Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Tryptophan Prevents the Development of Non-Alcoholic Fatty Liver Disease
Authors Yanko R , Levashov M, Chaka OG, Nosar V, Khasabov SG, Khasabova I
Received 11 October 2023
Accepted for publication 18 December 2023
Published 23 December 2023 Volume 2023:16 Pages 4195—4204
DOI https://doi.org/10.2147/DMSO.S444278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Roman Yanko,1,* Mikhail Levashov,1,* Olena Georgievna Chaka,1 Valentina Nosar,1 Sergey G Khasabov,2 Iryna Khasabova2,*
1Department of Clinical Physiology of Connective Tissue, Bogomoletz Institute of Physiology National Academy of Sciences of Ukraine, Kiev, Ukraine; 2Department of Diagnostic and Biological Sciences, School of Dentistry, University of Minnesota, Minneapolis, MN, USA
*These authors contributed equally to this work
Correspondence: Roman Yanko, Department of Clinical Physiology of Connective Tissue, Bogomoletz Institute of Physiology National Academy of Sciences of Ukraine, Bogomoletz Street 4, Kiev, 01024, Ukraine, Tel +380442562477, Email [email protected]
Purpose: The main aim of this research is to study the protective effects of tryptophan on the histomorphological and biochemical abnormalities in the liver caused by a high-calorie diet (HCD), as well as its ability to normalize mitochondrial functions in order to prevent the development of non-alcoholic fatty liver disease (NAFLD).
Methods: The study was conducted in male Wistar rats aged 3 months at the start of the experiment. Control animals (group I) were fed a standard diet. Group II experimental animals were fed a diet with an excess of fat (45%) and carbohydrates (31%) for 12 weeks. Group III experimental animals also received L-tryptophan at a dose of 80 mg/kg body weight in addition to the HCD. The presence of NAFLD, functional activity, physiological regeneration, and the state of the liver parenchyma and connective tissue were assessed using physiological, morphological, histo-morphometric, biochemical, and biophysical research methods.
Results: HCD induced the development of NAFLD, which is characterized by an increase in liver weight, hypertrophy of hepatocytes and an increase in the concentration of lipids, cholesterol and triglycerides in liver tissue. Increased alanine aminotransferase activity in the liver of obese rats also confirm hepatocytes damage. Tryptophan added to the diet lowered the severity of NAFLD by reducing fat accumulation and violations of bioelectric properties, and prevented a decrease in mitochondrial ATP synthesis.
Conclusion: The addition of tryptophan can have a potential positive effect on the liver, reducing the severity of structural, biochemical, mitochondrial and bioelectric damage caused by HCD.
Keywords: fatty liver disease, essential amino acids, obesity
Introduction
The most common complication of obesity is non-alcoholic fatty liver disease (NAFLD), which is characterized by a number of metabolic disorders, including the accumulation of triglycerides in hepatocytes and the development of oxidative stress.1–9 Together, these metabolic impairments lead to inflammation, fibrosis and cirrhosis of the liver.10 Low-calorie diets in combination with anorexigenic, hepatoprotective, membrane stabilizing and antioxidant drugs, as well as bariatric surgery are widely used to treat obesity and NAFLD.11,12 Since each of these methods has serious medical limitations and side effects, the search for safe approaches to reduce the severity of NAFLD in obesity is an urgent therapeutic task.
One such effective and safe approach may be to use the essential amino acid tryptophan, a vital component of the daily diet of children and adults. Tryptophan and its metabolites (serotonin, melatonin and niacin)13 play an important role in lipid metabolism, sleep, nutrition and emotional behavior, which in turn influence the development of obesity and NAFLD.14 Under normal physiological conditions, up to 90% of tryptophan is metabolized via the kynurenine pathway into nicotinamide adenine dinucleotide, which is actively involved in the mitochondrial functions. Residual tryptophan is used to produce serotonin and melatonin.15 High-calorie diet (HCD) has been shown to disrupt tryptophan metabolism, which contributes to inflammation, increased excitatory neurotransmission16–19 and leads to impaired mitochondrial function and fat accumulation in the liver.20,21
Despite the essential role of tryptophan in maintaining health, there is currently no clear answer to the question of the effectiveness of his supplements to reduce the severity of NAFLD and especially reduce mitochondrial dysfunction in hepatocytes. Tryptophan has been shown to reduce the symptoms of NAFLD, namely, it reduces the level of triglycerides and cholesterol in blood plasma, reduces the levels of pro-inflammatory cytokines and normalizes body weight in rats on HCD.22,23 The opposite results indicate that tryptophan in combination with a HCD contributes to the development of liver steatosis.14,24 The existing contradictions may reflect the differences in the species, age, and sex of the NAFLD models used, as well as a wide range of tryptophan doses.
