Back to Journals » Journal of Pain Research » Volume 13
Ultrasound-Guided Injection of High Molecular Weight Hyaluronic Acid versus Corticosteroid in Management of Plantar Fasciitis: A 24-Week Randomized Clinical Trial
Authors Raeissadat SA, Nouri F , Darvish M , Esmaily H , Ghazihosseini P
Received 28 May 2019
Accepted for publication 24 December 2019
Published 14 January 2020 Volume 2020:13 Pages 109—121
DOI https://doi.org/10.2147/JPR.S217419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Seyed Ahmad Raeissadat, 1, 2 Farshad Nouri, 2 Mahtab Darvish, 1, 2 Hadi Esmaily, 3 Parsa Ghazihosseini 1, 2
1Clinical Research Development Center, Shahid Modarres Hospital, Tehran, Iran; 2Physical Medicine and Rehabilitation Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Correspondence: Mahtab Darvish
Physical Medicine and Rehabilitation Research Center, Shahid Modarres Hospital, Saadat Abad St., Yadegare Imam Highway, Tehran 1998734383, Iran
Tel/Fax +982122832343
Email [email protected]
Hadi Esmaily
Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Niayesh Highway, Valiasr Ave, Tehran 6153-14155, Iran
Tel +98 9121579064
Email [email protected]
Background and Aims: Plantar fasciitis (PF) is the leading cause of heel pain in adults. This study was designed to evaluate the effect of hyaluronic acid (HA) injection in reducing the symptoms of PF, compared with corticosteroid (CS) injection as a conventional treatment.
Methods: In this triple-blind, randomized, clinical trial, 75 patients who had the symptoms of PF for at least 3 months were randomly divided into two groups of 38 and 37 individuals. Then, each patient received either a single injection of high molecular weight (> 2000 kDa) HA (1 mL HA 20 mg + 1 mL lidocaine 2%) or CS (1 mL methylprednisolone 40 mg + 1 mL lidocaine 2%) under the ultrasonography (US) guidance. Visual analog scale (VAS), foot ankle ability index (FAAI), pressure pain threshold (PPT), functional foot index (FFI), and plantar fascia thickness (PFT) were measured using US at baseline, 6 weeks and 24 weeks after the injection. Eventually, at the end of the treatment period, the patients’ satisfaction was measured. Intention to treat analysis was used to assess the results.
Results: After 24 weeks of follow-up, results from 60 subjects were fully obtained; however, results of 73 patients included into intention to treat analysis in the sixth-week follow-up. In both groups, VAS, PFT and FFI decreased, while FAAI and PPT increased significantly (P < 0.001). At the baseline and at the 24th-week, no significant difference between the two groups was observed in any of the variables. However, a comparison between the baseline and the sixth-week results shows a prominent decrease in PPT and PFT in the CS group compared to the HA group (P = 0.004 and P = 0.011). Finally, there were no statistical differences between the two groups in treatment satisfaction (P = 0.618).
Conclusion: Both CS and HA were effective modalities for PF and can improve pain and function with no superiority in 24th-week follow-ups, although CS seems to have a faster trend of improvement in the short term.
Keywords: plantar fasciitis, hyaluronic acid, pain, visual analog scale, patient satisfaction, ankle joint
Introduction
Plantar fasciitis (PF) is the leading cause of heel pain in adults. Nearly 1 million patients visit physicians for the diagnosis and management of PF symptoms each year.1 The prevalence of heel pain in public is 4–7% and about 80% of this is caused by PF.2 It is more prevalent among women versus men, in those aged 45–64 years versus those aged 18–44 years, and in the obese versus those with a body mass index (BMI) of less than 25 kg/m2.2,3 PF can cause significant morbidity and activity limitations in the affected patients.4 Nonsurgical treatment options include rest, ice application, stretching, exercise, proper footwear, arch supports, orthotics, night splints, extracorporeal shockwave therapy (ESWT),5 anti-inflammatory agents, and various injections including corticosteroid (CS), platelet-rich plasma (PRP),6,7 prolotherapy,8 autologous whole blood,9 botulinum toxin10 or ozone.11
CSs are the most common injection modality for PF. Based on a Cochrane review, CS injections have temporary effects for near 24 weeks.3 Some adverse effects associated with CS injection are plantar fascia rupture, fat pad atrophy, lateral plantar nerve injury, and calcaneal osteomyelitis.12,13
Hyaluronic acid (HA) injection has been used for many musculoskeletal disorders including osteoarthritis of the knee,14 persistent shoulder pain,15 osteoarthritis of the temporomandibular joint,16 knee pain in rheumatoid arthritis patients,17 and soft tissue and periarticular conditions such as lateral epicondylitis and patellar tendinopathy.18,19 Recently, Kumai and colleagues reported that a single injection of HA is clinically effective for the treatment of PF.20
