Back to Journals » Journal of Pain Research » Volume 16
Trigeminal Ganglion Electrical Stimulation for Trigeminal Nerve Postherpetic Neuralgia: A Retrospective Study
Authors Xu M, Liu J, Zhang H, Li R, Wei J
Received 24 August 2023
Accepted for publication 19 October 2023
Published 31 October 2023 Volume 2023:16 Pages 3633—3641
DOI https://doi.org/10.2147/JPR.S432842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Natalie Strand
Mengzhen Xu,1 Jin Liu,1 Hui Zhang,2 Ruiting Li,3 Junni Wei1
1School of Public Health, Shanxi Medical University, Taiyuan, People’s Republic of China; 2Department of Pain Management, First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 3Department of Pain Management, Taiyuan Central Hospital, Taiyuan, People’s Republic of China
Correspondence: Hui Zhang, Department of Pain Management, First Hospital of Shanxi Medical University, Taiyuan, 030000, People’s Republic of China, Tel +86 351 13934043126, Fax + 0351-4639114, Email [email protected]
Purpose: To investigate the clinical outcome of trigeminal ganglion electrical stimulation for the treatment of trigeminal postherpetic neuralgia (TPHN).
Patients and Methods: A retrospective analysis of clinical data was performed on six patients who suffered from severe postherpetic neuralgia involving the trigeminal nerve maxillary and mandibular branch. They were admitted under the Pain Management Department of the First Hospital of Shanxi Medical University from July 2022 to February 2023 and underwent trigeminal ganglion electrical stimulation therapy. Visual analogue scale (VAS) scores, pregabalin dosage, pittsburgh sleep quality index (PSQI), self-rating anxiety scale (SAS), and self-rating depression scale (SDS) were recorded before treatment, as well as after treatment at 1, 4, 8, 12, and 24-week. Adverse reactions related to the treatment were also documented.
Results: After trigeminal ganglion electrical stimulation therapy, the VAS scores, PSQI scores, anxiety scores, depression scores, and pregabalin dosage of six patients showed significant reductions at 1, 4, 8, 12, and 24 weeks. (P < 0.05). No serious adverse reactions occurred in any of the patients.
Conclusion: Trigeminal ganglion electrical stimulation effectively relieved postherpetic neuralgia in the distribution areas of the trigeminal nerve 2 and 3 branches, reduced the dosage of analgesics, improved the quality of sleep, and alleviated anxiety and depression symptoms in patients. Our data suggested that It was a safe and effective clinical.
Keywords: trigeminal postherpetic neuralgia, trigeminal ganglion stimulation, therapy, efficacy
Introduction
Herpes zoster (HZ) is a painful cutaneous disease resulting from the reactivation of the varicella-zoster virus in the dorsal root ganglia. It is characterized by persistent pain in the affected area lasting for a minimum of three months after the onset of shingles rash. PHN is internationally defined as persistent skin pain in the affected area for at least three months following the onset of shingles rash.1 Postherpetic neuralgia (PHN) is believed to result from peripheral nerve damage during shingles outbreaks. The estimated incidence of PHN among individuals with shingles ranges from 9% to 34%.2 Research has demonstrated that 10–15% of shingles cases involve the trigeminal nerve,3 being less common than chest, abdomen, and lower back. The pain may persist for several years or even decades, significantly affecting patients’ quality of life and work commitments.4 Conventional pharmacological and nerve block interventions for postherpetic neuralgia (PHN) frequently produce suboptimal outcomes. As a result, patients often experienceinadequate pain control when using orally administered medications, which can become even more complicated due to medication side effects.5 There have been more studies conducted on neuromodulation techniques including peripheral nerve electrical stimulation, pulsed radiofrequency, spinal cord stimulation, and deep brain electrical stimulation for treating PHN. In recent years, spinal cord electrical stimulation has attracted more attention in treating postherpetic neuralgia.6 However, there remains a need to further evaluate its role in treatment of trigeminal postherpetic neuralgia, as studies specific to this area are still limited.
The objective of treating trigeminal postherpetic neuralgia (TPHN) is to effectively control pain as early as possible, alleviate associated sleep and emotional disorders, and thus improve quality of life. PNS is a minimally invasive treatment for treating refractory trigeminal neuralgia. The technique involves placing a stimulating electrode (typically a spinal cord stimulation electrode) at the oval foramen to directly stimulate the trigeminal ganglion and nerve.7 In a recent case report by Lin et al,8 a novel treatment approach was proposed for elderly individuals suffering from trigeminal neuralgia resulting from herpes zoster. The implementation of peripheral nerve stimulation (PNS) resulted in significant pain relief and overall improvement among patients with postherpetic trigeminal neuralgia (TPHN). This electrical stimulation technique has been successfully used in the treatment of HZ-related trigeminal neuralgia.9,10 However, clinical studies of peripheral nerve stimulation (PNS) in the trigeminal nerve region were currently limited, with only a few small-sample clinical studies and case reports available. These studies demonstrated the potential use of PNS for treating trigeminal neuralgia caused by postherpetic neuralgia (PHN), particularly in patients with involvement of the first branch of the trigeminal nerve. However, there were few reports regarding the involvement of the second and third branches. Therefore, the aim of this study was to assess the effectiveness and safety of electrical stimulation at the trigeminal nerve’s semilunar junction in treating postherpetic neuralgia involving the 2nd and 3rd trigeminal nerve branches; with the goal of offering an alternative and potentially effective treatment approach for this painful condition.
