Back to Journals » Infection and Drug Resistance » Volume 13
Tryptophol Coating Reduces Catheter-Related Cerebral and Pulmonary Infections by Scedosporium apiospermum
Authors Kitisin T , Muangkaew W, Ampawong S, Sukphopetch P
Received 25 March 2020
Accepted for publication 9 July 2020
Published 22 July 2020 Volume 2020:13 Pages 2495—2508
DOI https://doi.org/10.2147/IDR.S255489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Thitinan Kitisin,1 Watcharamat Muangkaew,1 Sumate Ampawong,2 Passanesh Sukphopetch1
1Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; 2Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Correspondence: Passanesh Sukphopetch Email [email protected]
Introduction: Central venous catheter (CVC) is a medical device that is used to administer medication for a long duration. Colonization by an emerging opportunistic pathogen Scedosporium apiospermum in the CVC lumen is frequently reported to cause severe complications in patients. Here, we describe the effect of fungal quorum-sensing molecule (QSM) known as tryptophol (TOH) to control S. apiospermum colonization in catheter tube lumens in both in vitro and in vivo models.
Methods: Antifungal susceptibility of TOH against S. apiospermum was compared with voriconazole, and the colony diameter was determined on days 2, 4, and 6. Experimental catheterization rat model was conducted with pre-coating of TOH and voriconazole or an uncoated control and an infection with S. apiospermum. Biofilm formation on the catheter luminal surface was assessed using the scanning electron microscopy, crystal violet, and 2,3-bis(2-methoxy-4-ni-tro-5-sulfophenyl)-5-(phenylamino)-carbonyl-2H-tetra-zolium hydroxide (XTT) reduction assays. Brain and lung samples of catheterized rats were histopathologically assessed. Serum samples from catheterized rats were injected into Galleria mellonella larvae. Survival of catheterized rats and G. mellonella was determined.
Results: TOH impeded the growth of S. apiospermum by reducing the colony diameter in a dose-dependent manner. TOH coating remarkably lessened S. apiospermum biofilm formation and fungal cell viability on the catheter luminal surface. Additionally, TOH coating lessens cerebral edema that is associated with abscess and invasive pulmonary damages due to S. apiospermum catheter-related infection. Furthermore, TOH coating also lessened the virulence of S. apiospermum in sera of experimental catheterized rats and extended the survival rate of larvae Galleria mellonella infection model.
Conclusion: An alternative modification of catheter by coating with TOH is effective in preventing S. apiospermum colonization in vivo. Our study gives a new strategy to control catheter contamination and prevents nosocomial diseases due to S. apiospermum infection.
Keywords: Scedosporium apiospermum, central venous catheter, quorum-sensing molecules, tryptophol, fungal biofilms, antifungal susceptibility, scedosporiosis, Galleria mellonella
Introduction
Fungal infections in indwelling catheters, especially in long-term central venous catheters (CVC), are believed to be a chief cause of mortality among hospitalized patients.1 Several studies showed various images of microorganisms growing in the contaminated site as a biofilm environment. It is interesting to note that results from electron microscopy have proposed the correlation between biofilm-related microorganisms on the surface of contaminated catheters and soft-tissue injuries.2 Therefore, biofilm contamination on the luminal surface of the catheter can cause a high risk on human health for those who depend on the use of intravascular and other medical devices.
Scedosporium apiospermum was firstly isolated in Sardinia as the etiological cause of white mycetoma.3,4 Scedosporium apiospermum was once considered to be the anamorph of Scedosporium boydii (formerly known as Pseudallescheria boydii). Until 2005, these two species were proven to be distinctly different species based on molecular, pathological, and biochemical data.5 There is evidence that S. apiospermum is involved in opportunistic infections. In immunosuppressed patients, S. apiospermum can be the causative agent of invasive infections, which extremely spread to cause disseminated diseases.6 In Thailand, S. apiospermum has been reported in brain abscesses of near-drowning and renal transplant patients.7,8 Our previous study showed that S. apiospermum species complex can be found in soils across Bangkok and other major provinces of Thailand, with the predominance detection of S. apiospermum sensu stricto.9–11 Furthermore, the increased incidence of S. apiospermum infections was reported to be because of the extensive use of corticosteroids, immunosuppressants, antineoplastics, broad-spectrum antibiotics, and indwelling catheters including long-term CVC.1,12–16 In some cases, S. apiospermum infections following medical intervention were isolated from the insertion site of a catheter, indicating a significant evidence of nosocomial diseases.13
The recent study has exhibited that S. apiospermum can form biofilms on the surface of both polystyrene and polyurethane, a material of CVC.13,17 Scedosporium spp. are demonstrated to be fundamentally resistant to antifungal amphotericin B and frequently respond more effectively to voriconazole.18 On the contrary, biofilms formed by Scedosporium spp. are highly resistant to the azole class of antifungal drugs, such as caspofungin, fluconazole, itraconazole, and voriconazole,16,18 although voriconazole, which has been used as the first-line antifungal drug, complications including severe phototoxicity, visual impairment, and elevated liver enzymes have been reported.19 Because of antifungal resistance and complications, novel antifungal agents for prevention and/or control of Scedosporium spp. colonization in catheter tube lumens are poorly studied.
Fungal quorum-sensing molecules (QSMs) are signaling molecules that are used to communicate and/or control behavior among fungi, bacteria, and other microorganisms.20 Tryptophol (C10H11NO, TOH; molecular weight, 161.20) is a QSM that can be isolated from Candida albicans as well as Saccharomyces cerevisiae.20 In S. cerevisiae, TOH is a synthesized alcohol that is derived from aromatic amino acid tryptophan through the Ehrlich pathway under a low-nitrogen condition.21 In C. albicans, TOH was found to be an auto-antibiotic by inhibiting filamentation.22 Our present study showed that TOH suppressed C. albicans biofilm formation by inducing a programmed cell death.23 We also found that pre-treatment of C. albicans with TOH remarkably lessened the pathogenicity and virulence of C. albicans infection in larvae of Galleria mellonella. Even though TOH holds a promising antifungal property, little is known about whether TOH can be utilized as an antifungal agent against Scedosporium spp. Furthermore, the effect of TOH for controlling S. apiospermum colonization in catheter tube lumens has not been explained. Thus, the current study’s objective is to determine the antifungal effect of TOH against S. apiospermum infection. In addition, we explored the use of TOH as a coating agent for controlling catheter contamination and preventing nosocomial diseases due to S. apiospermum infection.