The plantar fascia is a thick aponeurosis with its origin at the medial calcaneal tubercle. The arch of the foot is supported by this fascia. Excessive running or standing for prolonged periods of time may cause an increased amount of tension along the plantar fascia which in turn makes changes in the aponeurosis that can be either acute or chronic. More recently, the term plantar fasciosis has been used instead of PF to show that the inflammation is not the main cause of the pain.21 Histopathologic investigation showed fibrous tissue has more disorganization rather than inflammation in PF, this finding is more likely to what happened in degenerative tendinosis.22,23
HA is a polysaccharide that can be found in the extracellular matrix of soft connective tissues and synovial fluid.24,25 It plays different roles in maintaining elasticity and viscosity of synovial fluid and integrity of connective tissues such as joints.26 HA has been reported to alleviate the pain,27 inhibit cartilage deterioration,28 avoid tissue adhesion,29 and inhibit the development of blood vessels and sensory nerves.30 Yoshida and colleagues31 reported that in a rat tendinopathy model, HA was effective for pain relief and partial repair of the patellar tendon. Wu et al32 also showed that in patients with tendinopathy of the long head of biceps, HA decreases metalloproteinase-1 and −3 expressions in tenocytes and would attenuate tendinopathy. Also, other researchers demonstrated that in patients with rotator cuff tears, intra-articular injections of HA provide immediate clinical improvement compared with ESWT.33
The aim of this clinical trial is to compare the outcomes of local administration of HA with CS injections for patients with chronic plantar fasciopathy.
Methods & Materials
Patients and Setting
The participants of this study were patients with signs and symptoms of PF who were referred to the Physical Medicine & Rehabilitation clinic of Shahid Modarres Hospital in Tehran from March 2017 to January 2019.
Inclusion Criteria
Inclusion criteria of this study were aging between 25 and 60, having clinical diagnosis of PF based on history and physical examination for at least 3 months, and finally not having responded to primary conservative managements such as rest, shoe insoles, conventional physical therapy, exercise therapy, and nonsteroidal anti-inflammatory drugs (NSAIDs).
Exclusion Criteria
Patients were excluded from the study if they had done ESWT or received any injection for PF within the past 3 months, had history of previous surgery for PF, had active bilateral PF, and had systemic inflammatory disease such as rheumatologic diseases, a history of vascular insufficiency and neuropathic heel pain, associated pathology involving the lower limb such as history of tarsal tunnel syndrome, effusion of the ankle indicating an intra-articular disease, old-healed calcaneal fracture, retrocalcaneal bursitis, achilles tendinopathy, ankle osteoarthritis, and finally, any deformity of foot and ankle, including pes planus or pes cavus. Other reasons for exclusion include pregnancy, uncontrolled diabetes mellitus, BMI >33, low back pain with radiation to lower limb, and infection or local trauma near the injection site.
Ethical Considerations
This trial was conducted in accordance with the Declaration of Helsinki; hence the process of the treatment was explained to the patients. Once the physician assures that the patient completely agrees with the study protocol, the written consent form was signed or fingerprinted by the patient. The institutional review board of Shahid Beheshti University of Medical Sciences (SBUMS) agreed on the protocol of this study. The treatment carried a low risk of adverse side effects and patients were advised that they can withdraw their participation at any time. Patients also had access to the project’s physician whenever they experience injection-related infection or fibrosis, persistent pain, and swelling, or any neuromuscular complications. This study was approved by ethical community of SBUMS (code: IR.SBMU.MSP.REC.1397.403) and was registered in the Iranian Center of Clinical Trials (www.irct.ir) (code: IRCT20130523013442N26).
Randomization and Patients’ Enrolment
A computer‑generated block randomization method was used to assign participants with a 1:1 ratio to both groups. It was presumed that participants were distributed almost equally with respect to gender and age in both intervention and positive control groups.34
Group 1. (HA) A syringe containing 20 mg in 1 mL high-molecular-weight HA (>2000 kDa) with 2% concentration of non-animal origin (Viscor®; Nikan Teb Kimia Pharmaceutical Co., Ltd., Tehran, Iran) and 1 mL Lidocaine 2% for a total of 2 mL volume in one syringe was used in this study.
Group 2. (CS) The patients in this group receive 40 mg in 1 mL methylprednisolone acetate (methylprednisolone acetate, 40 mg\mL vial, DEPO-MEDROL®, PFIZER) and 1 mL Lidocaine 2% for a total of 2 mL volume in one syringe.