Patients and Methods
Study Design and Selection of Patients
A retrospective analysis was conducted on the clinical data of patients with postherpetic neuralgia affecting the maxillary and mandibular branch of the trigeminal nerve, who underwent electrical stimulation at the trigeminal semilunar node. The study took place at the Pain Management Department of the First Hospital of Shanxi Medical University between July 2022 and February 2023. Written informed consent were obtained from enrolled patient. The study was approved by the Institutional Review Board of the First Hospital of Shanxi Medical University and was conducted according to the principles of the Declaration of Helsinki. All. The registration had finished (NO.KYLL-2023-127).
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) Patients who were diagnosed with PHN according to the clinical diagnostic criteria;11 (2) The pain was localized in the second and third branches of the trigeminal nerve. (3) Electrical stimulation was administered to the trigeminal nerve’s semilunar node. (4) Visual analog scale (VAS)score ≥5 points; and (5) Pain refractory to conventional pharmacological (such as opiate analgesics, tricyclic antidepressants, anticonvulsants, and topical analgesics) or physical therapies (such as percutaneous electrical nerve stimulation and acupuncture).
The exclusion criteria were as follows: (1) Severe organ dysfunction, including brain, heart, lung, kidney, and liver diseases; (2) Participants lost to follow-up for more than 60 days or with incomplete clinical data due to other reasons; and (3) Patients who had intellectual problems and were unable to complete self-evaluations.
Treatment Procedure
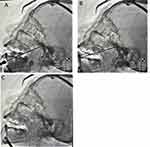
Upon admission to the operating room, patient’s ECGs were recorded and blood pressure, and oxygen saturation were checked; with a venous access established. The patients were in supine position on the operating table with a thin pillow placed under the shoulders, and general anesthesia was induced through tracheal intubation. The surgeon confirmed the pain area with the patient which was marked on the patient’s face. Following disinfection and positioning, the skin puncture point for the affected side was determined as the projection point of the foramina ovalis under DSA guidance (approximately 2cm from the corner of the mouth on the affected side). The puncture needle was then inserted slowly and precisely into the foramina ovalis, with the tip slightly beyond the inner opening of the foramina ovalis on the skull base revealed in the lateral DSA projection (Figure 1A). The puncture needle core was removed and substituted with a neurostimulation electrode (Medtronic 3861, Medtronic, Inc.) with eight contact leads. The DSA lateral view revealed a slight passage of the distal end of the electrode over the petrous ridge (Figure 1B and C) (with three inserted contacts). The puncture needle was then withdrawn, and the joint assembly was secured at the electrode’s skin puncture point (Figure 2), followed by the application of a sterile dressing. After the patient emerged from general anesthesia and extubated, they were transferred back to the ward. Once awake, the patient was connected to an external stimulator to adjust the parameters. Electrical stimulation parameters were tailored to the patient’s pain condition, with a frequency ranging from 2 to 1000Hz, pulse width ranging from 60 to 1000μs, and voltage ranging from 0 to 10.5V. A programming session was conducted to identify the optimal stimulation parameters for all implanted electrodes. In addition, patients were instructed on operating the external stimulator and the handheld controller. Patients had the capability to enable or disable the stimulation collectively, as well as adjust the amplitude individually for each implanted electrode. However, the active contacts, frequency, and pulse width were fixed and could only be modified by medical stuff. The intensity of the electrical stimulation was progressively increased to induce numbness and distension, effectively targeting the original pain area. After 14 days, the electrical stimulation was first stopped and observed for 6 hours. There were no apparent changes in the patient’s facial expression, and the analgesic effect was satisfactory, so the electrodes were removed.
 |
Figure 2 The fixation of electrode leads on the patient’s skin surface post-surgery. |
Evaluation and Outcome
Patient basic information, including gender, age, pain duration, nature of pain, history of underlying diseases, and baseline data at admission, was analyzed. Additionally, specific preoperative baseline data such as the pregabalin dose, visual analog scale (VAS), pittsburgh sleep quality index(PSQI), self-rating anxiety scale (SAS), self-rating depression scale (SDS), and response rate (defined as pain relief over 50% indicating effective treatment) were recorded. The primary outcomes assessed were postoperative VAS, PSQI, and response rate. The secondary outcomes measured included pregabalin dose, SAS, and SDS scores. These outcomes were further assigned again up at 1, 4, 8, 12, and 24-week after the surgery, respectively, allowing for comprehensive monitoring of any adverse reactions experienced by these patients.