Materials and Methods
Ethical Statement
All animal experiments in this study were carried out in accordance with the Animals for Scientific Purposes Act, B.E. 2558 (A.D. 2015), in Thailand. For rat experiments, all animal experiments were performed with assistance from Dr. Nichapa Sansurin at Northeast Laboratory Animal Center (NELAC), Khon Kaen University, Thailand and were approved by the Institutional Animal Care and Use Committee (IACUC) of Khon Kaen University, Thailand (approval number: 0514.1.75/34). For Galleria mellonella experiments, all relevant international, national, and institutional guidelines for the care and use of Galleria mellonella were followed, and the study was approved by the research ethics committee of Mahidol University, Thailand (approval certificate number: MU-IACUC 2018/015).
Fungal Strains and Growth Conditions
Scedosporium apiospermum CBS 117410 utilized in this study was kindly provided by Dr. Ana Alastruey-Izquierdo (Servicio de Micologίa, Instituto de Salud Carlos III, Madrid, Spain).10 Scedosporium apiospermum CBS 117410 was grown on Sabouraud dextrose agar (SDA) (Oxoid, Hampshire, UK) slant for 5 days at 37°C. Conidia were collected by washing with sterile phosphate-buffered saline (PBS, pH 7.2) and adjusted to a concentration of 105 conidia/mL.
Analysis of Tryptophol (TOH) on S. apiospermum Growth
A stock solution of 1 M tryptophol (TOH) was prepared by dissolving TOH in ethanol. The stock solution was then diluted to the desired concentration with the use of SDA. Twenty microliters of S. apiospermum CBS 117410 conidia suspension were placed in each well of a six-well plate containing serial dilutions (1, 10, 100, and 1000 μM) of TOH (Sigma-Aldrich, St Louis, MO) and serial dilutions (1, 10, 100, and 1000 μM) of voriconazole (Sigma-Aldrich, St Louis, MO) in SDA. The plate was incubated at 25°C, and the colony morphology was observed, and the colony diameter was measured on days 2, 4, and 6. The plates were monitored for 10 days. Wells that contain 1% (v/v) ethanol (TOH diluent) and TOH-free SDA were also included as controls for TOH experiments. In the case of voriconazole, groups that were prepared with voriconazole-free SDA and 1% (v/v) RPMI (voriconazole diluent) were used as controls.
In vivo Experimental Catheterization and Treatments
A sterilized polyethylene tube (1–1.5 mm in diameter) was utilized as a catheter in this study. The catheters were pre-coated with either 10 and 100 μM of tryptophol (TOH) or voriconazole overnight. Experimental catheterization rat model was conducted in female Mlac/Wistar rats (2-week-old, 350g of body weight, and 10 rats per group). Following the quarantine period, rats were anesthetized using an intraperitoneal injection (IP) (1 mg/kg) of a mixture of ketamine HCl 500 mg/10 mL at a concentration of 80 mg/kg (Bedford Laboratories, Bedford, OH) and xylazine at concentration of 8 mg/kg (Sigma-Aldrich, St Louis, MO), with a ratio of 1:2 (vol/vol). Next, the surgical incision was performed at the anterior neck just right to the midline, and a pre-coated catheter was inserted into the jugular vein.24,25 The pre-coated catheters were placed 24 h prior to infection, allowing a conditioning period for deposition of host protein on the catheter surface. The inoculum of S. apiospermum CBS 117410 at a concentration of 108 conidia/mL was instilled in the pre-coated catheter in a volume not greater than 700 µL (the entire catheter volume). Several inoculum conditions were instilled as follows: Group 1, pre-coated with TOH and inoculated with S. apiospermum CBS 117410; Group 2, pre-coated with voriconazole and inoculated with S. apiospermum CBS 117410; Group 3, uncoated catheter with inoculation of S. apiospermum CBS 117410; and Group 4, uncoated catheter with no S. apiospermum CBS 117410 inoculation. The inoculum was permitted to stabilize in the catheter lumen for 6 h, after which the catheter volume was withdrawn and the catheter was flushed and locked with sterile heparinized 0.85% NaCl. This infection was observed within 12 and 48 h. At the end point, the animals were sacrificed by CO2 asphyxiation. After sacrificing, the catheter samples were aseptically collected. Blood samples were then collected into Eppendorf tubes and permitted to clot for 30 min, followed by centrifugation at 3000 g for 10 mins at 4°C to collect the serum fraction. Catheterized rat sera and their control sera were utilized for additional treatments with Galleria mellonella.
To determine the presence of S. apiospermum CBS 117410 in TOH-coated catheter samples, the fungal elements were carried out following treatment with 15% potassium hydroxide (KOH). After 48 h post-inoculation of S. apiospermum in catheterization rats, the catheter samples were inoculated on SDA (Oxoid, Hampshire, UK) and incubated at 25°C with daily observation for fungal colonies. After 30 days, the tubes with no visible fungal colonies were discarded as negative. The identification of genera and species of the fungal colonies was carried out with the use of lactophenol cotton blue staining.
Analysis of S. apiospermum Biofilm Development by H-Score
To validate the presence of S. apiospermum germination inside the catheter lumen, a fine morphological study was conducted as formerly described.26 The catheter was cut perpendicular to the catheter length (as doughnut segments). The catheter segments were primary fixed in 2.5% glutaraldehyde for 1 h at room temperature and then washed with 0.1 M sucrose phosphate buffer (SPB) thrice. The catheter samples were secondary fixed in 1% osmium tetroxide in SPB and then washed again. The tissues were dehydrated in a series of ethanol solutions and air-dried overnight. The catheter segments were mounted on an aluminum stub and coated with a gold film (20 nm-thickness) using a sputter coater (Emitech K550, Ashford, UK). The luminal surface of catheter samples was imaged using a scanning electron microscope (JEOL JSM-6610LV, Japan) with 15 kV acceleration voltages.
