Back to Journals » Journal of Inflammation Research » Volume 16
Ulcerative Colitis Concomitant with Cytomegalovirus Infection, Bullous Sweet’s Syndrome, and Acute Myeloid Leukemia: A Case Report and Literature Review
Authors Zhu F, Hu Z, Yu W, Dai F, Jing D, Zhou G
Received 19 May 2023
Accepted for publication 11 August 2023
Published 28 August 2023 Volume 2023:16 Pages 3715—3723
DOI https://doi.org/10.2147/JIR.S422057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Fengqin Zhu,1,2 Zongjing Hu,1 Wei Yu,1 Fengxian Dai,1 Dehuai Jing,1 Guangxi Zhou1
1Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, Shandong, 272000, People’s Republic of China; 2Shandong University of Traditional Chinese Medicine, Jinan, Shandong, 250355, People’s Republic of China
Correspondence: Guangxi Zhou, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, Shandong, 272000, People’s Republic of China, Email [email protected]
Background: Ulcerative colitis (UC) is a chronic, relapsing progressive inflammatory immune disease. There is still no cure for it. Even worse, UC may predispose patients to opportunistic infections, and several extra-intestinal manifestations (EIMs) and comorbidities may antedate, occur with, or postdate the onset of UC, which may increase the mortality risk. But case reports of UC patients simultaneously concomitant with opportunistic infection, EIM, and comorbidity are extremely rare.
Case Presentation: We report a case of 51-year-old male patient with incipient UC accompanied by cytomegalovirus (CMV) infection and bullous Sweet’s syndrome (bSS, a cutaneous EIM of UC) after treatment with oral mesalazine and prednisolone for 3 weeks. After clearance of the CMV infection by using ganciclovir, the patient was administered two cycles of infliximab to cure UC and bSS; however, he developed acute myeloid leukemia (AML) a month later and died after two cycles of chemotherapy.
Conclusion: Based on this rare case of UC concomitant with CMV infection, bSS and AML, we recommend that it is important to distinguish between an acute UC flare and opportunistic infections, especially in patients receiving immunosuppressive therapy, and monitor EIMs and comorbidities timely. Particular attention should be paid to cancer surveillance. Clinicians should be mindful of these facts to adopt optimal therapeutic options to address all aspects of UC. Early initiation of biological therapy may be of benefit to patients with newly diagnosed severe UC.
Keywords: ulcerative colitis, cytomegalovirus, bullous Sweet’s syndrome, acute myeloid leukemia
Introduction
Ulcerative colitis (UC), is a subtype of chronic recurrent and remitting inflammatory bowel disease (IBD). The burden of UC is rising substantially worldwide. The incidence of UC is much higher in Western developed countries than in Eastern developing countries; however, it has risen rapidly in East while plateauing in West.1 UC can be observed across all age groups, with similar incidence between men and women.2,3 At present, the treatment of UC mainly includes 5-aminosalicylates, corticosteroids, immunosuppressants, biologics, and oral small-molecule therapies.4. Reportedly, a genetic predisposition and/or exposure to environmental risk factors, which trigger an aberrant immune response to enteric commensal microbes, are implicated as etiopathogenetic contributors.5 UC mainly affects the intestinal tract, and patients present with symptoms of bloody purulent diarrhea, increased frequency of bowel movements, and lower abdominal pain. However, organs other than the intestinal tract may occasionally be involved, which represent extra-intestinal manifestations (EIMs) of UC. EIMs most commonly include disorders of the joints, skin, eyes, and other organs such as the liver, lung, and pancreas, and EIMs may occur before or after diagnosis of UC.6 These factors tend to significantly affect patients’ quality of life. In addition to EIMs, UC is associated with several comorbidities, such as hematological disorders. Diagnosis and treatment of UC accompanied by comorbidities is challenging, and this condition increases the morbidity and mortality rates of patients with UC and significantly affects patients’ quality of life and the economic burden of the disease.7
Treatment (For example, administration of corticosteroids, biologics, or immunosuppressive agents), nutritional compromise, and/or underlying immune deficits result in immunosuppression in patients with UC, with a consequent increase in the risk of opportunistic infections.8 Common opportunistic infections in UC include bacterial (tuberculosis, Clostridium difficile, pneumococcal, and listeriosis), fungal (cryptococcosis, Pneumocystis jirovecii, and candidiasis), and viral infections (herpes simplex virus, cytomegalovirus [CMV], and Epstein–Barr virus).9 Opportunistic infections often aggravate UC symptoms and affect clinical treatment effects. Therefore, prompt initiation of treatment is warranted in patients diagnosed with UC accompanied by opportunistic infections.