Therefore, the present study was conducted to investigate the effectiveness of L-tryptophan supplements in reducing severity of NAFLD caused by obesity. Our results show that L-tryptophan attenuates histomorphological, biochemical and bioelectric damages in the liver induced by a diet high in fats and carbohydrates and protects the mitochondria of hepatocytes by supporting the coupling of respiration and oxidative phosphorylation.
Materials and Methods
Rats
Twelve-week-old Wistar rats (weight 360 ± 20 g), obtained from the vivarium of the Bogomoletz Institute of Physiology of the National Academy of Sciences of Ukraine were used in the experiments. The rats were kept at a temperature of 21°C and humidity of 40–60% with a 12-hour light/dark cycle individually in cages with mesh partitions to minimize stress from social isolation. All protocols were approved by the Committee on Biomedical Ethics of Animal Care and Use of the Bogomoletz Institute of Physiology National Academy of Sciences of Ukraine (protocol No. 5 dated 11/31/19). Rats were removed from the experiment by decapitation under isoflurane anesthesia in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986).
Rat Feeding
The rats were randomly assigned to 3 groups. Each rat of the control group (group I) received 20 g of standard feed daily, containing fats - 6%, proteins - 23% and carbohydrates - 55% (recipe K120-1 “Rezon-1”, Ukraine) and had unlimited access to water. The daily caloric intake was 66 kcal per rat. The daily diet of experimental rats (groups II and III) was supplemented with pork lard, white breadcrumbs and sunflower seeds (fats - 45%, proteins - 9% and carbohydrates - 31%). The daily caloric content of this diet was 116 kcal per rat. In addition, every second day, the experimental rats received a 10% fructose solution instead of water, which increased the calorie content to 140 kcal and accelerated the development of NAFLD. Such a drinking regime does not reduce the consumption of dry food and liquid by the experimental animals and does not threaten to disrupt the body’s water balance. The choice of fructose is based on the fact that its addition to the diet enhances the negative effect of a HCD on the development of NAFLD and on lipid metabolism in general. We patented the diet used in this study.25 Since a high-fat and carbohydrate diet mimics the Western human diet, it is widely used to model obesity and NAFLD in rodents.24,25
Group III rats, in addition to HCD, received daily L-tryptophan (Ajinomoto Eurolysine S.A.S, France) at a dose of 80 mg/kg of body weight added to the feed. This dose of tryptophan was selected based on literature data. The protective effect was more evident at doses that did not exceed 100 mg/kg,26,27 while high doses of L-tryptophan (250–400 mg/kg) often aggravated NAFLD.14,24
The duration of the experiment was 12 weeks. It was during such a period of time that all experimental rats, which were on HCD, showed marked signs of obesity and NAFLD. At the end of the experiment, liver and visceral fat were isolated and their absolute and relative weight (organ mass/body weight) were determined.
Histomorphological Analysis of the Liver
Histological slices of the livers were prepared according to the standard procedure.28 Liver tissue samples were fixed in a Buena solution (picric acid 75%, formalin 25%, acetic acid 5%), dehydrated in ethanols of increasing concentration, and embedded in paraffin. Paraffin slices (6 μm) were prepared on a microtome and stained with hematoxylin-eosin and by the Van Gieson method. The slices were analyzed using a microscope (“Nikon Eclipse E100”, Japan). “ImageJ 1.34” software was used for morphometric analysis. The average cross-sectional area of hepatocytes, their nucleus and cytoplasm were measured, and the nuclear-cytoplasmic ratio was calculated. The number of mono- and binuclear hepatocytes per 1000 μm2 of liver tissue and the number of nucleolus per 100 nucleus of hepatocytes were determined. We counted (per unit of area) lipid droplets with an area of more than 100 μm2 on liver microsections. Such large lipid drops occur with pronounced signs of NAFLD, at the site of dead hepatocytes. Numerous lipid droplets of smaller sizes are better represented not quantitatively, but qualitatively on microphotographs. After measuring the relative area of the sinusoids and liver parenchyma, the Vizotto coefficient (the ratio of the relative area of the sinusoids to the liver parenchyma) was calculated. The number of hepatocytes and connective tissue cells per unit area of liver tissue (23,000 μm2) was determined under X800 magnification. To facilitate cell counting, the studied section area was divided into sectors.29,30 10 fields of view of the liver of each rat were analyzed by blinded quantitative analysis.