Semi-quantitative examination of S. apiospermum CBS 117410 biofilm formation in the luminal surface of experimented catheters was measured using the H-score assay. The H-score was acquired from the biofilm coverage area (in percentage) and then multiplied by the score of biofilm thickness (1 = one layer, 2 = two layers, and 3 = three or more layers of biofilm thickness) using the ImageJ program (National Institutes of Health, Bethesda, MD, USA).
Analysis of S. apiospermum Biofilm Development by Crystal Violet (CV) Staining
Scedosporium apiospermum CBS 117410 biofilm formation in the luminal surface of experimented catheters was assessed further with the use of CV staining as formerly described with some alterations.26 The TOH-coated catheters and their controls were washed 2 times with PBS, air-dried, and then stained with 110 μL of 0.4% aqueous CV solution for 45 min. The catheter samples were washed 5 times with 350 μL sterile distilled water and destained with 200 μL of 95% ethanol for 45 min. The destaining solution in each sample (100 μL) was collected and then transferred into a new 96-well flat-bottom microtiter plate. The absorbance values were measured at 595 nm using a microtiter plate reader (Tecan, Sunrise, Austria). The absorbance values of treated groups were subtracted from those of the control groups to eradicate the background interference.
Analysis of S. apiospermum Biofilm Development by XTT Reduction Assay
Scedosporium apiospermum CBS 117410 biofilm formation in the luminal surface of experimented catheters was examined using tetrazolium salt 2,3-bis(2-methoxy-4-ni-tro-5-sulfophenyl)-5-(phenylamino)-carbonyl-2H-tetra-zolium hydroxide or XTT (Sigma-Aldrich, St Louis, MO) reduction assay.27 The XTT-menadione solution was freshly prepared by mixing the XTT solution (1 mg/mL in PBS) with the menadione solution (0.4 mM in acetone) at a 5:1 (v/v) ratio.28 12 μL of the XTT-menadione solution and 200 μL of PBS were added to the 96-well microtiter plate containing catheter samples and incubated in the dark for 2 h at 37°C. Next, 100 μL of the supernatant solution was transferred to a new microtiter plate, and absorbance values were measured at 490 nm. An arithmetic mean of the absorbance values was calculated. Any absorbance background was eliminated by subtracting the absorbance values of the control groups and the experimental groups.
Histopathology Analysis
Histopathology was used to determine the severity of brain and lung impairments after S. apiospermum infection. Dissected brains and lungs from catheterized animals were collected and fixed in 10% neutral buffer formalin for 48 h. All tissue samples were dehydrated and embedded in paraffin. All the brain and lung sections (5 µm) were stained with hematoxylin and eosin or Gomori methenamine silver (GMS) and examined under a light microscope. In the brain sections, the pathological changes were chiefly focused on microabscesses, perivascular cuffing with lymphocytes, perivascular edema, meningitis, and hemorrhage. In the lung sections, the pathological changes were chiefly focused on hemothorax, inflammatory zones, fibrosis, and granules. The amount of change in each histologic disease activity previously mentioned was scored on a four-tier system as follows: 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. Overall, the histopathologic score was calculated by the combination of each histological change.29
Galleria mellonella and Maintenance
Galleria mellonella larvae were provided from Jerry® Wax Worms (Thailand). Larvae were grown on a bedding medium that consisted of bran, all-purpose flour, dried bakery yeast, liquid honey, and glycerol at room temperature in plastic boxes with grids prior to experiments. During the last-stage larvae, the worms that stopped feeding and started to produce a thin silk body cover were chosen, which were monitored approximately 2 months following the hatching from their eggs. At this stage, G. mellonella larvae with 200–400 mg body weight and lightly colored cuticles with no gray areas were chosen and transferred to sterile Petri dishes for 24 h with no food. Furthermore, their nascent protective cocoons were removed.23
Survival Analysis of S. apiospermum-Infected Rat Serum on G. mellonella
Moth larvae were split into three treatment groups, each with nine larvae. Larvae were anesthetized by placing at 5–8°C in Petri dishes for 1 day and then injected with sera acquired from S. apiospermum-infected, catheterized rats in several types of coated catheters such as TOH, voriconazole, and uncoated controls. In brief, moth larvae were held carefully between the thumb, index finger, and middle finger, and the injection area was cleaned with the use of 70% ethanol. Sera from catheterized rats that were collected at 12 and 48 h following catheterization were injected into the larvae immediately. Sera were injected into the larval hemocoel through the last proleg with the use of a 30G single-use sterile syringe. The larvae were further incubated in Petri dishes with no food at 37°C in the dark for survival analysis.
The survival measurements of treated larvae were monitored every day following post-injection until all worms died. Larvae were counted as dead when there was no response to gentle stimulation with forceps and the cuticle color became totally black.
Statistical Analysis
Each experiment was carried out in triplicate. The survivals of G. mellonella in all treatments were assessed using GraphPad Prism. Kaplan–Meier lifespan analysis was carried out, and p-values < 0.05 were significantly different with the use of the Log-rank test. Data were presented as the mean ± standard deviation (SD). In other assays, significant differences at the p-values < 0.05 were calculated by two-way ANOVA or independent Student’s t-tests.