We report a rare case of UC patient accompanied by CMV infection, bullous Sweet’s syndrome (bSS, a cutaneous EIM), and concomitant acute myeloid leukemia (AML), together with a literature review. This study was approved by the Institutional Review Board for Clinical Research of the Affiliated Hospital of Jining Medical University, and written informed consent was obtained from each patient prior to study enrollment.
Case Description
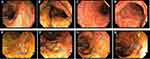
A 51-year-old man with an unremarkable medical history was admitted to a local hospital on January 7, 2022, for management of sudden onset of diarrhea and bloody purulent stool. Following comprehensive history-taking, physical examination, and blood tests, he underwent colonoscopy, which revealed mucosal erythema, edema, granular changes, and multiple small mucosal ulcerations, and he was diagnosed with UC (Figures 1A–D). The patient was administered an unknown dosage of oral mesalazine and prednisolone for 3 weeks; diarrhea gradually resolved, and examination showed fewer pus cells and lesser quantity of blood in the stool. However, on January 28, diarrhea and bloody purulent stool suddenly worsened without any apparent cause. Repeat colonoscopy performed to further evaluate intestinal mucosal inflammation revealed several round, large, and oval deep ulcers throughout the colon (Figures 1E–H). The patient was transferred to our hospital the same day for further evaluation and treatment.
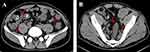
He denied a history of any other disease and drug abuse or food allergies. Physical examination showed mild tenderness in the left lower quadrant of the abdomen without any other remarkable signs. With regard to his family history, the patient’s parents died of esophageal and gastric cancer. Table 1 summarizes the laboratory data obtained on the day of admission. The patient showed an elevated erythrocyte sedimentation rate (ESR, 60 mm/hour) and elevated serum C-reactive protein (139.75 mg/dL), interleukin (IL)-6 (516.8 pg/mL), and IL-17A (31.27 pg/mL) levels, with hypoalbuminemia (serum albumin [Alb] 27.8 g/L) and a low platelet count (78 × 109/L), suggestive of severe acute inflammation. The CMV-immunoglobulin (Ig) G was 178.0 U/mL, and CMV-IgM measured 26.0 U/mL, with quantification of CMV DNA in plasma at 4.46◊103 copies/mL, which indicated active CMV infection in this patient with UC. We did not detect the antinuclear factor, Epstein–Barr virus, tuberculosis, C. difficile, or stool bacterial infection. Abdominal computed tomography (CT) revealed a thickened colonic wall with pericolonic exudates (Figure 2).
 |
Table 1 Laboratory Test Results of the Baseline and Follow-Up of the Patient |
Based on these findings, the patient was diagnosed with severe primary UC involving the entire colon accompanied by CMV infection and hypoproteinemia. Intravenous ganciclovir (0.5 g/day) was initiated with continued oral mesalazine (4.0 g/day) and prednisolone (50.0 mg/day) along with levofloxacin, probiotics, and other symptomatic and supportive treatment. We recommended Alb infusion therapy; however, the patient refused this treatment based on cost concerns. The patient’s bloody purulent diarrhea gradually improved following the aforementioned treatment.
