental Group Allocation for the Rat TMJ Inflammatory Experiments. All Treatments Were Administered Prior to Injection of 50 µL of 1% w/v Carrageenan to Induce Inflammation |
Carrageenan Induction in the Mice Hind-Paw and Treatment Delivery
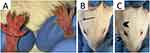
Mice were anesthetized with isoflurane and oxygen (3–5% induction, 2% for maintenance). Fifty microliters of 1% w/v Carrageenan (Sigma, Cat. 22049) solution in 0.9% saline was preloaded into a 30-gauge 0.3cc insulin syringe (Exel Int. 26014) for subcutaneous delivery into the plantar region of the right hind-paw,34 50 µL of CL or cells (preloaded at a density of 50,000 cells in 0.9% saline) were delivered in a caudal direction lateral to the Achilles tendon using a 0.3cc insulin syringe immediately prior to induction of inflammation. Edema was measured by wrapping a 2-ply cotton thread 360° around the paw from the first metatarsophalangeal joint to the fifth, prior to injections (0 hr) and 48 hr later (Figure 1A). Circumference measurements were used as a metric of edema.
Tissue Collection, Protein Isolation, and Purification
Following final hind-paw measurements, the right hind-paw was excised post-sacrifice, frozen in liquid nitrogen and ground into powder using a mortar and pestle. Cell lysis buffer supplemented with Benzonase Nuclease and Protease Inhibitor solution (Qiagen, Cat. 37901) was added to the powder (1 mL/40 mg of frozen tissue powder), followed by homogenization using ceramic beads (Omni International, Cat. 19–646) and a Mini-BeadBeater (MIDSCI, Cat. 607) for a total of 2 min (30-s intervals). Samples were centrifuged to remove all tissue debris, and the supernatant was aliquoted and stored at −80°C until further use.
Elisa Quantification
Supernatants were tested for MPO using MPO ELISA kit (Hycult Biotech, Cat HK210) and TNF-alpha using TNF-alpha Quantikine ELISA kit (R&D Systems, Cat. MTA00B). Total protein was measured to normalize values using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Cat. 23225). A standard curve for each cytokine was generated using the protein standard provided in individual kits. All samples were measured in duplicate. Sample absorbance was measured at 450 nm using a plate reader (Molecular Devices, SpectraMax i3x Multi-Mode Detection Platform).
Induction of TMJ-Inflammation in the TMJ-OA Rat Model and Experimental Design
Rats were anesthetized with isoflurane (3–5% for induction and 2% for maintenance), and oxygen for a total flow rate of 1 L/min. The pre-auricular region was then shaved to better localize the right TMJ. Rats were first injected with 50 µL of either 0.9% saline (control), HUCPVCs, or a 1% CL solution. These were followed by an injection of 50 µL 1% w/v carrageenan (Sigma; Cat. No. 22049) in 0.9% saline for induction of inflammation (Figures 1B and C). All solutions were pre-loaded in 0.3 cc 30-gauge needle insulin syringes (Exel Int. 26014). Localization of the TMJ was carried out using a technique described by Fuentes et al.37
Animal Sacrifice and Tissue Harvesting
Rats were euthanized using CO2 followed by cervical dislocation in accordance with standard operating protocols. TMJ synovial fluid and peri-articular tissue collection occurred immediately after sacrifice of the rats at 4 and 48-hr post-induction of inflammation. Overlying skin and temporalis muscle was dissected superficially until the articular capsule was visualized. Synovial fluid was collected by inserting a 0.3 cc 30-gauge insulin syringe (Exel Int. 26014) and depositing 100 µL of 10 mM ethylenediaminetetraacetic acid (EDTA) in a phosphate buffer solution (PBS) into the superior joint space and aspirating the fluid lavage. This synovial lavage was then stored at −80°C until analyzed. The TMJ joint capsule, associated peri-articular tissue, and mandibular condyle were dissected for further processing.