Assessment of Lipids and Enzyme Activity
Liver samples (100 mg) were homogenized in a mixture of chloroform: methanol (1:1). The resulting extract was filtered and the concentration of total lipids, triglycerides and cholesterol was determined by colorimetric-enzymatic method with standard reagent kits (Filisit-Diagnostics, Ukraine) using a biochemical analyzer (Sinnova, China). The activity of alanine aminotransferase in blood serum was determined by the Reitman-Frankel method.31 The activity of glucose-6-phosphatase was determined by the Swanson method, measuring the amount of phosphorus released from glucose-6-phosphate.32 Succinate dehydrogenase activity was measured by the Stinger and Kearney method in a suspension of mitochondria isolated from the liver by differential centrifugation.33,34.
Polarography
Mitochondria were isolated from the liver by differential centrifugation and stored on ice. The processes of respiration and oxidative phosphorylation of liver mitochondria were measured by polarography using a closed Clark electrode and an Oxigraph device (“Standart Oxygraf System, Hansatech”, England). Respiration was measured in a 1 mL polarographic cell with constant stirring of the incubation medium with the homogenate. Liver mitochondria were incubated in media containing sucrose (0.3 M), NaH2PO4 (3 mM), Tris HCl (10 mM). Mitochondrial oxidation substrate containing Na succinate (5 mM), Na glutamate (5 mM), Na pyruvate (3 mM), malate (2.5 mM) and palmitoyl-DL-carnitine (40 mM) was added prior to conducting polarography. Respiration was stimulated by adding 200 µL of ATP to a polarographic cell. Chronoamperographic curves were recorded (reflecting the dynamics of oxygen tension in the incubation medium) and the parameters of mitochondrial respiration were calculated: V4s – resting respiratory rate; V4ATP – frequency of controlled respiration (when ADP injected into the cell begins to run out); V3 – active respiration with ADP; V3/V4ATP – respiratory control, an indicator of the effectiveness of mitochondrial breathing; ADP/O is a phosphorylation efficiency coefficient reflecting the quantitative relationship between O2 consumption and ATP synthesis.35
Bioimpedance Analysis
To assess the polarization properties of tissue the multi-frequency bioelectrical impedance analysis (BIA) was used.36 The BIA method was used as one of the most informative methods for assessing the viability of biological tissues, their functional and metabolic activity and can be used to diagnose NAFLD.37,38 BIA testing was carried out ex tempore after liver isolation using LCR meter QuadTech 1920 (Bell Electronics, Renton USA) in the parallel equivalent circuit mode. The absolute values of the electrical parameters were determined at frequencies between 103 Hz – 106 Hz. Bioelectric properties were measured in the left lobe of the liver using 2 flat silver electrodes with an area of 25 mm2. The distance between the electrodes was 4 mm. The values of impedance and reactance obtained at the frequencies of minimum (103 Hz) and maximum (106 Hz) polarization were used for subsequent analysis. The coefficient of impedance dispersion was calculated according to the formula “DZ = Z104/Z106”, where Dz is coefficient of impedance dispersion, Z104 is the value of impedance at the frequencies of 104 Hz, and Z106 is the value of impedance at the frequencies of 106 Hz. The coefficient of reactance dispersion was calculated according to the formula “DXc = Xc104/Xc106”, where Dxc is coefficient of reactance dispersion, Xc104 is the value of reactance at the frequencies of 104 Hz, and Xc106 is the value of reactance at the frequencies of 106 Hz.
Statistical Analysis
Statistical analyses were performed using “SigmaPlot 14.5” software (Inpixon, CA). Data are reported as the mean ± SEM when normally distributed. Multiple groups were analyzed by one-way analysis of variances (ANOVA), followed by Bonferroni t tests for multiple comparisons. The Shapiro–Wilk test was used to determine the normality of the distribution. Sample sizes and power were determined by ANOVA the sample size and power analysis. Statistical power π=0.80 and criterion P<0.05 were considered statistically significant.
Results
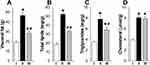
HCD for 12 weeks (group II) led to obesity and NAFLD in rats, as evidenced by an increase in visceral fat weight by 142%, as well as an increase in the amount of total lipids, triglycerides, and cholesterol in the liver by 173%, 123% and 105% respectively, compared with control rats (Figure 1A–D). In obese rats, the liver weight increased by 34% compared to the control (p<0.05, One-way ANOVA followed by Bonferroni post hoc test, N=10 rats/group) and had a viscous consistency as well as reduced turgor pressure within the parenchyma. Consistent with NAFLD, there were multiple diffuse yellow spots on the surface of the liver, alternating with areas of normal red-brown color.