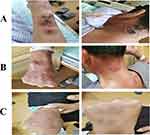
On the 6th day of admission, the patient had well-delineated, erythematous, and mildly tender plaques across the face, neck, trunk, arms, and hands, accompanied by fever (Tmax 38.5°C) (Figure 3A). We initiated oral ebastine (10.0 mg/day) with local application of mometasone furoate cream. Owing to fever, levofloxacin was switched to intravenous cefoperazone-sulbactam (3.0 g/12 hours) as intensive anti-infection therapy. However, the skin lesions extended and worsened with partial coalescence, accompanied by bullae or blister formation over the following 5 days (Figure 3B and C). Considering the clinical findings, we diagnosed these lesions as those of SS. The Following a Dermatology consultation, thalidomide (100.0 mg/day) was added to the patient’s regimen on February 6 to suppress an abnormal immune response. Simultaneously, we obtained blister secretions for culture studies; however, no abnormalities were detected. The patient’s fever persisted, and laboratory test results showed an elevated white blood cell count of 12.21 × 109/L, decreased platelet count of 73 × 109/L, anemia with serum hemoglobin 97.0 g/L, ESR of 82 mm/hour, and serum Alb of 29.1 g/L. Physical examination showed no oral or genital mucosal ulcer or erythema. Therefore, we consulted the Infectious Disease Department, and antibiotic therapy was switched to intravenous impenem and cilastatin sodium (0.5 g/6 hours). We performed metagenomic sequencing of genomic DNA extracted from whole blood for pathogen detection, which revealed no abnormality.
The patient showed defervescence on February 8, and repeat CMV DNA quantification in blood, urine, and stool showed negative results, which indicated clearance of active CMV infection. Therefore, the patient was administered intravenous infliximab (IFX, 300.0 mg), and we discontinued oral administration of mesalazine and thalidomide, as well as intravenous antibiotic therapy, and oral prednisone was tapered at 5 mg/week. The patient was discharged one week later and had no abdominal pain or bloody purulent stool, with significantly improved skin lesions (Figure 4A).
Two weeks later, the patient returned to our hospital for a second course of IFX. He was asymptomatic; the original skin lesions had subsided (Figure 4B), and he underwent routine blood tests (Table 1). His serum hemoglobin level was 81.0 g/L, red blood cells (RBCs) 2.45 × 109/L, with a low platelet count (98.0 × 109/L). He received the second course of IFX (300.0 mg). During follow-up, he was prescribed prednisolone 30.0 mg/day, which was gradually tapered to 7.5 mg/day.
A month later on March 30, the patient was rehospitalized for the third course of IFX. He was asymptomatic, and residual pigmentation was observed at the site of the original skin lesions, with no new eruption (Figure 4C). Table 1 summarizes routine blood test results. Microscopic examination showed 4.0% primitive immature cells with fine chromatin and nucleoli identified in some cells and tear-like RBCs and rarely platelets, which was highly suggestive of leukemia. Therefore, the patient was immediately transferred to the hematology department for further evaluation. Bone marrow aspirate smear evaluation revealed significant active granulocytic hyperplasia with 34.0% myeloid primitive cells. Flow cytometry of the bone marrow aspirate showed 13.48% immature myeloid cells (blasts) positive for CD117, CD13, and CD38 but negative for CD34, CD14, CD15, and HLA-DR expression. Next-generation sequencing analysis of genomic DNA extracted from the bone marrow revealed mutations of the NPM1, TP53, TET2, and DNMT3A genes, which indicated poor prognosis. Based on these results, the patient was diagnosed with AML and was administered combination chemotherapy using the homoharringtonine, cytarabine, and granulocyte colony-stimulating factor regimen, and steroids were withdrawn. The patient developed severe bacterial and fungal infections during chemotherapy; however, stool test results were unremarkable, and skin lesions did not recur.
Re-examination of the bone marrow aspirate following a chemotherapy cycle showed no remission; therefore, the chemotherapy regimen was switched to include chidamide, venetoclax, and azacitidine. On June 14 (<3 months after AML onset), the patient was rehospitalized for evaluation of high fever and died of ventricular fibrillation and cardiopulmonary arrest the following day.