Histological Analysis
TMJ samples were fixed in 4% paraformaldehyde in PBS for 24 hr, followed by demineralization in 10% EDTA for 2 weeks. Tissues were dehydrated using ascending concentrations of ethanol (70%, 95%, 100%), for 2 hours in each solution. Tissue samples were placed in toluene for clearing for 12 hr. Tissue infiltration and embedding was carried out using paraffin wax heated for 1 hr at 58°C, followed by two 1-hr cycles of paraffin wax infiltration at 58°C under vacuum. Embedded samples were cooled using a cooling plate. All samples were oriented by identifying the root of the zygomatic arch overlying the joint capsule and sectioned along the sagittal plane into 5 µm slices. Tissue sections were mounted onto glass slides, with 1–2 sections per slide. Qualitative histological analysis by light microscopy and photomicrography were carried out using a digital camera (Zeiss Axiocam ICc 5, Germany) coupled to a Leitz Aristoplan microscope (Leica, Germany). Acquired images were then formatted and saved using Zeiss Zen 2.3 lite (Carl Zeiss Microscopy GmbH, Germany).
Synovial Aspirate Cell Counts
Synovial fluid aspirate, collected from the articular capsule of the TMJ, was diluted with PBS (5µL:95µL). Cell counts were determined using an automated cell counter (Vi-cell XR, Beckman Coulter Life Sciences) with cell-size parameters set at 8–12 µm, based on the average size distribution of leukocytes found in rats.38 Lavage was performed at selected timepoints since carrageenan is known to produce a biphasic inflammatory response peaking at 4 hr, followed by a second peak in inflammatory response, at low administrative doses at 48 hr.
Statistical Analysis
MPO, TNF-alpha, and paw edema measurement data are presented as a mean ± standard deviation (SD) with 95% confidence interval (CI) after a one-way ANOVA followed by a post-hoc Tukey’s test for pairwise comparisons between means of groups. Paw edema and MPO measurement data were analyzed as is, but TNF-alpha results were transformed by taking the cube root of the measurements. Statistical analysis was performed using 36 GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). Statistical analysis of the cell counts were conducted in R (R Core Team, 2017) using linear modeling and were transformed using y’ = log(y). The significance level of 5% was utilized for all statistical analyses. Cell count graphs were produced using IBM SPSS Statistics (IBM Corp. Released 2019. IBM SPSS Statistics for Macintosh, Version 26.0.0.0 Armonk, NY: IBM Corp). Data transformations were determined using Box-Cox transformations, normality of residuals was checked by using the Shapiro–Wilk test, and equality of variances between groups was verified using Bartlett’s test. A p value of <0.05 was considered significant.
Results
Reduction of Edema and Inflammatory Cytokines in the Mouse Paw
Edema was recorded as a change in circumference from the original size of the paw prior to injection (Figure 2). Paw circumference of control mice increased by 5.3 mm on average (n = 6) throughout the 48 hr, contrary to the significant edema reduction seen in all treated paws. There were no statistical differences between the different treatments. However, the lowest values belonged to the cell and the 1% CL groups at a 1.9 mm and 2.1 mm increase from baseline, respectively. Both MPO and TNF-alpha were highest in control mice, with an average of 1.8×10−4 mg/mL and 7.7×10−8 mg/mL, respectively (Figures 3 and 4). Analysis of transformed TNF-alpha concentrations (determined by Box-Cox transformations) demonstrated statistically significant reductions in TNF-alpha concentrations in all groups treated with cell lysate, although no significant difference was seen comparing the control group to viable cells (Figure 4). When analyzing raw TNF-ɑlpha concentrations, treatment with 100% cell lysate resulted in a 65% reduction compared to the control, with an average of 9.4×10−5 mg/mL, whereas viable cells exhibited the poorest performance amongst the 4 treatments with only a 43% reduction. Both the 100% and 1% CL reduced MPO by 48% compared to control. However, treatment with viable cells demonstrated a 53% MPO reduction compared to the control mice (Figure 3).