Although CS and HA were filled in same shaped prefilled syringes, because of the physical differences (viscosity of the solution) between treatments, accidental unblinding of the injector would be unpredictable; hence, we decided not to include the physician who did the injections in the investigation team, and effective blinding of the assessment physician was achieved by the physical separation of the injecting and assessment department and by document control protocols. Patients and the physicians performing the assessments were blinded to the groups. Every prefilled syringe placed in a sealed envelope by a nurse to cover the turbidity of the CS suspension.
Injection Technique
US is an accurate, reliable, and non-invasive technique for measuring PF thickness, monitoring effects of different interventions, and guiding therapeutic interventions in patients with PF.35 It can visualize increases in thickness of the plantar fascia, hypoechoic changes, perifascial fluid collections, and calcaneal spur.36 The US-guided injection is better than palpation-guided injection because the latter provides better relief in pain (VAS scores) and control of PFT by enhancing the accuracy of the injection site by exact localization of the plantar fascia during the injection. Kane et al claimed that the US-guided injection of four heels with recalcitrant PF is highly effective.37
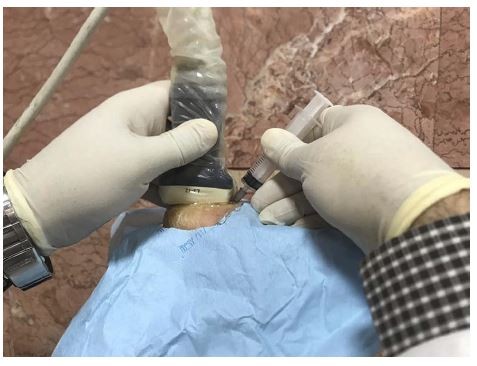
In this study, the plantar fascia was examined with a 3- to 12-MHz real-time linear-array transducer (Ecube7 US system (Alpinion, Seoul, Korea)) with the patient lying prone with 90° of knee flexion and neutral ankle position. The injection was performed under aseptic conditions using a 22-gauge needle. Plantar fascia thickening of >4 mm and/or abnormal hypoechoic areas in plantar fascia were targeted under the longitudinal plane of US-guidance. Figure 1 presents our injection practice. Also, the needle was inserted through the medial heel along with the long-axis view (in-plane technique) toward the target area. Then, 2 mL of HA or CS were injected using a peppering technique, which involves a single skin portal followed by several penetrations of the fascia. Patients were kept in the sitting position without moving their foot for 30 min after the injection. They were sent home with instructions to limit the use of the affected foot for approximately 72 h and to use acetaminophen in case of pain. Moreover, they were taught to perform stretching exercises. It should be noted that the use of NSAIDs was not allowed.
Outcome Measures
Visual Analog Scale (VAS)
Patients were asked to rate pain by marking a horizontally positioned 10-cm VAS with anchor points of “no pain” and “worst possible pain”. Patients were also asked to verbally rate their pain “on a scale of 0 to 10“, with 0 indicating no pain and 10 showing the worst possible pain.38
Pressure Pain Threshold (PPT)
The PPT is a popular model for inducing acute, experimental pain.39 Algometry is a useful technique in determining PPT measures that is used widely in both clinical and laboratory settings.40,41
Pressure algometry is mainly a manual procedure that requires a perceptual response from the participant or patient.39 The results of previous studies have shown that this test has very good measurement reliability, with intra-class correlations (ICC) greater than 0.8 for the PPT measurements, which indicates that participants were indeed capable of judging their pain and discomfort thresholds.42 An analog algometer (SM100 Sundoo®) was used to measure the tenderness threshold (TT). It is a force gauge fitted with an elastic disc with a surface area of 1 cm2 and is calibrated in kg/cm2. The algometer is applied on the medial calcaneal tuberosity by placing the algometer at a 90° vertical angle to the skin surface. The maximum pressure applied is up to 11 kg/cm2. If no pain was recorded in the maximum pressure, the TT is defined as 11 kg/cm2. The minimum pressure to provoke pain was written down. Then, the measurement was done three times with 30-s intervals at the same location and the average value was recorded.
Foot Function Index-Revised (FFI-R)
The FFI-R is a 17-item questionnaire in which each item is scored on a 10-point Likert scale. FFI is used widely worldwide. This instrument that establishes a quantifiable measure of foot health has changed the approach of outcome measurement to subjective, patient-centered, valid, reliable, and responsive hard data endpoints. Editing FFI-R into four response categories will enhance its user-friendliness for measuring foot health.43
Foot and Ankle Ability Measure (FAAM ADL)
The FAAM is a 29-item questionnaire divided into two categories: activities of daily living (ADL) with 21 items and SPORTS with 8 items.44 Each item is scored on a 5-point Likert scale that represents different levels of difficulty (no difficulty at all, slight difficulty, moderate difficulty, extreme difficulty and inability to do). The ADL and SPORTS subscales have a total score of 84 and 32, respectively. In this study, we used the Persian-translated version of FAAM that formerly was validated in a study by Mazaheri et al and showed good reliability and validity for patients with foot and ankle musculoskeletal conditions.45 Because most of our patients were not athletes, we only use the ADL subgroup with a total score of 84.