In contrast to control animals (group I), histological sections of the liver of obese rats showed signs of fatty degeneration of hepatocytes (Figure 2A and B) and a decrease in the relative area of the sinusoidal network by 60%, as calculated by the Visotto coefficient (Table 1), reflecting impaired blood and lymph circulation.39 Fatty degeneration of hepatocytes due to the formation of numerous lipid droplets of different sizes in the cytoplasm led to an increase in the area of the cytoplasm by 42% and, as a result, to a decrease in the nuclear-cytoplasmic ratio. Sometimes lipid drops merged into one large one, which leads to the peripheral location of the nucleus, stretching of the cytoplasmic membrane, and subsequently its rupture and cell death. The total number of hepatocytes in obese rats decreased due to the death of mainly mononuclear forms (Table 1). The voids of dead hepatocytes were replaced by large lipid droplets (area > 100 µm2). Hepatocyte nucleus had reduced numbers of nucleolus by 41% compared to control rats, reflecting a decrease in synthetic activity.40 The total number and density of connective tissue cells (per unit area of the liver) were lower in the liver of obese rats than in control (Table 1).
 |
Table 1 Histomorphometric Parameters of the Liver |
Obesity in rats was accompanied by an increase in serum alanine aminotransferase activity by 37% compared with the control, indicating damage to hepatocytes.41 Also a by 34% decrease in glucose-6-phosphatase activity (p<0.05, One-way ANOVA followed by Bonferroni post hoc test, N=10 rats/group) was observed in the liver homogenate of obese rats compared to control rats, which is probably due to a decrease in anaerobic ATP synthesis. Succinate dehydrogenase activity in suspensions of mitochondria from obese rat hepatocytes was decreased by 57% when compared to control. Succinate dehydrogenase (also known as complex II) connects two major pathways in mitochondria – the citric acid cycle and the respiratory chain, both of which are necessary for oxidative phosphorylation. A decrease in oxygen consumption during succinate oxidation (V3) by 31% and a decrease in the respiratory control coefficient (V3/V4ATP) by 74% indicated the uncoupling of respiration and oxidative phosphorylation in the liver mitochondria of obese rats (Table 2). Mitochondrial respiration was impaired due to both a decreased rate of ADP-stimulated respiration and decreased coupling between respiration and oxidative phosphorylation.
 |
Table 2 Parameters of Respiration and Oxidative Phosphorylation of Hepatocyte Mitochondria |
HCD resulted in significant changes in bioimpedance measurements of liver tissue. The impedance and reactance values increased at both low and high frequencies. However, at a frequency of 106 Hz, these indicators increased more significantly, which led to a decrease in the coefficient of frequency dispersion of the reactance by 28% (Figure 3A–E). Such changes indicate a deterioration in the electrical conductivity of liver tissue due to intracellular fat accumulation. All of the listed biochemical, histomorphological and electrical changes found in the liver of rats on HCD indicated the development of NAFLD.
L-tryptophan added to HCD (group III) alleviated the hepatic disturbances. L-tryptophan reduced visceral fat accumulation by 43% and decreased total liver lipids and triglycerides by 38% and 27%, respectively, compared to HCD rats but did not affect cholesterol levels (Figure 1A–D), although it was previously reported to reduce total blood cholesterol levels in patients.42 The livers of rats that received L-tryptophan in combination with HCD had a predominantly red-brown color with alternating single yellow-white areas. The number of large lipid droplets in the liver parenchyma was 25% less compared to obese rats (group II) (Table 1). The number of mononuclear hepatocytes, as well as the number of nucleolus were higher in L-tryptophan-treated rats compared to HCD-only rats, although not reaching the levels of control rats. The size of hepatocytes, the area of the cytoplasm and the nuclear-cytoplasmic ratio in rats treated with L-tryptophan in combination with HCD remained at the level of control rats. There were no changes in the area of sinusoids and Visotto coefficient compared to control rats, indicating the preservation of perfusion and functional activity of the liver (Table 1, Figure 2C).
L-tryptophan additionally prevented the disruption of enzyme activities and electrical properties of liver tissue. In rats treated with L-tryptophan in combination with HCD, the activities of alanine aminotransferase, glucose-6-phosphatase and succinate dehydrogenase returned to the levels of control rats. The magnitude of the impedance and reactance of the liver tissue in rats treated with L-tryptophan was lower than in rats treated only with HCD, and the frequency dispersion coefficient of reactivity increased by 20% and approached the control values (Figure 3A–E).