Reductions in Synovial Fluid Aspirate Cell Counts in the Rat TMJ
Cell counts were highest in the control group at 4 hr (18.91×106 cells/mL) followed by the control group at 48 hr (8.13×106 cells/mL) (Figure 5). 1% cell lysate-treated joints (3.20×106 cells/mL) and cell-treated joints (3.73×106 cells/mL) demonstrated significantly lower cell counts compared to the control at 4 hr. Although both treatments lowered cell counts compared to the control, no significant differences were detected at 48 hr. Overall, total cell counts decreased over time between the 4-hr and 48-hr measurements.
 |
Figure 5 Boxplot representing logarithmic transformation: y’ = log(y) of cell counts (8–12 µm in diameter) isolated from lavages of the TMJs at the indicated timepoints. (n=8 for each sample). |
Histologic Findings of Inflammatory Changes in the TMJ
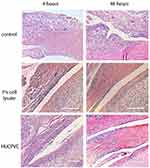
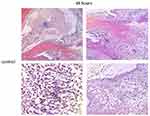
Controls treated with injections of normal saline (NS) and carrageenan exhibited histologic signs consistent with TMJ inflammation (Figures 6–8). In the 4-hr control specimen, early signs of inflammation, such as the presence of numerous capillaries dispersed throughout the loose fibrous connective tissue in the retrodiscal region and subsynovial lining, were observed (Figure 7). At 48 hr, control specimens demonstrated focal regions of dense lymphocytic cell infiltration in the superior joint space, increased cell density along the synovium and within the subsynovium, and the formation of villous-like projections secondary to infolding of the synovial membrane (Figure 8). These characteristics were not observed in the 1% CL and cell-treated TMJ specimens (Figures 6 and 7).
 |
Figure 6 Photomicrographs of H&E stains of the peri-articular tissues of the control specimens compared to the 1% cell lysate and the HUCPVC-treated specimens at the 4-hour and 48-hour timepoints. White arrows indicate the anterior (left arrows in photomicrographs) discal attachments and the retrodiscal tissues (right arrows in photomicrographs) where increases in cell density in the synovial lining were observed in the controls and not the treated specimens. These regions are shown in higher magnification in Figures 7 and 8. Scale bar = 200 µm. |
Discussion
The goal of this preliminary study was to compare the acute anti-inflammatory effects of a lysate derived from HUCPVCs with the cells themselves in both a standard drug-testing inflammation model33,39 and the inflamed TMJ. The choice of two immune competent rodent species was predicated on the established mouse hind-paw model and the increased size of the rat, and its similar structural and histological characteristics compared to the human TMJ, which facilitated administering intra-articular injections in comparison to mice.40
The use of carrageenan, as a phlogistic agent to produce edema in the rodent hind paw, was first described as a method to screen the anti-inflammatory drugs hydrocortisone, phenylbutazone, and acetylsalicylic acid by Winter et al in 1962.41 They described swelling that peaked within 3–5 hr. By 2013 Morris, who confirmed the early 5-hr maximal edema, had described the method as a widely used, well-established, and highly reproducible test for the screening of anti-inflammatory drugs,42 although Henriques et al34 had shown in 1987 that the response was biphasic with a second peak at low drug administrative doses at 48 hr.
Indeed, the relevance of testing over this short 48-hr time-frame was also demonstrated by Radhakrishnan et al43 who showed that rats exhibited spontaneous pain behaviours for 24–48 hr after carrageenan injection into knee joints, which was not seen after 48 hr. Carrageenan is therefore employed in short-term studies monitoring the anti-inflammatory properties of drugs such as that of Loram et al who tracked changes for up to 72 h after carrageenan injection and showed that Tumour Necrosis Factor alpha (TNF-α) and Interleukin-1β (IL-1β) were significantly elevated in the hind paw after 6 hr, and that IL-1 β, Interleukin-6 (IL-6) and Cytokine-induced neutrophil chemoattractant 1 (CINC-1) peaked at 2 days and decreased thereafter.44,45 Thus, in accord with these studies, we chose 48 hr as our maximum time point. Of course, whether the physical and biochemical changes we have observed can be translated into longer term beneficial outcomes would have to be the subject of future experiments. However, as with others who have employed these carrageenan models, we believe that abrogating an acute inflammatory response may reduce the probability of more chronic sequelae.