Results
Effect of Tryptophol (TOH) on S. apiospermum Growth
Colonies of S. apiospermum CBS 117410 were observed following 2 days of incubation. SDA media containing either 1% RPMI (v/v) or 1% ethanol (v/v) were utilized as controls for voriconazole or tryptophol (TOH), respectively. For voriconazole treatment, the colony diameter increased from day 2 to day 4 in the same manner in 1% RPMI-treated group as in the colonies grown on SDA alone (Table 1). Treatment of vericonazole at the concentration ranging from 1 to 10 μM remarkably lessened the growth rate of S. apiospermum CBS 117410. Nonetheless, the colony diameter remarkably lessened following treatment with moderate-to-high concentrations of vericonazole (100–1000 μM, p < 0.05). Therefore, voriconazole at 100 μM was chosen for further subsequent experiments. For TOH treatment, the colony diameter increased from day 2 to day 4 in the same manner with the 1% ethanol-treated group as in the colonies grown on solely SDA (Table 1). Treatment of TOH at the concentration ranging from 1 to 10 μM remarkably lessened the growth rate of S. apiospermum CBS 117410. Additionally, the colony diameter was remarkably lessened following treatment with 100–1000 μM of TOH (p < 0.05). Therefore, TOH at 100 μM was chosen for further subsequent experiments.
 |
Table 1 Colony Diameters of Tryptophol (TOH)-Treated Scedosporium apiospermum CBS 117410 Following Incubation at 25°C |
General Animal Well-Being and Catheter Site Observations
Following catheterization, rats appeared well in the 96 h period of study (24 h pre-inoculation and up to 72 h post-inoculation). The surgical site and catheter exit site in all studied animals still had no signs of inflammation or purulence.
Catheter Biofilm Imaging
Catheter samples following 48 h post-injection with S. apiospermum CBS 117410 had a fungal growth on SDA. In an uncoated catheter, the colony diameter of S. apiospermum increased from day 2 to day 6 similar to the S. apiospermum inoculum alone (Table 1). Pre-coated catheter with voriconazole (100 μM) remarkably lessened the S. apiospermum colony diameter from day 2 to day 6 in comparison to the uncoated control catheter (p < 0.05). Nonetheless, pre-coated catheter with TOH (100 μM) remarkably lessened the S. apiospermum colony diameter from day 2 to day 4 in comparison with voriconazole-coated catheter (Table 1, p < 0.05).
Colony and microscopic pictures of the S. apiospermum from TOH-coated catheter samples are shown in Figure 1A and B, and C using lactophenol cotton blue staining. Fungal biofilm on the luminal surface of the catheter segments was clearly visualized using a scanning electron microscopy (SEM). Uninoculated catheter with no infection was utilized as the control (Figure 2A). Scedosporium apiospermum biofilm was observed following 48 h post-injection from the top of the intraluminal biofilm surface (Figure 2B–D). Mature biofilm was characterized by both yeast and hyphal cell forms on the extracellular matrix networks. Reduction in S. apiospermum biofilm was observed in catheter lumen following treatments with 100 μM of vericonazole or TOH. Nevertheless, it was clear that biofilm was remarkably lessened in the catheter coating with TOH in comparison with vericonazole (Figure 2C and D).
Effect of TOH on S. apiospermum Biofilm Development
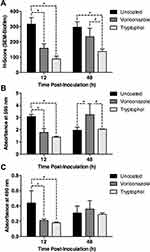
The fungal biofilm on the luminal surface of the catheter segments was further evaluated with SEM observations using the H-score determination. H-Scores were calculated using the percent coverage area of biofilm multiplied with the layer score of the S. apiospermum CBS 117410 biofilm. In the current study, the means of H-scores in uncoated catheter, voriconazole-coated-catheter (100 μM), and TOH-coated catheter (100 μM) were slowly decreased in a time-dependent manner from 12 h to 48 h post-inoculation. At 12 h post-inoculation, the results showed that the mean H-score of biofilm development in TOH-coated-catheter (88.66 ± 18.09) remarkably lessened in comparison with that in the uncoated catheter (316.39 ± 42.63, p < 0.05) but not with voriconazole-coated catheter (Figure 3A, 158.07 ± 29.58, p > 0.05). Furthermore, the mean H-score of biofilm development in TOH-coated-catheter (137.29 ± 16.74) remarkably lessened in comparison with that in the uncoated catheter (296.64 ± 35.41) and voriconazole-coated catheter (234.11 ± 55.03) following 48 h post-inoculation (Figure 3A, p < 0.05). The results proposed that TOH can be utilized as a coating agent to lessen the biofilm formation of S. apiospermum CBS 117410.
Fungal biofilm on the luminal surface of the catheter segments was further examined with the use of CV staining. At 12 h post-inoculation, the mean of A595 in TOH-coated catheter (100 μM) (1.40 ± 0.07) was remarkably lessened in comparison with that in the uncoated catheter (3.08 ± 0.18, p < 0.05) but not voriconazole-coated catheter (100 μM) (Figure 3B, 1.78 ± 0.33, p > 0.05). At 48 h post-inoculation, the mean of A595 in TOH-coated catheter (2.05 ± 0.07) was remarkably lessened in comparison with that in the uncoated catheter (1.96 ± 0.25, p < 0.05). Nonetheless, the mean of A595 in voriconazole-coated catheter (3.23 ± 0.90) was remarkably increased in comparison with that in the uncoated catheter (Figure 3B, p < 0.05). Therefore, the results proposed that TOH can be utilized as a coating agent to reduce the biofilm formation of S. apiospermum CBS 117410.
Biofilm metabolic activity on the luminal surface of the catheter segments was further examined with the use of XTT reduction assay. At 12 h post-inoculation, the mean of A490 in TOH-coated catheter (100 μM) (0.18 ± 0.01) was remarkably lessened in comparison with the uncoated catheter (0.44 ± 0.16, p < 0.05) but not voriconazole-coated-catheter (100 μM) (Figure 3C, 0.21 ± 0.02, p > 0.05). At 48 h post-inoculation, however, the means of A490 in uncoated catheter, voriconazole-coated-catheter, and TOH-coated-catheter were not statistically significant (Figure 3C, 0.31 ± 0.09, 0.36 ± 0.11, and 0.29 ± 0.02, respectively, p > 0.05). Therefore, the results proposed that TOH can be utilized as a coating agent to reduce the biofilm formation of S. apiospermum CBS 117410.