Discussion
The liver plays an important role in lipid metabolism. Approximately 50% of cholesterol is synthesized in the liver. It has been shown that alimentary obesity in almost all patients is accompanied by the development of NAFLD, when more than 5% of the liver mass is fat, which accumulates in hepatocytes in the form of triglycerides.43
The medical community has long discussed the possible protective effect of tryptophan.44 The protective effect is mainly due to its derivatives – serotonin and melatonin.45 An increased level of serotonin reduces stress, thereby contributing to a decrease in food intake and body weight.46 Serotonin activates gluconeogenesis, suppresses glucose uptake by the liver and accelerates bile acid metabolism, which reduces calorie intake.46,47 The concentration of melatonin in the blood is inversely proportional to the level of leptin and food intake.48 Melatonin reduces lipid peroxidation, the activity of hepatic lipogenic enzymes and fatty acid synthesis enzymes, and prevents de novo lipogenesis in the liver.49,50 In addition, melatonin eliminates the loss of mitochondrial respiratory function, blocks oxidative cell damage during HCD, thereby protecting mitochondria.51
The protective mechanisms caused by tryptophan are complex and have not yet been fully elucidated. This is confirmed by contradictory results obtained by a number of authors. It has been shown that tryptophan reduces triglyceride and cholesterol levels, reduces inflammation and hypertrophy of hepatocytes and normalizes body weight in rats on HCD.22,23,52 In patients with steatohepatitis (the stages of NAFLD), it was shown that one month of taking L-tryptophan (500 mg daily) led to a decrease in the level of triglycerides in the liver.53 Oral administration of tryptophan for 8 weeks reduced the ratio of liver weight to body weight and reduced the accumulation of fat in the liver of mice with NAFLD.23 However, some authors generally deny the existence of a protective effect of tryptophan, indicating that tryptophan in combination with HCD contributes to the development of liver steatosis.14,24
The use of our patented model (Patent № 150511. 23.02.2022),25 characterized by excessive consumption of fats and carbohydrates for 12 weeks, allowed us to obtain well-expressed manifestations of NAFLD in experimental rats. This was indicated by an increase in liver weight, hypertrophy of hepatocytes and an increase in the total content of lipids and triglycerides in liver tissues. Increased alanine aminotransferase activity in the liver of obese rats also confirms damage to hepatocytes. The addition of L-tryptophan to HCD decreased the amount of fat in the liver of rats.
The pathogenesis of NAFLD includes mitochondrial dysfunction, which leads to a violation of the energy balance. For this reason, some researchers consider NAFLD as a mitochondrial disease.54 Mitochondrial succinate dehydrogenase catalyzes the oxidation of succinate in the Krebs cycle and provides electrons to the respiratory chain. Confirming earlier data, the activity of this enzyme decreased with NAFLD, which indicates a decrease in the energy potential of mitochondria.55,56 A decrease in the activity of glucose-6-phosphatase, one of the key enzymes of gluconeogenesis, probably indicates a decrease in the processes of anaerobic synthesis of ATP by the liver, which is confirmed by a decrease in respiration and oxidative phosphorylation of hepatocyte mitochondria.57 L-tryptophan reduces the negative effect of HCD on oxidative phosphorylation in the liver mitochondria and prevents a decrease in the processes of aerobic and anaerobic synthesis of ATP by the liver. Since tryptophan has antioxidant properties by absorbing oxygen radicals, it has been found that it improves the recovery of mitochondrial metabolism and prevents NAFLD.58,59
It has been documented that the development of NAFLD in patients is accompanied by a violation of bioelectric properties.60 Bioelectric impedance analysis is widely used as a simple and reliable screening of fatty liver disease with high accuracy and reproducibility comparable to transient elastography and ultrasound.61 Our rat model of NAFLD is also characterized by changes in electrical conductivity and the ability to polarize under the influence of electric current of different frequencies. An increase in the impedance and reactance of liver tissue at both low and high frequencies of the tested current indicates fatty liver degeneration in NAFLD, since fat has a high electrical resistance in contrast to normal liver tissue, which has a relatively high electrical conductivity.62 L-tryptophan, by reducing the fat content, decreased the impedance and reactance of liver tissue, as well as their frequency dispersion coefficients.