Ultrasonography Thickness Values (USV)
In clinical settings, USV is a very valuable diagnostic tool for detecting PF via direct observation of the thickness and echogenicity of the plantar fascia.46 USV assessment is relatively fast, inexpensive, and widely available. It may detect relatively small differences in plantar fascia even in clinically undetected cases.47 Increased thickness and hypo-echogenicity in plantar fascia are consistent sonographic findings in patients with PF. USV greater than 4 mm will be considered as abnormal.46 PF does not affect the thickness and echogenicity of the heel pad; therefore, USV may help to make a difference between heel pad pathologies and PF.47 Sabir et al48 suggested that US imaging could be as valuable as magnetic resonance image for detecting PF. The efficacy of US imaging is often limited by examiner-dependent error.49 However, previous studies have shown the reproducibility of measurements of plantar fascia thickness by US, with high intra-and inter-observer reliability.46,50 The examination of each plantar fascia included B-mode scanning was performed using an Ecube 7 US system (Alpinion, Seoul, Korea) with a 3–12-MHz linear transducer (L3-12H; Alpinion). All examinations were conducted by a physiatrist. Each subject was examined lying prone with 90° of knee flexion and neutral ankle position. In a longitudinal view, the thickness of the plantar fascia was measured from the anterior edge of the inferior calcaneal border vertically to the inferior border of the plantar fascia. Finally, local or diffuse hypo-echogenicity at the calcaneal insertion of the plantar fascia were evaluated.
Satisfaction
Patients’ satisfaction was assessed at the end of the study based on the Likert scale from 1 to 5 (1= Not satisfied at all, 5= Very satisfied).
Data Analysis
The intention‐to‐treat (ITT) patients were defined as all included patients after randomization and before treatment commenced at 24-week follow-up. Statistical analysis of data was performed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA). Chi-square (χ2) method and Fisher’s exact test were used to compare qualitative data between groups. Student’s t-test and ANOVA were used for comparing quantitative data, within and between groups. Comparison of the pre- and post-treatment data was done using paired t-test and repeated measurement methods. The two-tailed p-values less than 0.05 were considered as significant.
Results
A total of 94 patients were initially enrolled, of which 75 were included in this study. Using a computer program for random allocation, participants were randomly divided into two parallel groups of CS and HA injection (38 in the HA group and 37 in the CS group). However, within the time to 6-week follow-up, one patient in each group lost to follow-up and four patients in the HA group and six patients in the CS group discontinued the study due to lack of satisfactory efficacy despite the intervention (CS or HA) and continuation of the bothersome PF pain. They were eager to receive another intervention. These last patients’ results recorded, and after excluding from the study, they received ESWT, physical therapy or CS injection depends on their first interventions. The endpoint results of these patients included into ITT analysis. As a result, collected data from 60 patients including 29 participants in the CS group and 31 ones in the HA group were finally analyzed at 24-week time (CONSORT flow chart Figure 2). In this study, 40 patients (53.4%) were women and 35 (46.6%) were men with a mean age of 41.04 ± 8.96 years (22–60 years). With respect to the difficulty level of the job, 9 patients were categorized in difficult job group (12.0%), 26 were housewives (34.6%), 5 were unemployed (6.6%) and 35 (46.6%) had jobs that were categorized as non-difficult. The average BMI calculated as weight/(height)2 was 27.01 ± 1.97 in HA group and 27.71 ± 1.44 kg/m2 in CS group. Mean duration of pain was 7.30 ± 3.50 (in months) in HA group and 6.92 ± 3.64 in CS group.
As presented in Table 1, no significant difference was observed in the two groups with respect to their demographic data including age, sex, height, weight, BMI, job difficulty, level of education and pain duration. Thus, we could conclude that the population was homogeneously distributed in the groups.
 |
Table 1 Baseline Characteristics of the Participants in the Groups |
VAS, PPT, FFI, FAAM and plantar fascia thickness by US was measured at baseline, 6 weeks and 24 weeks after the intervention. Also, satisfaction based on the Likert 5-point scale was measured in the 24th week.