Discussion
UC is a chronic inflammatory disorder of the colon characterized by an exaggerated systemic immune response.10 Therefore, immunosuppressants that temper the host inflammatory response serve as the cornerstone of UC therapy. However, these medications are known to predispose patients to opportunistic infections.11,12 Notably, UC is associated with several comorbidities and EIMs, which is attributable to potential genetic risk factors, environmental factors, and immune disorders.2,13–15 We report a case of UC accompanied by CMV infection, bSS, and concomitant AML. CMV, a ubiquitous double-stranded DNA virus that belongs to the Herpesviridae family remains latent within the host hematopoietic cells under normal conditions. However, this virus may undergo reactivation and trigger systemic infections in immunocompromised hosts.16,17 The first case report on CMV infection in UC was described in 1961.18 Since then, the correlation between CMV infection and UC has been reported in a wide range of studies. In UC, mucosal inflammation and T helper cell 2 type cytokines (interleukin [IL]-5, IL-6, and IL-13), together with immunomodulatory agents may favor CMV reactivation within the gut, with consequent aggravation of mucosal inflammation and impaired steroid efficacy.19 Some authors have reported a 25.0–30.0% prevalence of CMV infection among patients with UC.20–22 On the other hand, CMV infection may play a role in exacerbating the severity of UC, evidenced by a higher prevalence of endoscopically severe UC in CMV infected patients compared to non-infected patients.23 Therefore, it is important to differentiate between UC flares and CMV infection. CMV infection is diagnosed based on histopathological evaluation, serological tests for IgM and IgG, the CMV DNA viral load in the blood or intestinal tissue, and CMV pp65 antigenemia assays.24 Management of patients with UC and concomitant CMV infection includes administration of antiviral agents (For example, ganciclovir and foscarnet).25 High serum CMV-IgM and CMV-DNA levels favored diagnosis of CMV infection in our patient with UC. Moreover, ganciclovir treatment was effective in this case. It is now well established that corticosteroids and immunomodulators are associated with increased risk of CMV reactivation in UC.26,27 Conversely, biotherapy against TNF-α such as infliximab or adalimumab does not increase the risk of CMV reactivation.28 CMV infection in this patient may be attributable to an immunocompromised state secondary to previous corticosteroid treatment. And we wonder that the initial application of biological agents against TNF-α may circumvent CMV infection.
SS, also termed “acute febrile neutrophilic dermatosis”, is a rare type of cutaneous EIM associated with UC, and presents as an abrupt onset of painful erythematous plaques or nodules, often with fever and elevated inflammatory markers, with histopathological evidence of neutrophil-rich infiltrates without vasculitis.29,30 SS may be associated with pro-inflammatory signals in UC, which could activate neutrophils in the skin, such as TNF-α and IL-8.31,32 SS often occurs alongside UC diagnosis and correlates with active UC, thus, it can signal a UC diagnosis early. Similarly, reassessment for disease activity in known UC is essential once SS occurs.33 bSS is quite an uncommon clinical variant of SS, with bullous and blisters. Only a few cases of bSS associated with UC have been described.34–36 Corticosteroids and TNF-α inhibitors have demonstrated efficacy in treating SS in UC, and the resolution of bullous lesions does not result in scars.33 In addition to its association with IBD, bSS can be associated with infections. Investigations did not reveal any pathogen in the blister secretion or in whole blood, which therefore ruled out infection in our patient. As a limitation, we did not perform the skin biopsy because rejection of the patient, which will be helpful for better diagnosis. However, based on the typical clinical presentation of skin lesions and their parallel to intestinal disease activity, we considered them most likely bSS associated with UC.
AML, a well-known hematological malignancy, is a heterogeneous clonal disorder of hematopoietic progenitor cells that lose their ability to differentiate normally and fail to respond to normal regulators of proliferation, which leads to fatal infection, bleeding, or organ infiltration.37 Previous studies have reported that the risk of AML among patients with UC is higher than that in the general population.38,39 However, few studies have reported the incidence and outcome of AML in the UC population. We searched the PubMed database for English articles, reviews, and case reports using the following words: “Acute Myeloid Leukemia”, “AML”, “leukemia and inflammatory bowel disease”, and “myeloid leukemias and autoimmune disorders;” however, our search yielded only 13 cases of AML in patients with UC (Table 2).38,40–43 The latent period between UC and AML ranges from 1 year to three decades. Our patient was diagnosed with AML < 3 months after diagnosis of UC, which is the shortest interval between the diagnosis of the two diseases to date.