The innovation of this study is the optimal diet, which made it possible to simulate visceral obesity and NAFLD in rats in a short time, reflecting the pathogenesis of the disease in patients.25 Although L-tryptophan at a dose of 80 mg/kg reduced the severity of NAFLD, the lack of a dose-dependent effect for the model used limits the final conclusion.
Conclusion
The addition of tryptophan can have a potential benefit on the liver, reducing the severity of structural, biochemical, mitochondrial and bioelectric damage caused by HCD, and can be used in combination with other methods to improve the effectiveness of treatment. Enhancing the positive effects of tryptophan by combining dietary supplements with physical activity will be the focus of our future research and may benefit patients with NAFLD caused by obesity.
Abbreviations
NAFLD, non-alcoholic fatty liver disease; HCD, high-calorie diet; BIA, bioelectrical impedance analysis.
Data Sharing Statement
The datasets used and analyzed during the current study are included in this published article.
Ethics Approval
This study was approved by the Committee on Biomedical Ethics of Animal Care and Use of the Bogomoletz Institute of Physiology National Academy of Sciences of Ukraine (protocol No. 5 dated 11/31/19).
Acknowledgments
The authors would like to thank Samuel Zorn for editing the article.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The work was performed as part of the state assignment “Bogomoletz Institute of Physiology” (No. 0119U103965). This work was supported by the grant NIH R01 CA263777.
Disclosure
The authors reports no conflicts of interest in this work.
References
1. Galvan-Martinez DH, Bosquez-Mendoza VM, Ruiz-Noa Y, Ibarra-Reynoso LDR, Barbosa-Sabanero G, Lazo-de-la-Vega-Monroy ML. Nutritional, pharmacological, and environmental programming of NAFLD in early life. Am J Physiol Gastrointest Liver Physiol. 2023;324(2):G99–G114. doi:10.1152/ajpgi.00168.2022
2. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. doi:10.1016/j.freeradbiomed.2020.02.025
3. Pérez-Carreras M, Del Hoyo P, Martín MA, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007. doi:10.1053/jhep.2003.50398
4. Lee K, Haddad A, Osme A, et al. Hepatic mitochondrial defects in a nonalcoholic fatty liver disease mouse model are associated with increased degradation of oxidative phosphorylation subunits. Mol Cell Proteomics. 2018;17(12):2371–2386. doi:10.1074/mcp.RA118.000961
5. Golubeva JA, Sheptulina AF, Yafarova AA, Mamutova EM, Kiselev AR, Drapkina OM. Reduced quality of life in patients with non-alcoholic fatty liver disease may be associated with depression and fatigue. Healthcare. 2022;10(9):1699. doi:10.3390/healthcare10091699
6. Shea S, Lionis C, Kite C, et al. Non-alcoholic fatty liver disease (NAFLD) and potential links to depression, anxiety, and chronic stress. Biomedicines. 2021;9(11):1697. doi:10.3390/biomedicines9111697
7. Lazarus JV, Colombo M, Cortez-Pinto H, et al. NAFLD - sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol. 2020;17(7):377–379. doi:10.1038/s41575-020-0315-7
8. Rinaldi L, Pafundi PC, Galiero R, et al. Mechanisms of non-alcoholic fatty liver disease in the metabolic syndrome. A narrative review. Antioxidants. 2021;10:270. doi:10.3390/antiox10020270
9. Iqbal U, Perumpail BJ, Akhtar D, Kim D, Ahmed A. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines. 2019;6(1):41. doi:10.3390/medicines6010041
10. Schiavo L, Busetto L, Cesaretti M, Zelber-Sagi S, Deutsch L, Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24(30):3330–3346. doi:10.3748/wjg.v24.i30.3330
11. Mantovani A, Dalbeni A. Treatments for NAFLD: state of art. Int J Mol Sci. 2021;22(5):2350. doi:10.3390/ijms22052350
12. Głuszyńska P, Lemancewicz D, Dzięcioł JB, Razak Hady H. Non-alcoholic fatty liver disease (NAFLD) and bariatric/metabolic surgery as its treatment option: a review. J Clin Med. 2021;10(24):5721. doi:10.3390/jcm10245721
13. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401. doi:10.1038/s41573-019-0016-5
14. Shipelin VA, Trusov NV, Apryatin SA, et al. Effects of tyrosine and tryptophan in rats with diet-induced obesity. Int J Mol Sci. 2021;22:24–29. doi:10.3390/ijms22052429
15. Chen J, Vitetta L, Henson JD, Hall S. Intestinal dysbiosis, the tryptophan pathway and nonalcoholic steatohepatitis. Int J Tryptophan Res. 2022;15:11786469211070533. doi:10.1177/11786469211070533
16. Sun P, Wang M, Liu YX, et al. High-fat diet-disturbed gut microbiota-colonocyte interactions contribute to dysregulating peripheral tryptophan-kynurenine metabolism. Microbiome. 2023;11(1):154. doi:10.1186/s40168-023-01606-x
17. Madella AM, Van Bergenhenegouwen J, Garssen J, Masereeuw R, Overbeek SA. Microbial-derived tryptophan catabolites, kidney disease and gut inflammation. Toxins. 2022;14(9):645. doi:10.3390/toxins14090645
18. Hezaveh K, Shinde RS, Klötgen A, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324–340.e8. doi:10.1016/j.immuni.2022.01.006
19. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi:10.1038/nrn3257
20. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11:557. doi:10.3389/fimmu.2020.00557
21. Tanaka M, Szabó Á, Spekker E, Polyák H, Tóth F, Vécsei L. Mitochondrial impairment: a common motif in neuropsychiatric presentation? The link to the tryptophan-kynurenine metabolic system. Cells. 2022;11(16):2607. doi:10.3390/cells11162607
22. Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol. 2014;65(1):75–82.