Survival Time
Following S. apiospermum CBS 117410 inoculation in the catheter lumen for 6 h, experimental catheterized rats were further monitored within 12 to 48 h. Severe neurological symptoms, moribund, and death were seen in S. apiospermum-infected rats. However, rat mortality rate of 100% was noted in the uncoated catheter group, while TOH-coated and voriconazole-coated catheter groups have shown a mortality rate of 40% and 50%, respectively (data not shown).
Histopathological Changes of the Brain and Lungs
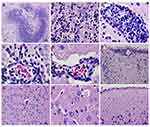
Scedosporium apiospermum CBS 117410 infection in rats with an uncoated catheter prompted severe pathological changes in the brain in comparison with TOH-coated catheter groups as presented in Figure 4. Following 48 h post-inoculation, histopathological studies of brains from the uncoated catheter group showed meningitis, hemorrhage, brain abscess surrounded with edema, and cerebritis with no encapsulation (Figure 4A–F). The histological tissues had S. apiospermum hyphae (Figure 4B) and perivascular cuffing principally with neutrophil and mononuclear infiltrates around the site of infection (Figure 4B–E) and in the meninges (Figure 4F). The infected brain lesions have also shown a central necrotic area surrounded with inflammatory cells, unlike the brain with no abscess (Figure 4G–I). Additionally, the number of brain abscesses was examined in the cerebral cortex, midbrain, hippocampus, and hypothalamus. Nevertheless, olfactory bulb, diencephalon, and cerebellum did not present any abscess.
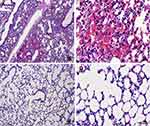
The lungs of S. apiospermum CBS 117410-infected rats with an uncoated catheter also prompted severe pathological changes in comparison with TOH-coated catheter groups as presented in Figure 5. Invasive pulmonary scedosporiosis was characterized by severe hemorrhage (hemothorax) in experimental rat from the uncoated catheter group (Figure 5A and B). Furthermore, large inflammatory zones, fibrosis, and granules were noted in uncoated catheter groups (data not shown). The infected lung lesions in the uncoated catheter groups have also shown the presence of inflammatory cells such as lymphocytes and macrophages unlike the TOH-coated catheter groups (Figure 5C and D). Histopathological studies of brains and lungs from voriconazole-coated catheter groups displayed similar to TOH-coated catheter groups (data not shown), proposing that TOH can be utilized as a coating agent, which exhibited antifungal effects similar to voriconazole. The presence of S. apiospermum hyphae in brains and lungs from uncoated catheter groups was also validated with the use of GMS staining (Supplementary Figure 1).
Effect of S. apiospermum-Infected Rat Serum on the Survival of G. mellonella
Colonies of S. apiospermum CBS 117410 from infected rat sera in TOH-coated catheter, voriconazole-coated catheter, and uncoated catheter groups were observed following 48 h post-infection on SDA. The colony diameter of S. apiospermum from rat sera of the uncoated catheter group increased from day 2 to day 6 (Table 1). It is interesting to note that the colony diameter of S. apiospermum from rat sera of TOH-coated catheter (100 μM) group was remarkably smaller than the uncoated catheter group (p < 0.05) similar to the voriconazole-coated catheter (100 μM) group from day 2 to day 6 (Table 1).
The ability of TOH to lessen the S. apiospermum infection was further determined in G. mellonella. Scedosporium apiospermum-infected rat sera from TOH-coated, voriconazole-coated, and uncoated catheter groups were injected in G. mellonella larvae, and the survival rate was determined. Normal saline (0.9%) was injected in G. mellonella as a control. Following 12 h post-injection, the mean lifespan of normal saline treatment (144 h) was remarkably highest among other treated groups (Table 2). However, rat sera from TOH-coated catheter (100 μM) remarkably increased the mean lifespan (106.40 ± 22.45 h, 35.34%, p < 0.0001) in comparison to sera from voriconazole-coated catheter (100 μM) (84.00 ± 25.79 h, 22.09%, p < 0.05) or uncoated catheter (68.80 ± 20.65 h) (Figure 6A, Table 2). Following 48 h post-injection, the mean lifespan of normal saline treatment (132.00 ± 16.97 h) was remarkably highest among other treated groups (Table 2). However, rat sera from TOH-coated catheter remarkably increased the mean lifespan (94.40 ± 29.52 h, 131.37%, p < 0.0001) in comparison to sera from voriconazole-coated catheter (72.00 ± 25.21 h, 76.47%, p < 0.0001) or uncoated catheter (40.80 ± 15.63 h) (Figure 6B, Table 2). These findings indicated that TOH-coated catheter lessens the pathogenicity of S. apiospermum infection in G. mellonella.
 |
Table 2 Effect of S. apiospermum-Infected Rat Serum on the Survival of G. mellonella |
 |
Figure 6 S. apiospermum infected rat sera from TOH-coated catheter extended G. mellonella lifespan. Data represented percentages of survival of G. mellonella larvae treated with S. apiospermum infected rat sera after (A) 12 h post-injection and (B) 48 h post-injection. A percentage of survival in each condition was also compared to normal saline (0.9%). (C) G. mellonella larvae showed melanized in 1–2 h post-injection. (D) Melanization of G. mellonella larvae cuticle was darker and (E) completely black near the time of death. Scare bar = 1 mm. Lifespan was analyzed using Kaplan–Meier analysis and p - values were calculated using Log-rank test. Statistical details of lifespan are summarized in Table 2. |
The S. apiospermum infection was also correlated with the skin melanization of G. mellonella larvae. The cuticle color of G. mellonella that is injected with S. apiospermum-infected rat sera from the uncoated-coated catheter group was stated to change from lightly colored to dark within 2–8 h post-injection. An accumulation of melanin was initially seen following 1–2 h post-infection (Figure 6C and D), and it became totally black near the time of death (Figure 6E). Consistent with the mortality results, however, larvae injected with S. apiospermum-infected rat sera from TOH-coated catheter group showed delay melanization (12–24 h) in comparison with voriconazole-coated catheter group (6–12 h).