An additional rationale for limiting the current experiments to only 48 hr was the xenogeneic use of human cells and a human cell lysate. We, and others, have shown that the delivery of human cells into immune competent rodents results in the delivered cell number reducing, in vivo, to zero as a result of the host immune system within 5–7 days,45,46 so at 48 hr we could be reasonably assured that the delivered human cells were both present and viable in the host.47
In all metrics recorded, both cell lysate and viable cells demonstrated a reduction in acute inflammation compared to negative controls. Given the numerous publications attesting to the immune modulatory phenotype of MSCs, our observed anti-inflammatory effects of viable MSCs were to be expected, and corroborate the findings of others.2,14 However, it was of interest to note that viable cells exhibited the poorest performance with respect to TNF-alpha reduction but the greatest reduction of MPO, possibly indicating different underlying mechanisms. Furthermore, given the key observation of Kim et al23 that a lysate produced from their human umbilical cord cells contained at least one known anti-inflammatory mediator and the plethora of data attesting to the immune modulatory effects of exosomes, we reasonably expect our lysate to have a similar effect.
However, it was surprising that a highly diluted form of lysate (produced to contain the same number of cells as employed in the viable cell injectates, but then diluted to 1% of this original concentration) was equally effective as the cells themselves in reducing mouse edema, MPO and TNF-alpha levels, and rat TMJ synovial fluid leukocyte count, and histological evidence of inflammatory peri-articular tissue in the latter compared to the controls. Interestingly, Kim et al23 had also found that inflammatory cytokines were reduced in all three concentrations of viable MSCs they delivered (1, 5 and 10×105 cells in 200μL saline) although they reported only one sacrifice time point of 4 weeks, at which time the cells would no longer be viable in an immune competent lagomorph. A similar lack of dose-dependence has also been reported by others.47 However, to our knowledge, our results are the first to demonstrate that a lysate derived from umbilical cord MSCs can produce a similar therapeutic anti-inflammatory effect compared to their viable cell counterparts. Indeed, the 1% CL was also greater at reducing TMJ cell counts compared to the 10% CL derivative.
This finding of equivalence of viable cells and a 1% lysate remains intriguing.
It is now well established that, upon intravenous administration of MSCs, they become rapidly trapped in the lung microvasculature,19 are degraded and that as a result their degradation products are phagocytosed by lung macrophages48 that undergo polarization from an M1 to M2 phenotype.49,50 These findings have been a stimulant to the burgeoning field of extracellular vesicle (EV),51 and particularly exosome, therapy as cell-free drug delivery systems.52 Exosomes are produced by live cells; different types can be produced by the same cell, and they represent an important means of cellular communication for many cell types,52 and have been shown to contain proteins, miRNAs, and lipids.53 MSC-exosomes are also heterogeneous and are thought to act through multiple mechanisms to effect a therapeutic response.54,55 To reduce such variability and focus the mechanism of action of exosomes on particular constituents, and also in part to address regulatory challenges, exosomes have been engineered with specific contents, so-called “designer exosomes” for specific applications,56 which has resulted in increasingly challenging production methods.51 However, as mentioned earlier,22 several sources of both live and dead cell microparticles have been shown to have immunotherapeutic properties, but little is currently known about the mechanisms of action of such disparate microparticle factions.
Clearly, the cell lysate employed herein differs from an exosome population since it contains the complete contents of the cell including cell membrane particles from both plasma and other cell membrane-bound organelles and cytoplasmic content in addition to the secretome produced during the final 24 hr of cell culture. The resultant lysate is therefore highly complex in both range of particle sizes (below the 0.22 micron filter cut-off) and molecular composition (see Figure S1), although our purpose herein was to show that the far simpler cell processing approach, of whole-cell micronization, can create a complex lysate that has potent anti-inflammatory effects rather than exploring its mechanisms of action.