Table 2 summarizes the changes of the mean VAS, PPT, FAAM, FFI, and USV within groups at baseline, 6 weeks and 24 weeks after the treatment. It also showed the mean satisfaction in each group at the end of the 24-week follow-up. At the baseline and the 24th week, no significant difference was observed between the two groups for any of the variables (P> 0.05). However, between baseline and the sixth week, the increase in PPT and decrease in plantar fascia USV was significantly higher in the CS group compared to HA group (P = 0.004 and P = 0.011, respectively). Table 3 compares the results between the two groups over the time.
 |
Table 2 Within Group Analysis of Outcomes Over the Time |
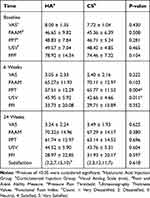 |
Table 3 Between Group Analysis of Outcomes Over the Time |
Participants’ satisfaction, measured at the end of the study, was relatively similar for both groups (P= 0.618). We defined success rates for both interventions as any decrease in about 30% or higher from the baseline scores on the third assessment (24 weeks after intervention). According to this definition, success rates 30% for each scale are as follows (HA vs CS): VAS 93.5% vs 86.2% (P = 0.304), FAAM 77.4% vs 75.9% (P = 0.564), PPT 51.6% vs 62.1% (P = 0.289), and FFI 87.1% vs 79.3% (P = 0.322). Table 4 compares success rates between the two groups.
30% for each scale are as follows (HA vs CS): VAS 93.5% vs 86.2% (P = 0.304), FAAM 77.4% vs 75.9% (P = 0.564), PPT 51.6% vs 62.1% (P = 0.289), and FFI 87.1% vs 79.3% (P = 0.322). Table 4 compares success rates between the two groups.
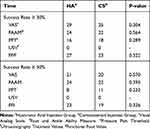 |
Table 4 Comparing the Success Rate Between Groups Over the Time |
The VAS, PPT, USV, FFI and FAAM trend of changes in plantar fascia thickness in the HA and the CS injection groups are shown in Figures 3–7.
Discussion
To the best of our knowledge, this is the first study that compares the effects of US-guided injection of CS and HA in patients with chronic PF at 6 weeks and 24th-week follow-ups.
The results of this study show that there was no significant difference between the two groups for VAS, PPT, FAAM, FFI and fascia thickness measured by the US before treatment. However, 6 weeks after the treatment, plantar fascia thickness measured by US (P <0.001) and PPT (P <0.001) became significantly better in the CS group compared to the HA group. Also, 24 weeks after the treatment, no significant difference between any variable was observed in both groups. In this study, no serious adverse events were seen in any of the groups.
CS and HA were both effective treatments for PF and can improve pain and function with no superiority in 24-week follow-up; however, CS seems to have a faster trend of improvement in the short term.
Steroid injection is commonly used to treat PF. There is some evidence that injected CS were effective in providing transitory pain relief.51 The rapid effects of steroid injection on pain inspire its common use in treating PF. However, current scientific evidence shows that steroid injection should be avoided in treating PF in athletes because of its greater risk of causing spontaneous ruptures.52
The efficacy of CS in treating chronic inflammation has been well demonstrated.53,54 Intralesional injection of CS in chronic PF (defined as persistence of symptoms more than 8 weeks despite conservative care) has been shown to be effective.55 In a review by Ang TW,56 the effectiveness of CS injection in the treatment of PF was evaluated. All placebo-controlled randomized controlled trials (RCTs) showed a significant reduction in pain with the use of CS injections. However, it is evident from these studies that the effects of CS injections are usually short term, lasting only for a duration of 4–12 weeks.56
Kumai et al20 evaluated the efficacy of high molecular weight HA (H-HA), low molecular weight HA (L-HA) and a 0.01% HA (control group) injections in the treatment of recalcitrant PF. Patients were evaluated at baseline and 5 weeks after injection. Based on the obtained results, the improvement in the VAS score for pain in patients with PF was significantly greater in the H-HA group than in the control group. More noticeable improvement was achieved in the H-HA group compared with the L-HA group. Other measures such as Roles and Maudsley score, local symptoms, and FAAM, were also improved in each group, with the H-HA group having better results. Outcomes were also improved by an injection of 0.01% HA (control group); therefore, the injection itself may have possible improvement in pain and FAAM.
In summary, it can be stated that HA may be a proper choice for treating PF. These findings, like our study, showed the effectiveness of high molecular weight HA in the improvement of pain and function of patients with chronic PF.
So far, only one other study has been conducted to evaluate a single HA injection in the treatment of chronic PF.
Advantages of this study were using different outcome measures including VAS, FAAM, FFI, and PPT and measuring changes of plantar fascia thickness by US. Also, US-guided injection had the advantage of more precisely localizing the exact site of injection and doing the intervention with more accuracy.
For future studies, we recommend evaluating the efficacy of HA injection in special groups such as athletes with higher risk of plantar fascia rupture due to CS injection. Also, it is recommended enrolling larger number of patients. Although our follow-up was for 24 weeks, longer follow-ups and assessing the recurrence rate of PF would be recommended in upcoming studies.