Discussion
The nosocomial outbreak caused by Scedosporium spp. in immunocompromised hosts and in transplant recipients has been continually increased over the past decade.6,30 In spite of the antifungal therapy, mortality rates of patients with Scedosporium infections are still incredibly high as 50–70%.31 Several studies have proposed that S. apiospermum infections are linked to intravenous catheters, injections, or blood collection.13,16,32–35 The disease pathogenesis of catheter-related Scedosporium spp. infections is complex. Various factors have been highlighted in the disease pathogenesis such as the duration of catheter placement, catheter materials, and the pathogen vs host properties.36 Thus, the strategy to remove fungal biofilm on the catheter lumen is urgently needed to prevent or cure catheter-related fungal infections.
Here, we reported that a fungal QSM known as tryptophol (TOH) hampered the growth of S. apiospermum CBS 117410 in a concentration-dependent manner. A moderate concentration (100 μM) of TOH remarkably hampered the growth of S. apiospermum. Furthermore, TOH appeared to be as effective as voriconazole, a standard antifungal drug for Scedosporium spp. infection.8,37 Our current study showed that S. apiospermum manifested an ability to form biofilms on the surface of catheter lumen, which is similar to prior reports on the biofilm formation covering polystyrene surfaces.17 Scedosporium apiospermum biofilm formation in the catheter was remarkably lessened when the catheter was pre-coated with TOH rather than voriconazole. Quantifications of S. apiospermum biofilms were performed using H-scores, CV staining, and XTT reduction assays. The results demonstrated that TOH appeared to be effective in controlling S. apiospermum biofilm formation than known antifungal agents of voriconazole. CV staining and reduction of XTT are used to explain all the cells in the biofilm community and determine cell viability as the reduction of formazan by mitochondrial dehydrogenase in living cells.27,28 Our results showed that biofilm bulk and level of XTT metabolic activity in TOH-coated catheter were lower than in voriconazole-coated catheter following 48 h post-inoculation, proposing that cell viability in the biofilm community was lessened when coating catheter with TOH. Furthermore, TOH has been reported to lessen filamentation in Aspergillus spp. and in C. albicans.23,38 Therefore, our study gives the use of QSM of TOH for surface coating to prevent biofilm formation in the catheter lumen. However, the molecular mechanisms of anti-biofilm in TOH against S. apiospermum infection are still to be further studied in depth.
Degree severity of Scedosporium infection has been thoroughly studied, showing a wild range from mild lymphocutaneous (LC) infections to severe life-threatening disseminated infections involving the central nervous system (CNS).39 Invasive S. apiospermum infection in distant organs including the brain and lungs was noted in the experimental rat with uncoated catheter in our study. Invasive CNS infections were characterized by cellular damages in various areas of the meninges, cerebral cortex, midbrain, hippocampus, and hypothalamus. We also found that pulmonary infection by S. apiospermum was observed consistently with CNS infection in the uncoated catheter group. Several inflammatory areas, hemothorax, fibrosis, and granules in pleural cavity and lung tissues, characterized as invasive pulmonary scedosporiosis, were also observed in the experimental rat in the uncoated catheter group and were resolved in the TOH-coated catheter group. Additionally, a mortality rate of 100% in the uncoated control group was lessened to 50% in the TOH-coated catheter group. The catheter from catheterization further determined the presence of S. apiospermum. The growth of S. apiospermum from TOH-coated catheter was lessened in comparison to that from the voriconazole-coated catheter. Furthermore, the similar phenomenon of S. apiospermum growth inhibition was noted in the culture of rat sera acquired from the TOH-coated catheter group.
It is possible that conidia of S. apiospermum on the catheter lumen may enter the brain and lungs through the bloodstream.6 These conidia are germinated, causing hyphal invasion in the target organs. Prior study has shown that S. apiospermum generates a 33-kDa extracellular serine protease peptidase, which degrades human fibrinogen, proposing that it has a role in lung invasion and inflammation.40 Apart from that, S. apiospermum has been reported to display a siderophore activity, making it iron dependent.41 Therefore, it may also explain S. apiospermum neurotropism because the CNS has free iron unlike the serum. Scedosporium spp. can tolerate temperature between 37°C and 42°C and easily adapt themselves to a wide variety of environmental stresses such as osmotic stress, halo-tolerance, and low-oxygen environments.39,42 Furthermore, Scedosporium frequently shows resistance in antifungal therapy, elucidating their rapid hematogenous dissemination, which affected very distant organs and render among high percentages of blood cultures.34 Even though the precise mechanism of how Scedosporium passes the blood-brain-barrier still is unknown, one hypothesis illuminated that alveolar macrophages infected with Scedosporium conidia may be the principal cause of disease pathogenesis. Scedosporium-infected macrophages can pass the blood-brain-barrier, and then conidia succeedingly germinate and damage brain tissue. This hypothesis was well recognized, as macrophages are generally known vehicles for eukaryotic pathogens such as Leishmania and Toxoplasma that can pass through the blood-brain-barrier.43 Our recent study also showed that S. apiospermum infection induced severe cerebral edema correlated with abscess in immunocompromised mice. Elevation of tumor necrotic factor-α and downregulation of aquaporin-4 and nuclear factor erythroid-2 related factor in neuronal cells and myelin degeneration induced by S. apiospermum were found in non-treated mice, proposing the cause of water imbalance and oxidative stress which lead to cerebral edema and abscess.29 Thus, our results proposed that a pre-coating catheter with TOH lessens the risk of cerebral and pulmonary complications due to S. apiospermum catheter-related infection.