 |
Table 2 Summary of 13 Cases of AML in Patients with UC |
The hypothetical association between UC and AML is mainly attributable to immune dysfunction and the iatrogenic effect of UC medications such as thiopurines, methotrexate, and biological agents.44,45 NK cell function is decreased in both UC and AML. Impaired immune surveillance function in UC increases the immune escape of malignant hematopoietic cells, leading to hematopoietic malignancy (eg, AML, CMML and lymphoma).46,47 Tumor necrosis factor (TNF)-α can inhibit the growth of human leukemia progenitor cell colonies and various leukemic cell lines; therefore, anti-TNF-α agents may serve as triggers in patients with a genetic predisposition to some hematological malignancies.48 Studies have reported AML in patients with Crohn’s disease or other immune diseases, who received anti-TNF-α agents (For example, adalimumab and IFX).49–51 Despite the close temporal association between IFX exposure and AML in our patient, we could not conclusively establish a causal association. Because our patient received only two short cycles of IFX therapy (total dose 600.0 mg). Many other underlying factors may affect AML development. Our patient had gene mutations including those observed in the TET2, and DNMT3A genes, which are frequently detected in AML and may also be associated with UC. Studies have shown that TET2 loss is associated with elevated cytokine/chemokine levels, which results in increased susceptibility to colitis.52 Additionally, TET2, which is involved in pre-mRNA splicing, is associated with UC.53 A recent study reported that DNMT3A could induce enterocyte barrier dysfunction to facilitate the interaction between enterocytes and B cells, which promotes UC progression.54 Notably, researchers have observed that DNMT3A was the most frequently mutated gene in patients with IBD associated with hematological malignancies.55 Thus, we hypothesized that patients with UC and concomitant AML may share a genetic predisposition. However, this hypothesis remains untested.
Limitation
There were no pathological images of intestinal mucosa biopsy under colonoscopy, which were done in another hospital, and we have no access to obtain them.
Conclusion
To our knowledge, this is the first report that describes a case of UC accompanied by CMV infection, bSS, and concomitant AML detected <3 months after diagnosis of UC. Based on our observations of the patient’s clinical course and subsequent diagnosis and treatment, following are the key findings of this study: (a) Immunosuppressive therapy is beneficial in patients with UC; however, it is associated with several adverse effects. Therefore, close monitoring for opportunistic infections is important. (b) Early initiation of biological therapy may be useful for young patients with newly diagnosed severe UC. (c) Further researches focused on common immune pathway and genetic locus is warranted to gain deeper insight into the pathogenesis and treatment of UC concomitant with leukemia. Cancer surveillance is essential to closely monitor patients with UC.
Data Sharing Statement
The data and material underlying this article are all available in the article.
Ethics Approval and Consent to Participate
The study received approval from the ethics committees of the Affiliated Hospital of Jining Medical University (2021-09-C002). Participant’s written informed consent was obtained from the patient’s next of kin.
Consent for Publication
Publication consents of case details and accompanying images were obtained from the patient’s next of kin.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82270562, 82200591).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Taku K, Britta S, Chen WS, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):73.
2. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi:10.1053/j.gastro.2011.01.055
3. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–e30. doi:10.1053/j.gastro.2011.10.001
4. Mao R, Chen M. Precision medicine in IBD: genes, drugs, bugs and omics. Nat Rev Gastroenterol Hepatol. 2022;19(2):81–82. doi:10.1038/s41575-021-00555-w
5. Chang JT, Longo DL. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–2664. doi:10.1056/NEJMra2002697
6. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. 2021;161(4):1118–1132. doi:10.1053/j.gastro.2021.07.042
7. Argollo M, Gilardi D, Peyrin-Biroulet C, et al. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. 2019;4(8):643–654. doi:10.1016/S2468-1253(19)30173-6
8. Dave M, Purohit T, Razonable R, et al. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2014;20(1):196–212. doi:10.1097/MIB.0b013e3182a827d2
9. Rahier JF, Magro F, Abreu C, et al. European Crohn’s and Colitis Organisation (ECCO) second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443–468. doi:10.1016/j.crohns.2013.12.013
10. Dave M, Papadakis KA, Faubion WA Jr. Immunology of inflammatory bowel disease and molecular targets for biologics. Gastroenterol Clin North Am. 2014;43(3):405–424. doi:10.1016/j.gtc.2014.05.003
11. Chapman TP, Gomes CF, Louis E, Colombel JF, Satsangi J. De-escalation of immunomodulator and biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2020;5(1):63–79. doi:10.1016/S2468-1253(19)30186-4
12. Wheat CL, Ko CW, Clark-Snustad K, Grembowski D, Thornton TA, Devine B. Inflammatory Bowel Disease (IBD) pharmacotherapy and the risk of serious infection: a systematic review and network meta-analysis. BMC Gastroenterol. 2017;17(1):52. doi:10.1186/s12876-017-0602-0
13. Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11(46):7227–7236. doi:10.3748/wjg.v11.i46.7227
14. Kaine J, Song X, Kim G, et al. Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using US administrative claims data. J Manag Care Spec Pharm. 2018;24:01–11.