In this study, we used a single injection of 20 mg high-molecular-weight, linear HA (>2000 kDa) of non-animal origin in 1 mL (Viscor® 2%; Nikan Teb Kimia Pharmaceutical Co., Ltd., Tehran, Iran). Based on the brand of the HA and its properties such as molecular weight, concentration, linear or reticulated HA, different results may be achieved, and the results of this study may not be generalizable to other HA products. Investigating the molecular weight effect and dose response on the efficacy of HA in PF would be a research target for future studies. Another limitation of this study is that no economic analysis between two injections was directed in our trial.
Conclusion
Both corticosteroid and hyaluronic acid are effective interventions for plantar fasciitis and can improve pain and function with no superiority in 24-week follow-up, although corticosteroid seems to have a faster trend of improvement in the short term. No serious adverse effects were seen in any groups.
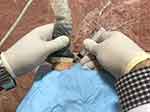 |
Figure 1 Ultrasound-Guided Injection Practice. |
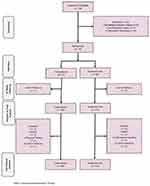 |
Figure 2 The Consolidated Standards of Reporting Trials (CONSORT) diagram of the current study. |
 |
Figure 3 The visual analog scale (VAS) trend of changes in the hyaluronic acid (HA) and corticosteroid injection (CS) groups. |
 |
Figure 4 The pressure pain threshold (PPT) trend of changes in the hyaluronic acid (HA) and corticosteroid injection (CS) groups. |
 |
Figure 5 The ultrasonographic ultrasonography thickness values (USV) trend of changes in plantar fascia in the hyaluronic acid (HA) and corticosteroid injection (CS) groups. |
 |
Figure 6 The functional foot index (FFI) trend of changes in the hyaluronic acid (HA) and corticosteroid injection (CS) groups. |
 |
Figure 7 The foot and ankle ability measure (FFAM) trend of changes in the hyaluronic acid (HA) and corticosteroid injection (CS) groups. |
Data Sharing Statement
The authors intend to share participants' data collected during the trial, after deidentification. This includes, study protocol, statistical analysis plan, informed consent forms, clinical study report, and analytic code. It will be available based on the request of investigators whose proposed use of the data has been approved by an independent review committee by sending an email to the corresponding author. Data are available immediately after publication and with no end date.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25:303–310. doi:10.1177/107110070402500505
2. Beeson P. Plantar fasciopathy: revisiting the risk factors. Foot Ankle Surg. 2014;20:160–165. doi:10.1016/j.fas.2014.03.003
3. David JA, Sankarapandian V, Christopher PRH, Chatterjee A, Macaden AS. Injected corticosteroids for treating plantar heel pain in adults. Cochrane Database Syst Rev. 2017. doi:10.1002/14651858.CD009348.pub2
4. Roerdink RL, Dietvorst M, van der Zwaard B, der Worp H, Zwerver J. Complications of extracorporeal shockwave therapy in plantar fasciitis: systematic review. Int J Surg. 2017;46:133–145. doi:10.1016/j.ijsu.2017.08.587
5. Vahdatpour B, Mokhtarian A, Raeissadat SA, et al. Enhancement of the effectiveness of extracorporeal shock wave therapy with topical corticosteroid in treatment of chronic plantar fasciitis: a randomized control clinical trial. Adv Biomed Res. 2018;7.
6. Singh P, Madanipour S, Bhamra JS, Gill I. A systematic review and meta-analysis of platelet-rich plasma versus corticosteroid injections for plantar fasciopathy. Int Orthop. 2017;41:1169–1181. doi:10.1007/s00264-017-3470-x
7. Raeissadat SA, Babaee M, Rayegani SM, et al. An overview of platelet products (PRP, PRGF, PRF, etc.) in the Iranian studies. Futur Sci OA. 2017;3:FSO231. doi:10.4155/fsoa-2017-0045
8. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM&R. 2014;6:152–158. doi:10.1016/j.pmrj.2013.07.003
9. Karimzadeh A, Raeissadat SA, Erfani Fam S, Sedighipour L, Babaei-Ghazani A. Autologous whole blood versus corticosteroid local injection in treatment of plantar fasciitis: a randomized, controlled multicenter clinical trial. Clin Rheumatol. 2017;36:661–669. doi:10.1007/s10067-016-3484-6
10. Díaz-Llopis IV, Rodriguez-Ruiz CM, Mulet-Perry S, et al. Randomized controlled study of the efficacy of the injection of botulinum toxin type A versus corticosteroids in chronic plantar fasciitis: results at one and six months. Clin Rehabil. 2012;26:594–606. doi:10.1177/0269215511426159
11. Bahrami MH, Raeissadat SA, Barchinejad M, Elyaspour D, Rahimi-Dehgolan S. Local ozone (O2-O3) versus corticosteroid injection efficacy in plantar fasciitis treatment: a double-blinded RCT. J Pain Res. 2019;12:2251–2259. doi:10.2147/JPR.S202045
12. Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91–97. doi:10.1177/107110079801900207
13. Tahririan MA, Motififard M, Tahmasebi MN, Siavashi B. Plantar fasciitis. J Res Med Sci. 2012;17:799–804.
14. Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951–956.