If, as hypothesized, the mode of action of MSCs relies, in part, on subcellular particles released from them that provide the effector function in inflammatory in vivo environments,22 then administering a cell lysate comprising nanometer size-ranged cell particles, which is facile to produce compared to exosomes, may be a more efficient delivery of the bioeffector than the cells themselves. However, we should caution that preliminary observations (unpublished) have shown that lysate contains particles of varying size and morphology, two important parameters that have been shown to effect immune responses.57 Furthermore, despite the ease of production of such a lysate, and its evident efficacy as demonstrated herein, considerable analysis will be required to isolate the roles of its various constituents. Indeed, such a product would face many of the same regulatory challenges of characterization as have already been described for exosomes.58 Nevertheless, we believe our study contributes to a growing body of evidence that demonstrates that MSC derivatives are therapeutic and that a localized injection, either via deep subcutaneous delivery as in the hind-paw model or via intra-articular injection, can produce an anti-inflammatory effect.
Conclusion
The administration of viable human umbilical cord perivascular cells or a 1% CL derived therefrom were able to abrogate inflammation in both the hind-paw and TMJ models employed herein. These results suggest that a lysate derived from human umbilical-cord derived perivascular cells could be developed as a putative therapeutic for TMJ-OA.
Acknowledgments
We acknowledge the support of Tissue Regeneration Therapeutics Inc. that kindly provided the mesenchymal cells and the cell lysate used in this study and financial support from the Dept. of Oral Surgery, Faculty of Dentistry, University of Toronto. We would also like to thank Zhen-Mei Liu for her technical assistance throughout this project, and also our colleagues who assisted with sample acquisition and processing.
Disclosure
CKW, RGG, and RSL report no conflicts of interest in this work. JED is a stockholder and officer of Tissue Regeneration Therapeutics Inc., which provided the cells and lysate described herein. In addition, JED has a patent wo/20210222377A1 pending to Tissue Regeneration Therapeutics Inc.
References
1. Wang XD, Zhang JN, Gan YN, et al. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94(5):666–673. doi:10.1177/0022034515574770
2. Cui D, Li H, Xu X, et al. Mesenchymal stem cells for cartilage regeneration of TMJ osteoarthritis. Stem Cells Int. 2017;2017(2017):5979741. doi:10.1155/2017/5979741
3. Arinci A, Ademoglu E, Aslan A, et al. Molecular correlates of temporomandibular joint disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):666–670. doi:10.1016/j.tripleo.2004.08.029
4. Liapaki A, Thamm JR, Ha S, et al. Is there a difference in treatment effect of different intra-articular drugs for temporomandibular joint osteoarthritis? A systematic review of randomized controlled trials. Int J Oral Maxillofac Surg. 2021;50(9):1233–1243. PMID: 33642154. doi:10.1016/j.ijom.2021.01.019
5. Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. PMID: 26674652; PMCID: PMC4622344. doi:10.1177/2325967115581163
6. El-Hakim IE, Abdel-Hamid IS, Bader A. Temporomandibular joint (TMJ) response to intra-articular dexamethasone injection following mechanical arthropathy: a histological study in rats. Int J Oral Maxillofac Surg. 2005;34(3):305–310. doi:10.1016/j.ijom.2004.05.004
7. Hegab AF, Ali HE, Elmasry M, Khallaf MG. Platelet-rich plasma injection as an effective treatment for temporomandibular joint osteoarthritis. J Oral Maxillofac Surg. 2015;73(9):1706–1713. PMID: 25882438. doi:10.1016/j.joms.2015.03.045
8. Bouloux GF, Chou J, Krishnan D, et al. Is hyaluronic acid or corticosteroid superior to lactated ringer solution in the short-term reduction of temporomandibular joint pain after arthrocentesis? Part 1. J Oral Maxillofac Surg. 2017;75(1):52–62. PMID: 27632069. doi:10.1016/j.joms.2016.08.006
9. Chung PY, Lin MT, Chang HP. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):106–116. PMID: 30449691. doi:10.1016/j.oooo.2018.09.003
10. Bousnaki M, Bakopoulou A, Koidis P. Platelet-rich plasma for the therapeutic management of temporomandibular joint disorders: a systematic review. Int J Oral Maxillofac Surg. 2018;47(2):188–198. PMID: 29066000. doi:10.1016/j.ijom.2017.09.014
11. Luk F, de Witte SF, Wm B, et al. Efficacy of immunotherapy with mesenchymal stem cells in man: a systematic review. Expert Rev Clin Immunol. 2015;11(5):617–636. doi:10.1586/1744666X.2015.1029458
12. Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. 2021;13(2):448–461.
13. Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150. doi:10.1002/term.299
14. Gong S, Emperumal CP, Enciso R, Enciso R. Regeneration of temporomandibular joint using in vitro human stem cells: a review. J Tissue Eng Regen Med. 2022;16(7):591–604. doi:10.1002/term.3302
15. Saad FA, Abdellah AM. Does stem cells approach ameliorate the prospective alterations of mandibular joint histology and acetylcholinesterase expression in experimentally induced alzheimer’s disease? Egypt J Histol. 2021;44(2):349–367.
16. Köhnke R, Ahlers MO, Birkelbach MA, et al. Temporomandibular joint osteoarthritis: regenerative treatment by a stem cell containing advanced therapy medicinal product (ATMP)- An in vivo animal trial. Int J Mol Sci. 2021;22(1):443. doi:10.3390/ijms22010443
17. Patel R, Kong D, Eisig SB, et al. Improving TMJ Regeneration. JOMS. 2013;71(9):E25–26. doi:10.1016/j.joms.2013.06.046
18. Zhou Y, Xei L. An update on mesenchymal stem cell-centered therapies in temporomandibular joint arthritis. Stem Cells Int. 2021;2021:1–15. doi:10.1155/2021/6619527
19. Lee RH, Pulin AA, Sao MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi:10.1016/j.stem.2009.05.003
20. Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res. 2019;37(6):1229–1235. doi:10.1002/jor.24343
21. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi:10.1038/nbt.2816
22. Weiss DJ, English K, Krasnodembskaya A, isaza-Correa JM, Hawthorne IJ, Mahon BP. The necrobiology of mesenchymal stromal cells affects therapeutic efficacy. Front Immunol. 2019;10:1228. doi:10.3389/fimmu.2019.01228
23. Kim H, Yang G, Park J, Choi J, Kang E, Lee B-K. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci Rep. 2019;9:13854. doi:10.1038/s41598-019-50435-2
24. Kou X, Xu X, Chen C, et al. The Fas/Fap-1/Cav-1 complex regulates Il-1RA secretion in mesenchymal stem cells to accelerate healing. Sci Transl Med. 2018;10(432). doi:10.1126/scitranslmed.aai8524
25. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi:10.1002/stem.2575
26. Song I, Seol DR, Kim EM, Lim T-H, Martin J, Shin K. Therapeutic effects of MSC-exosomes on fibroblasts and synoviocytes in TMJ.
27. Hamidian JS, Li Y, Davies JE. Effect of tumor necrosis factor alpha dose and exposure time on tumor necrosis factor-induced gene-6 activation by neonatal and adult mesenchymal stromal cells. Stem Cells Dev. 2018;27(1):44–54. doi:10.1089/scd.2017.0179
28. Forbes S, Bond AR, Thirlwell KL, et al. Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci Transl Med. 2020;12(526). doi:10.1126/scitranslmed.aan5907
29. Davies JE, Walker JT, Keating A. Concise review: Wharton’s jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6(7):1620–1630. doi:10.1002/sctm.16-0492
30. L-l L, Liu Y-J, Yang S-G, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026.
31. Sarugaser R, Lickorish D, Baksh D, Hosseini M, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220–229. doi:10.1634/stemcells.2004-0166
32. Schugar RC, Chirieleison SM, Wescoe KE, et al. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol. 2009;2009:789526. doi:10.1155/2009/789526
33. Di Rosa M, Willoughby DA. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971;23(4):297–298. doi:10.1111/j.2042-7158.1971.tb08661.x