15. Boehncke W-H. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9:579. doi:10.3389/fimmu.2018.00579
16. Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2(1):6. doi:10.1186/2042-4280-2-6
17. Berry R, Watson GM, Jonjic S, et al. Modulation of innate and adaptive immunity by cytomegaloviruses. Nat Rev Immunol. 2020;20(2):113–127. doi:10.1038/s41577-019-0225-5
18. Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30(2):334–340. doi:10.1016/0002-9343(61)90105-X
19. Tun GSZ, Raza M, Hale MF, et al. Polymerase chain reaction for detection of mucosal cytomegalovirus infection in patients with acute ulcerative colitis. Ann Gastroenterol. 2019;32(1):81–87. doi:10.20524/aog.2018.0318
20. Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1756–1767. doi:10.1053/j.gastro.2011.02.016
21. Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106(11):2001–2008. doi:10.1038/ajg.2011.202
22. Wethkamp N, Nordlohne EM, Meister V, et al. Identification of clinically relevant cytomegalovirus infections in patients with inflammatory bowel disease. Mod Pathol. 2018;31(3):527–538. doi:10.1038/modpathol.2017.149
23. Wada Y, Matsui T, Matake H, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46(10 Suppl):S59–S65. doi:10.1097/01.DCR.0000087486.21981.C6
24. Yerushalmy-Feler A, Padlipsky J, Cohen S. Diagnosis and management of CMV colitis. Curr Infect Dis Rep. 2019;21(2):5. doi:10.1007/s11908-019-0664-y
25. Jones A, McCurdy JD, Loftus EV Jr, et al. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin Gastroenterol Hepatol. 2015;13(5):949–955. doi:10.1016/j.cgh.2014.09.042
26. Gauss A, Rosenstiel S, Schnitzler P, et al. Intestinal cytomegalovirus infection in patients hospitalized for exacerbation of inflammatory bowel disease: a 10-year tertiary referral center experience. Eur J Gastroenterol Hepatol. 2015;27(6):712–720. doi:10.1097/MEG.0000000000000361
27. McCurdy JD, Jones A, Enders FT, et al. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13(1):131–e7. doi:10.1016/j.cgh.2014.05.026
28. Shukla T, Singh S, Tandon P, McCurdy JD. Corticosteroids and thiopurines, but not tumor necrosis factor antagonists, are associated with cytomegalovirus reactivation in inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2017;51(5):394–401. doi:10.1097/MCG.0000000000000758
29. Su WP, Liu HN. Diagnostic criteria for sweet’s syndrome. Cutis. 1986;37(3):167–174.
30. Ayyash AM, Sampath R. Acute Febrile Neutrophilic Dermatosis. N Engl J Med. 2021;385(4):e14. doi:10.1056/NEJMicm2033219
31. Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108(5):763–768. doi:10.1111/1523-1747.ep12292156
32. Belhadjali H, Marguery MC, Lamant L, Giordano-Labadie F, Bazex J. Photosensitivity in sweet’s syndrome: two cases that were photoinduced and photoaggravated. Br J Dermatol. 2003;149(3):675–677. doi:10.1046/j.1365-2133.2003.05487.x
33. Sleiman J, Hitawala AA, Cohen B, et al. Systematic review: sweet syndrome associated with inflammatory bowel disease. J Crohns Colitis. 2021;15(11):1864–1876. doi:10.1093/ecco-jcc/jjab079
34. Esposito I, Fossati B, Peris K, De Simone C. A rare case of bullous sweet’s syndrome in a patient with inactive ulcerative colitis. J Eur Acad Dermatol Venereol. 2019;33(10):e380–e381. doi:10.1111/jdv.15674
35. Giannoni M, Rizzetto G, Sapigni C, et al. Bullous sweet’s syndrome in a patient with ulcerative colitis: a rare case report. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29(3):153–155.