15. Moskowitz RW, Blaine TA. An overview of treatment options for persistent shoulder pain. Am J Orthop (Belle Mead. NJ). 2005;34:10–15.
16. Iturriaga V, Bornhardt T, Manterola C, Brebi P. Effect of hyaluronic acid on the regulation of inflammatory mediators in osteoarthritis of the temporomandibular joint: a systematic review. Int J Oral Maxillofac Surg. 2017;46:590–595. doi:10.1016/j.ijom.2017.01.014
17. Matsuno H, Yudoh K, Kondo M, Goto M, Kimura T. Biochemical effect of intra-articular injections of high molecular weight hyaluronate in rheumatoid arthritis patients. Inflamm Res. 1999;48:154–159. doi:10.1007/s000110050439
18. Muneta T, Koga H, Ju Y-J, Mochizuki T, Sekiya I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J Orthop Sci. 2012;17:425–431. doi:10.1007/s00776-012-0225-9
19. Petrella RJ, Cogliano A, Decaria J, Mohamed N, Lee R. Management of Tennis Elbow with sodium hyaluronate periarticular injections. BMC Sports Sci Med Rehabil. 2010;2:4. doi:10.1186/1758-2555-2-4
20. Kumai T, Samoto N, Hasegawa A, et al. Short-term efficacy and safety of hyaluronic acid injection for plantar fasciopathy. Knee Surgery Sport Traumatol Arthrosc. 2018;26:903–911. doi:10.1007/s00167-017-4467-0
21. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heel pain: a clinical practice guideline–revision 2010. J Foot Ankle Surg. 2010;49:S1–S19. doi:10.1053/j.jfas.2010.01.001
22. MacAuley D, Best TM. Evidence-Based Sports Medicine. Wiley Online Library; 2007.
23. Schwartz EN, Su J. Plantar fasciitis: a concise review. Perm J. 2014;18:e105–7. doi:10.7812/TPP/13-113
24. Nakamura H, Gotoh M, Kanazawa T, et al. Effects of corticosteroids and hyaluronic acid on torn rotator cuff tendons in vitro and in rats. J Orthop Res. 2015;33:1523–1530. doi:10.1002/jor.22921
25. Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. doi:10.1096/fasebj.6.7.1563592
26. Chen LH, Xue JF, Zheng ZY, et al. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: a review of recent developments and critical appraisal of preclinical and clinical investigations. Int J Biol Macromol. 2018;116:572–584. doi:10.1016/j.ijbiomac.2018.05.068
27. Tamoto K. High molecular weight hyaluronic acids inhibit interleukin-1-induced prostaglandin E_2 generation and prostaglandin E_2-elicited cyclic AMP accumulation in human rheumatoid arthritic synovial cells. Jpn J Rheumatol. 1994;5:227–236.
28. Kikuchi T. Effects of hyaluronan on proteoglycan metabolism of rabbit articular chondrocytes in culture. Jpn J Rheumatol. 1994;3:207–215.
29. Weiss C, Levy HJ, Denlinger J, Suros JM, Weiss HE. The role of Na-hylan in reducing postsurgical tendon adhesions. Bull Hosp Jt Dis Orthop Inst. 1986;46:9–15.
30. Tang T, Muneta T, Sekiya I. Fibrous change of the infrapatellar fat pad due to strenuous running exercise and its treatment with intraarticular hyaluronan injection in a rat model. J Med Dent Sci. 2008;55:163–173.