34. Henriques MG, Silva PM, Martins MA, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20(2):243–249.
35. Goulart AC, Dos Santos Correia FA, de Sousa SCOM, de Cerqueira Luz JG. Study of the inflammatory process induced by injection of carrageenan or formalin in the rat temporomandibular joint. Braz Oral Res. 2005;19(2):99–105. doi:10.1590/S1806-83242005000200005
36. Sarugaser R, Ennis J, Stanford WL, Davies JE. Isolation, propagation, and characterization of Human Umbilical Cord Perivascular Cells (HUCPVCs). Methods Mol Biol. 2009;482:269–279.
37. Fuentes R, Veuthey C, Arias A, et al. Injection in temporomandibular joint of rats. Description of technical protocol. Pol J Vet Sci. 2017;20(2):207–211. doi:10.1515/pjvs-2017-0025
38. Berke HL, Wilson G, Berke ES. Size distribution changes in peripheral lymphocytes of the rat after X-irradiation. Rad Res. 1969;37(1):181–191. doi:10.2307/3572762
39. Willoughby DA, DiRosa M. Studies on the mode of action of non-steroid anti-inflammatory drugs. Ann Rheum Dis. 1972;31(6):540. doi:10.1136/ard.31.6.540
40. Porto GG, Vasconcelos BC, Andrade ES, et al. Comparison between human and rat TMJ: anatomic and histopathologic features. Acta Cir Bras. 2010;25(3):290–293. doi:10.1590/S0102-86502010000300012
41. Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi:10.3181/00379727-111-27849
42. Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–121. doi:10.1385/1-59259-374-7:115
43. Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104(3):567–577. doi:10.1016/S0304-3959(03)00114-3
44. Loram LC, Fuller A, Gayle Fick L, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8(2):127–136. doi:10.1016/j.jpain.2006.06.010
45. Loram LC, Themistocleous AC, Fick LG, Kamerman PR. The time course of inflammatory cytokine secretion in a rat model of postoperative pain does not coincide with the onset of mechanical hyperalgesia. Can J Physiol Pharmacol. 2007;85(6):613–620. doi:10.1139/Y07-054
46. Grinnemo H, Månsson A, Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127(5):1293–1300. doi:10.1016/j.jtcvs.2003.07.037
47. Kwon DR, Park G-Y, Lee SC. Regenerative effects of mesenchymal stem cells by dosage in a chronic rotator cuff tendon tear in a rabbit model. Regen Med. 2019;14(11):1001–1012. doi:10.2217/rme-2018-0125
48. Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi:10.3389/fimmu.2014.00148
49. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi:10.1016/j.stem.2013.09.006
50. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi:10.1038/nri3209
51. Lässer C, Jang SC, Lötvall J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med. 2018;60:1–14. doi:10.1016/j.mam.2018.02.002
52. Muthu S, Bapat A, Jain R, Jeyaraman N, Jeyaraman M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021;8:7. doi:10.21037/sci-2020-037
53. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
54. Liang W, Han B, Hai Y, Sun D, Yin P. Mechanism of action of mesenchymal stem cell-derived exosomes in the intervertebral disc degeneration treatment and bone repair and regeneration. Front Cell Dev Biol. 2022;9:833840. doi:10.3389/fcell.2021.833840
55. Hu JC, Zheng CX, Sui BD, Liu WJ, Jin Y. Mesenchymal stem cell-derived exosomes: a novel and potential remedy for cutaneous wound healing and regeneration. World J Stem Cells. 2022;14(5):318–329. doi:10.4252/wjsc.v14.i5.318
56. Jafari D, Shajari S, Jafari R, et al. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34:567–586. doi:10.1007/s40259-020-00434-x
57. Baranov MV, Kumar M, Sacanna S, Thutupalli S, van den Bogaart G. Modulation of immune responses by particle size and shape. Front Immunol. 2021;11. doi:10.3389/fimmu.2020.607945
58. Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906. doi:10.1016/j.jconrel.2020.10.020
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.