36. Miranda E, Meza R, Kolbach M, et al. Sweet syndrome in pediatric active ulcerative colitis. Am J Gastroenterol. 2021;116(2):234. doi:10.14309/ajg.0000000000000645
37. Saultz J, Garzon R. Acute myeloid leukemia: a concise review. J Clin Med. 2016;5(3):33. doi:10.3390/jcm5030033
38. Fabry TL, Sachar DB, Janowitz HD. Acute myelogenous leukemia in patients with ulcerative colitis. J Clin Gastroenterol. 1980;2(3):225–227. doi:10.1097/00004836-198009000-00003
39. Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54:617–622. doi:10.1136/gut.2004.051771
40. Cuttner J. Increased incidence of acute promyelocytic leukemia in patients with ulcerative colitis. Ann Intern Med. 1982;97:864–865. doi:10.7326/0003-4819-97-6-864
41. Hanauer SB, Wong KK, Frank PH, et al. Acute leukemia following inflammatory bowel disease. Dig Dis Sci. 1982;27(6):545–548. doi:10.1007/BF01296735
42. Mir Madjlessi SH, Farmer RG, Weick JK. Inflammatory bowel disease and leukemia. A report of seven cases of leukemia in ulcerative colitis and Crohn’s disease and review of the literature. Dig Dis Sci. 1986;31(10):1025–1031. doi:10.1007/BF01300254
43. Suzuki Y, Nakase K, Nagaya S, et al. Acute promyelocytic leukemia following ulcerative colitis. Rinsho Ketsueki. 1995;36(7):707–709.
44. Lopez A, Mounier M, Bouvier AM, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndrome in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(8):1324–1329. doi:10.1016/j.cgh.2014.02.026
45. Khan N, Patel D, Trivedi C, et al. Incidence of acute myeloid leukemia and myelodysplastic syndrome in patients with inflammatory bowel disease and the impact of thiopurines on their risk. Am J Gastroenterol. 2021;116(4):741–747. doi:10.14309/ajg.0000000000001058
46. Scoville SD, Nalin AP, Chen L, et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood. 2018;132(17):1792–1804. doi:10.1182/blood-2018-03-838474
47. Yadav PK, Chen C, Liu Z. Potential role of NK cells in the pathogenesis of inflammatory bowel disease. J. Biomed Biotechnol. 2011;2011:348530.
48. Murase T, Hotta T, Saito H, Ohno R. Effect of recombinant human tumour necrosis factor on the colony growth of human leukemia progenitor cells and normal haematopoietic progenitor cells. Blood. 1987;69:467–472. doi:10.1182/blood.V69.2.467.467
49. Nair B, Raval G, Mehta P. TNF-alpha inhibitor etanercept and hematologic malignancies: report of a case and review of the literature. Am J Hematol. 2007;82(11):1022–1024. doi:10.1002/ajh.20926
50. Cesarini M, Vernia P, Angelucci E. Acute lymphoid leukemia in a Crohn’s disease patient during treatment with Adalimumab after a prolonged treatment with azathioprine and steroids. Inflamm Bowel Dis. 2010;16(3):371–372. doi:10.1002/ibd.21005
51. Li B, Zhu Z, Long S, et al. Acute myeloid leukemia with myelodysplasia-related changes in a patient with Crohn’s disease treated with immunosuppressive therapy. Case Rep Oncol. 2018;11(2):573–576. doi:10.1159/000491573
52. Zhang Q, Zhao K, Shen Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525(7569):389–393. doi:10.1038/nature15252
53. Zhao LP, Boy M, Azoulay C, et al. Genomic landscape of MDS/CMML associated with systemic inflammatory and autoimmune disease. Leukemia. 2021;35:2720–2724. doi:10.1038/s41375-021-01152-1
54. Cheng B, Rong A-M, Li W, et al. DNMT3a-mediated enterocyte barrier dysfunction contributes to ulcerative colitis via facilitating the interaction of enterocytes and B cells. Mediators Inflamm. 2022;2022:4862763. doi:10.1155/2022/4862763
55. Cumbo C, Tarantini F, Zagaria A, et al. Clonal hematopoiesis at the crossroads of inflammatory bowel diseases and hematological malignancies: a biological link? Front Oncol. 2022;12:873896. doi:10.3389/fonc.2022.873896
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.