Biofilm formation and fungal germination are considered to be important risk factors in patients with a life-threatening systemic infection, especially in the long-term use of inserted devices including S. apiospermum-caused catheter-related infections.13 Contaminated catheter and infected sera with S. apiospermum can foster fungal growth and therefore create a continuous cycle of reinfection, permitting a great potential to cause opportunistic infection in healthcare units. By pre-coating the catheter with TOH, our results indicated that S. apiospermum-infected rat sera appeared to be less virulent in vivo than voriconazole-coated catheter group. In fact, injection of rat sera from the uncoated catheter group generated immediate melanization in G. mellonella, leading to a high mortality rate in comparison with the TOH-coated catheter group. Melanization and nodulation are considered to be the chief immune responses of G. mellonella against pathogenic fungal infection.23 Enhanced survival rate of G. mellonella after being injected with S. apiospermum-infected rat sera was also noted in longer post-inoculation periods in the TOH-coated catheter group (48 h post-inoculation). Our results propose that delayed mortality of G. mellonella injected with rat sera from TOH-coated catheter group is consistent with reduced S. apiospermum virulence. We formerly reported that TOH mediates the reduction of pathogenicity and induction of cellular apoptosis in C. albicans.23 We showed that TOH treatment remarkably increases CARD-9 and Noxa and reduces Bcl-2 of C. albicans mRNA expressions. Generally, CARD-9 is correlated with Bcl-10 to positively regulate the apoptosis by activating the nuclear factor-kappa B (NF-κB) stress pathway.44 Furthermore, Noxa (a pro-apoptotic protein of Bcl-2) is directly cooperated with anti-apoptotic Bcl-2 protein to regulate oxidative stress-induced apoptosis.45 Therefore, it is possible that TOH can prompt the cellular apoptosis and lessen the virulence of S. apiospermum similar to C. albicans. Thus, our results propose that TOH can be utilized as a coating agent in medical devices, which may help lessen the risk of opportunistic infections especially the virulence of S. apiospermum. Additionally, we plan to examine the molecular mechanisms of TOH-mediated reduction in S. apiospermum pathogenicity in the future.
Conclusion
Our current study shows that a QSM known as tryptophol (TOH) can be coated on the luminal surface of the CVC to lessen catheter-related cerebral and pulmonary infections by S. apiospermum. Decreased growth and virulence of S. apiospermum were related to the decreased biofilm formation of S. apiospermum and increased mortality rate of experimented animals in TOH-coated catheter group. The current study shows that TOH can be more effective in controlling S. apiospermum colonization on catheter lumen than voriconazole, as an antifungal agent. Nonetheless, further extensive studies are required to determine the mechanisms of TOH involved in the antifungal susceptibility of S. apiospermum biofilms and to use TOH as an alternative catheter coating agent for antifungal property.
Acknowledgments
We thank Dr. Nichapa Sansurin from the Northeast Laboratory Animal Center (NELAC), Khon Kaen University, Thailand, for preparing and processing specimens in the electron microscope. This work was funded by the Health Systems Research Institute (Grant number: HSRI 63-005), Passanesh Sukphopetch; National Research Council of Thailand and Mahidol University (Grant number: NRCT-2782), Sumate Ampawong; and Faculty of Tropical Medicine, Mahidol University.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Donlan RM. Biofilms on central venous catheters: is eradication possible? Curr Top Microbiol Immunol. 2008;322:133–161. doi:10.1007/978-3-540-75418-3_7
2. Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993;168:400–407. doi:10.1093/infdis/168.2.400
3. Ajello L. The isolation of Aliescheria boydii shear, an etiologic agent of mycetomas, from soil. Am J Trop Med Hyg. 1952;1:227–238. doi:10.4269/ajtmh.1952.1.227
4. Tarozzi G. Ricerche anatomo-patologiehe, baceriologiehet e sperimentali sopra un caso di actinomicosi del piede. Arch Perle Sc Med. 1909;33:553–632.
5. Gilgado F, Cano J, Gené J, Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol. 2005;43:4930–4942. doi:10.1128/JCM.43.10.4930-4942.2005
6. Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21:157–197. doi:10.1128/CMR.00039-07
7. Larbcharoensub N, Chongtrakool P, Wirojtananugoon C, et al. Treatment of a brain abscess caused by Scedosporium apiospermum and Phaeoacremonium parasiticum in a renal transplant recipient. Southeast Asian J Trop Med Public Health. 2013;44:484–489.
8. Leechawengwongs M, Milindankura S, Liengudom A, Chanakul K, Viranuvatti K, Clongsusuek P. Multiple Scedosporium apiospermum brain abscesses after near-drowning successfully treated with surgery and long-term voriconazole: a case report. Mycoses. 2007;50:512–516. doi:10.1111/j.1439-0507.2007.01410.x
9. Luplertlop N, Pumeesat P, Muangkaew W, Wongsuk T, Alastruey-Izquierdo A. Environmental screening for the Scedosporium apiospermum species complex in public parks in Bangkok, Thailand. PLoS One. 2016;11:e0159869. doi:10.1371/journal.pone.0159869
10. Luplertlop N, Muangkaew W, Pumeesat P, Suwanmanee S, Singkum P. Distribution of Scedosporium species in soil from areas with high human population density and tourist popularity in six geographic regions in Thailand. PLoS One. 2019;14:e0210942. doi:10.1371/journal.pone.0210942
11. Wongsuk T, Pumeesat P, Luplertlop N. Genetic variation analysis and relationships among environmental strains of Scedosporium apiospermum sensu stricto in Bangkok, Thailand. PLoS One. 2017;12:e0181083. doi:10.1371/journal.pone.0181083