23. Ritze Y, Bárdos G, Hubert A, Böhle M, Bischoff SC. Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br J Nutr. 2014;112(1):1–7. doi:10.1017/S0007114514000440
24. Osawa Y, Kanamori H, Seki E, et al. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286(40):34800–34808. doi:10.1074/jbc.M111.235473
25. Янко РВ, Зінченко АС, Чака ОГ, Левашов МІ. Спосіб моделювання аліментарного жирового гепатозу у лабораторних щурів. Патент України № 150511. 2022 лютого 23. [Yanko RV, Zinchenko AS, Chaka OG, Levashov MI. Method of modeling alimentary fatty liver disease in laboratory rats. Ukraine patent No. 150511. 2022 Feb 23]. Ukraine.
26. Badawy AA, Bano S. Tryptophan metabolism in rat liver after administration of tryptophan, kynurenine metabolites, and kynureninase inhibitors. Int J Tryptophan Res. 2016;9:51–65. doi:10.4137/IJTR.S38190
27. Ayinde TO, Olayaki LA, Ojulari LS, Oluwasola A. Hepatoprotective effect of tryptophan in carbontetrachloride-induced hepatotoxicity in male Wistar rats. Int J Basic Appl Physiol. 2021;10(2):25–30.
28. Rehfeld A, Nylander M, Karnov K. Histological methods. In: Compendium of Histology. Cham: Springer; 2017. doi:10.1007/978-3-319-41873-5_2
29. Mamontov I, Ivakhno I, Tamm T, Panasenko V, Padalko V, Zulfugarov I. Morphometric parameters of hepatocytes in experimental complete extrahepatic bile duct obstruction. ScienceRise. 2020;1(34):51–56. doi:10.15587/2519-4798.2020.193845
30. Yanko RV, Chaka OG, Levashov MI. Influence of methionine on morphofunctional changes of rat liver parenchyma. Fiziol Zh. 2020;66(5):38–45. doi:10.15407/fz66.05.038
31. Reitman S, Frankel S. The structure and function of subcellular components. Am J Clin Pathol. 1957;28:56. doi:10.1093/ajcp/28.1.56
32. Swanson MA. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950;184(2):647–659.
33. Singer TP, Kearney EB, Bernath P. Studies on succinic dehydrogenase. Isolation and properties of the dehydrogenase from beef heart. J Biol Chem. 1956;223:599–613. doi:10.1016/S0021-9258(18)65059-8
34. Sumbalova Z, Fontana M, Krumschnabel G. Isolation of rat liver mitochondria. Mitochondr Physiol Network. 2016;20(08(02)):1–2.