31. Yoshida M, Funasaki H, Kubota M, Marumo K. Therapeutic effects of high molecular weight hyaluronan injections for tendinopathy in a rat model. J Orthop Sci. 2015;20:186–195. doi:10.1007/s00776-014-0650-z
32. Wu P-T, Kuo LC, Su FC, et al. High-molecular-weight hyaluronic acid attenuated matrix metalloproteinase-1 and −3 expression via CD44 in tendinopathy. Sci Rep. 2017;7:40840. doi:10.1038/srep40840
33. Frizziero A, Vittadini F, Barazzuol M, et al. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: preliminary results. J Sports Med Phys Fitness. 2017;57:1162–1168. doi:10.23736/S0022-4707.16.06408-2
34. Vahdatpour B, Kianimehr L, Moradi A, Haghighat S. Beneficial effects of platelet-rich plasma on improvement of pain severity and physical disability in patients with plantar fasciitis: a randomized trial. Adv Biomed Res. 2016;5:179. doi:10.4103/2277-9175.192731
35. Mohseni-Bandpei MA, Nakhaee M, Mousavi ME, et al. Application of ultrasound in the assessment of plantar fascia in patients with plantar fasciitis: a systematic review. Ultrasound Med Biol. 2014;40:1737–1754. doi:10.1016/j.ultrasmedbio.2014.03.001
36. DiMarcangelo MT, Yu TC. Diagnostic imaging of heel pain and plantar fasciitis. Clin Podiatr Med Surg. 1997;14:281–301.
37. Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O. Ultrasound guided injection of recalcitrant plantar fasciitis. Ann Rheum Dis. 1998;57:383–384. doi:10.1136/ard.57.6.383
38. Bijur PE, Latimer CT, Gallagher EJ Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Available from: www.aemj.org.
39. Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23:760–766. doi:10.1097/AJP.0b013e318154b6ae
40. Isselée H, De Laat A, Bogaerts K, Lysens R. Short-term reproducibility of pressure pain thresholds in masticatory muscles measured with a new algometer. J Orofac Pain. 1998;12.
41. Fischer AA. Pressure threshold measurement for diagnosis of myofascial pain and evaluation of treatment results. Clin J Pain. 1986;2:207–214. doi:10.1097/00002508-198612000-00001
42. Xiong S, Goonetilleke RS, Jiang Z. Pressure thresholds of the human foot: measurement reliability and effects of stimulus characteristics. Ergonomics. 2011;54:282–293. doi:10.1080/00140139.2011.552736
43. Budiman-Mak E, Conrad KJ, Mazza J, Stuck RM. A review of the foot function index and the foot function index – revised. J Foot Ankle Res. 2013;6:5. doi:10.1186/1757-1146-6-5
44. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of Validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26:968–983. doi:10.1177/107110070502601113
45. Mazaheri M, Salavati M, Negahban H, et al. Reliability and validity of the Persian version of Foot and Ankle Ability Measure (FAAM) to measure functional limitations in patients with foot and ankle disorders. Osteoarthritis Cartilage. 2010;18:755–759. doi:10.1016/j.joca.2010.03.006
46. Cheng J-W, Tsai W-C, Yu T-Y, Huang K-Y. Reproducibility of sonographic measurement of thickness and echogenicity of the plantar fascia. J Clin Ultrasound. 2012;40:14–19. doi:10.1002/jcu.v40.1
47. Karabay N, Toros T, Hurel C. Ultrasonographic evaluation in plantar fasciitis. J Foot Ankle Surg. 2007;46:442–446. doi:10.1053/j.jfas.2007.08.006
48. Sabir N, Demirlenk S, Yagci B, Karabulut N, Cubukcu S. Clinical utility of sonography in diagnosing plantar fasciitis. J Ultrasound Med. 2005;24:1041–1048. doi:10.7863/jum.2005.24.8.1041
49. Scheel AK, Schmidt WA, Hermann KA, et al. Interobserver reliability of rheumatologists performing musculoskeletal ultrasonography: results from a EULAR “Train the trainers” course. Ann Rheum Dis. 2005;64:1043–1049. doi:10.1136/ard.2004.030387
50. Rathleff MS, Moelgaard C, Olesen JL. Intra-and interobserver reliability of quantitative ultrasound measurement of the plantar fascia. J Clin Ultrasound. 2011;39:128–134. doi:10.1002/jcu.20787
51. Crawford F, Thomson CE. Interventions for treating plantar heel pain. In: Crawford F, editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd;2003. doi:10.1002/14651858.CD000416
52. Lee HS, Choi YR, Kim SW, et al. Risk factors affecting chronic rupture of the plantar fascia. Foot Ankle Int. 2014;35:258–263. doi:10.1177/1071100713514564
53. journal, T. A.-S. Medical & 2015, undefined. The effectiveness of corticosteroid injection in the treatment of plantar fasciitis. Available from: ncbi.nlm.nih.gov.
54. Foster ZJ, Voss TT, Frimodig A. Corticosteroid Injections for Common Musculoskeletal Conditions. Vol. 92; 2015. Available from: www.aafp.org/afp.
55. Mahindra P, Yamin M, Selhi HS, Singla S, Soni A. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics. 2016;39:e285–e289. doi:10.3928/01477447-20160222-01
56. Hsiao M-Y, Hung CY, Chang KV, et al. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: a network meta-analysis. Rheumatology. 2015;54:1735–1743. doi:10.1093/rheumatology/kev010
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