12. Kusne S, Ariyanayagam-Baksh S, Strollo DC, Abernethy J. Invasive Scedosporium apiospermum infection in a heart transplant recipient presenting with multiple skin nodules and a pulmonary consolidation. Transpl Infect Dis. 2000;2:194–196. doi:10.1034/j.1399-3062.2000.020405.x
13. Eldin C, Chiche L, Thomas G, et al. Scedosporium apiospermum catheter-related soft-tissue infection: a case report and review of the literature. Med Mycol. 2012;50:627–630. doi:10.3109/13693786.2011.639035
14. Noh SH, Na GH, Park K, Kim EJ. A Case of localized cutaneous infection caused by Scedosporium apiospermum presenting as cellulitis. Ann Dermatol. 2017;29:640–642. doi:10.5021/ad.2017.29.5.640
15. Tóth EJ, Nagy GR, Homa M, et al. Recurrent Scedosporium apiospermum mycetoma successfully treated by surgical excision and terbinafine treatment: a case report and review of the literature. Ann Clin Microbiol Antimicrob. 2017;16:31. doi:10.1186/s12941-017-0195-z
16. Rollin-Pinheiro R, de Meirelles JV, Vila TVM, et al. Biofilm formation by Pseudallescheria/Scedosporium species: a comparative study. Front Microbiol. 2017;8:1568. doi:10.3389/fmicb.2017.01568
17. Mello TP, Aor AC, Goncalves DS, Seabra SH, Branquinha MH, Santos AL. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling. 2016;32:737–749. doi:10.1080/08927014.2016.1192610
18. Goldman C, Akiyama MJ, Torres J, Louie E, Meehan SA. Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: A case report and review of the literature. Med Mycol Case Rep. 2016;11:40–43. doi:10.1016/j.mmcr.2016.04.005
19. Vöhringer S, Schrum J, Ott H, Höger PH. Severe phototoxicity associated with long-term voriconazole treatment. J Dtsch Dermatol Ges. 2011;9:274–276. doi:10.1111/j.1610-0387.2010.07563.x
20. Mehmood A, Liu G, Wang X, Meng G, Wang C, Liu Y. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: a review. Molecules. 2019;24:E1950. doi:10.3390/molecules24101950
21. Hazelwood LA, Daran JM, Van Maris AJA, Pronk JT, Dickinson JR. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol. 2008;74:2259–2266. doi:10.1128/AEM.02625-07
22. Kruppa M. Quorum sensing and Candida albicans. Mycoses. 2009;52:1–10. doi:10.1111/j.1439-0507.2008.01626.x
23. Singkum P, Muangkaew W, Suwanmanee S, Pumeesat P, Wongsuk T, Luplertlop N. Suppression of the pathogenicity of Candida albicans by the quorum-sensing molecules farnesol and tryptophol. J Gen Appl Microbiol. 2019;65(6):277–283. doi:10.2323/jgam.2018.12.002
24. Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–6031. doi:10.1128/IAI.72.10.6023-6031.2004
25. Nett JE, Marchillo K, Andes DR. Modeling of fungal biofilms using a rat central vein catheter. Methods Mol Biol. 2012;845:547–556. doi:10.1007/978-1-61779-539-8_40
26. Pumeesat P, Muangkaew W, Ampawong S, Luplertlop N. Candida albicans biofilm development under increased temperature. New Microbiol. 2017;40:279–283.
27. Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med Mycol. 2011;49:253–262. doi:10.3109/13693786.2010.530032
28. Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003;41:2961–2967. doi:10.1128/jcm.41.7.2961-2967.2003
29. Ampawong S, Luplertlop N. Experimental scedosporiosis induces cerebral oedema associated with abscess regarding Aquaporin-4 and Nrf-2 depletions. Biomed Res Int. 2019;2019:6076571. doi:10.1155/2019/6076571
30. Husain S, Muñoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis. 2005;40:89–99. doi:10.1086/426445
31. Troke P, Aguirrebengoa K, Arteaga C, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52:1743–1750. doi:10.1128/AAC.01388-07
32. Bernstein EF, Schuster MG, Stieritz DD, Heuman PC, Uitto J. Disseminated cutaneous Pseudallescheria boydii. Br J Dermatol. 1995;132:456–460. doi:10.1111/j.1365-2133.1995.tb08683.x
33. Severo LC, Oliveira Fde M, Londero AT. Subcutaneous scedosporiosis. Report of two cases and review of the literature. Rev Inst Med Trop Sao Paulo. 1997;39:227–230. doi:10.1590/S0036-46651997000400009
34. Guarro J, Kantarcioglu AS, Horré R, et al. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 2006;44:295–327. doi:10.1080/13693780600752507
35. Beier F, Kittan N, Holzmann T, et al. Successful treatment of Scedosporium apiospermum soft tissue abscess with caspofungin and voriconazole in a severely immunocompromised patient with acute myeloid leukemia. Transpl Infect Dis. 2010;12:538–542. doi:10.1111/j.1399-3062.2010.00537.x
36. Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis. 2002;34:1232–1242. doi:10.1086/339863
37. Lat A, Thompson GR
38. Wongsuk T, Pumeesat P, Luplertlop N. Fungal quorum sensing molecules: role in fungal morphogenesis and pathogenicity. J Basic Microbiol. 2016;56(5):440–447. doi:10.1002/jobm.201500759
39. Lackner M, Guarro J. Pathogenesis of Scedosporium. Curr Fungal Infect Rep. 2013;7:326–333. doi:10.1007/s12281-013-0157-7
40. Larcher G, Cimon B, Symoens F, et al. A 33 kDa serine proteinase from Scedosporium apiospermum. Biochem J. 1996;315(Pt1):119–126. doi:10.1042/bj3150119
41. de Hoog GS, Marvin-Sikkema FD, Lahpoor GA, et al. Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses. 1994;37(3–4):71–78. doi:10.1111/j.1439-0507.1994.tb00780.x
42. Kirk PW. A comparison of saline tolerance and sporulation in marine and clinical isolates of Allescheria boydii Shear. Mycopathol Mycol Appl. 2010;33:65–75. doi:10.1007/BF02049792
43. Mack D, Estes R, McLeod R. Administration of intraliposomal CP20961 to mice activates peritoneal macrophages and augments serum antibody response to Toxoplasma gondii. J Eukaryot Microbiol. 1994;41:12.
44. Bertin J, Guo Y, Wang L, et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem. 2000;275:41082–41086. doi:10.1074/jbc.C000726200
45. Eno CO, Zhao G, Olberding KE, Li C. The Bcl-2 proteins Noxa and Bcl-xL co-ordinately regulate oxidative stress-induced apoptosis. Biochem J. 2012;444:69–78. doi:10.1042/BJ20112023
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.