35. Chance B, Williams G. Respiratory enzymes in oxidative phosphorylation. The steady state. J Biol Chem. 1955;217:409–427. doi:10.1016/S0021-9258(19)57191-5
36. Shchelykalina SP, Nikolaev DV, Kolesnikov VA, Korostylev KA, Starunova OA. Technology of two-dimensional bioimpedance analysis of the human body composition. J Electr Bioimpedance. 2021;12(1):17–25. doi:10.2478/joeb-2021-0004
37. Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014;14(6):10895–10928. doi:10.3390/s140610895
38. Hanna DJ, Jamieson ST, Lee CS, et al. Bioelectrical impedance analysis in managing sarcopenic obesity in NAFLD. Obes Sci Pract. 2021;7(5):629–645. doi:10.1002/osp4.509
39. Karschau J, Scholich A, Wise J, et al. Resilience of three-dimensional sinusoidal networks in liver tissue. PLoS Comput Biol. 2020;16(6):e1007965. doi:10.1371/journal.pcbi.1007965
40. Dubois ML, Boisvert FM. The nucleolus: structure and function. Funct Nucleus. 2016;23:29–49. doi:10.1007/978-3-319-38882-3_2
41. Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783.e4. doi:10.1053/j.gastro.2014.07.018
42. Wang W, Liu L, Tian Z, Han T, Sun C, Li Y. Dietary tryptophan and the risk of metabolic syndrome: total effect and mediation effect of sleep duration. Nat Sci Sleep. 2021;13:2141–2151. doi:10.2147/NSS.S337171
43. Lian CY, Zhai ZZ, Li ZF, Wang L. High fat diet-triggered non-alcoholic fatty liver disease: a review of proposed mechanisms. Chem Biol Interact. 2020;330:109199. doi:10.1016/j.cbi.2020.109199
44. Akiba Y, Takahashi K, Horiguchi M, Ohtani H, Saitoh S, Ohkawara H. L-Tryptophan alleviates fatty liver and modifies hepatic microsomal mixed function oxidase in laying hens. Comp Biochem Physiol Comp Physiol. 1992;102(4):769–774. doi:10.1016/0300-9629(92)90738-c
45. Mangge H, Summers KL, Meinitzer A, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity. 2014;22(1):195–201. doi:10.1002/oby.20491
46. van Galen KA, Ter Horst KW, Serlie MJ. Serotonin, food intake, and obesity. Obes Rev. 2021;22(7):e13210. doi:10.1111/obr.13210
47. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16(5):588–600. doi:10.1016/j.cmet.2012.09.014
48. Guan Q, Wang Z, Cao J, Dong Y, Chen Y. Mechanisms of melatonin in obesity: a review. Int J Mol Sci. 2021;23(1):218. doi:10.3390/ijms23010218
49. Ou TH, Tung YT, Yang TH, Chien YW. Melatonin improves fatty liver syndrome by inhibiting the lipogenesis pathway in hamsters with high-fat diet-induced hyperlipidemia. Nutrients. 2019;11:748. doi:10.3390/nu11040748
50. Sato K, Meng F, Francis H, et al. Melatonin and circadian rhythms in liver diseases: functional roles and potential therapies. J Pineal Res. 2020;68:e12639. doi:10.1111/jpi.12639
51. Zhou H, Du W, Li Y, et al. Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. 2018:64. doi:10.1111/jpi.12450
52. Watanabe H, Akasaka D, Ogasawara H, et al. Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinolog. 2010;151:4776–4786. doi:10.1210/en.2009-1349
53. Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol. 2010;61(5):577–580.
54. Nassir F, Ibdah JA. Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:8713–8742. doi:10.3390/ijms15058713
55. Staňková P, Kučera O, Peterová E, et al. Western diet decreases the liver mitochondrial oxidative flux of succinate: insight from a murine NAFLD model. Int J Mol Sci. 2021;22(13):6908. doi:10.3390/ijms22136908
56. Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase - assembly, regulation and role in human disease. Mitochondrion. 2010;10(4):393–401. doi:10.1016/j.mito.2010.03.001
57. van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;362(Pt 3):513–532. doi:10.1042/0264-6021:3620513
58. Nayak BN, Buttar HS. Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J Complement Integr Med. 2016;13(2):129–136. doi:10.1515/jcim-2015-0051
59. Zhao F, Liu ZQ, Wu D. Antioxidative effect of melatonin on DNA and erythrocytes against free-radical-induced oxidation. Chem Phys Lipids. 2008;151:77–84. doi:10.1016/j.chemphyslip.2007.10.002
60. Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:26. doi:10.1186/1475-2891-7-26
61. Choi JW, Yoo JJ, Kim SG, Kim YS. Bioelectrical impedance analysis can be an effective tool for screening fatty liver in patients with suspected liver disease. Healthcare. 2022;10(11):2268. doi:10.3390/healthcare10112268
62. Schloerb PR, Forster J, Delcore R, Kindscher JD. Bioelectrical impedance in the clinical evaluation of liver disease. Am J Clin Nutr. 1996;64(3):510S–514S. doi:10.1093/ajcn/64.3.510S
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.