Visual Analogue Scale (VAS)
The VAS was a 100-mm horizontal line labeled no pain at one end and worst imaginable pain at the other end. The patients were asked to mark on this line where the intensity of the pain was felt. The distance from no pain to the patients’ mark numerically quantifies the pain. The VAS is a simple and efficient method that correlates well with other reliable methods.
Self-Rating Anxiety Scale (SAS)
A tool used in clinical evaluation of anxiety state, which was applied to evaluate the degree of anxiety state induced by PHN in patients in this study. The total score ranges from 20 to 80 points. In general, the higher the score is, the greater the anxiety.
Self-Rating Depression Scale (SDS)
A clinical tool evaluating the depressive state of patients was used in this study to evaluate the depressive state of patients induced by PHN. The total score ranges from 20 to 80 points. The higher the score is, the more severe the depression.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI was adopted clinically used to assess the sleep quality of the subjects in the last 1 month. It consisted of 19 self-evaluation items and 5 other evaluation items, and 18 items were composed of 7 components. Each component was scored according to the level of 0 ~ 3, and the cumulative score of each component was the total score of PSQI, which ranged from 0 to 21. The higher the score, the worse the sleep quality.
Adverse Events
Observed adverse reactions included various postoperative complications associated with general anesthesia, including nausea, vomiting, and complications arising from the puncture site, such as bleeding, infection, cerebrospinal fluid leakage, electrode displacement, or dislodgement.
Statistical Analysis
The analysis of all data was conducted using SPSS 26.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 9.0 (GraphPad Software, Inc.). The measurement data were reported as the mean ± standard error (mean ± SEM). To compare variable data at different time points, a repeated measures analysis of variance (RM-ANOVA) test was utilized. A P-value <0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients
The study included a total of six patients after the inclusion and exclusion criteria were applied. Among the participants, four were male and two were female. The mean age was 68.5±6.1 years. One patient had the disease for over two years, while three patients had for three months, and the rest two patients had it for four months. In four patients, the pain was described as stabbing, with two of them experiencing accompanied burning pain and itching symptoms. The remaining two patients reported pain characterized as electric shock-like and tactile allodynia. Three patients had comorbidities such as hypertension. In addition to medication treatment, all patients received nerve blocks, and two patients underwent pulsed radiofrequency therapy as well (Table 1).
 |
Table 1 Patient Characteristics, Types of Trigeminal Neuropathic Pain and Previous Invasive Treatment Modalities |
Response Rate of Treatment
The average response rate of the six patients was 33.3% at 1 week after surgery, which increased to 83.3% at the 24-week follow-up. Compared to 1 week post-surgery (T1), the response rate of trigeminal ganglion electrical stimulation therapy for trigeminal postherpetic neuralgia showed significant improvement at T2, T3, T4, and T5 (P < 0.05). Among these six patients, there was one case in whom the degree of pain relief was less than 50%, which was considered as ineffective (Table 2).
 |
Table 2 Comparison of Postoperative Response Rate |
VAS Score, PSQI Score and Pregabalin Dosage
The initial visual analog scale (VAS) scores at T0 for the six patients were recorded as (8.5±0.2). After a 24-week follow-up, the patients’ VAS scores had decreased to 2.67. In addition, there were significant reductions in VAS scores at all postoperative periods (T1, T2, T3, T4, and T5) compared to the preoperative period T0 (P<0.01), indicating a noteworthy decrease in pain intensity (Figure 3).
Furthermore, the patients’ preoperative Pittsburgh Sleep Quality Index (PSQI) score was reported as (13.7±1.0). At the final 24-week postoperative follow-up, the PSQI score had decreased to (5.2±1.0) for the six patients. Moreover, there was a consistent downward trend in PSQI scores at 1 week, 4 weeks, 8 weeks, 12 weeks, and 24 weeks postoperatively, with significant differences compared to the preoperative period (P<0.01). This indicates a substantial improvement in sleep quality (Figure 3).
In this study, the dosages of pregabalin administered to patients were examined. The average preoperative dosage of pregabalin was 300 mg per day, and there was no alteration in medication dosage at the 1-week postoperative stage. However, notable reductions in pregabalin dosage were observed during the postoperative periods T2, T3, T4, and T5, indicating significant disparities in comparison to the preoperative phase T0 (P<0.01). Further information can be found in Figure 4.
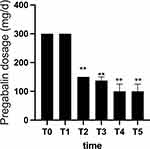 |
Figure 4 Changes in pregabalin dosage. **Repeated-measures ANOVA test, P<0.01. Notes: T0: before treatment, T1: one week, T2: 4 weeks, T3: 8 weeks, T4: 12 weeks, T5: 24 weeks. |
Comparison of SAS and SDS Scores in Different Postoperative Periods
This study aimed to investigate the changes in SAS and SDS scores among patients before and after treatment. Prior to the intervention, patients presented with an average SAS score of (49.2±6.1) and an SDS score of (46.2±5.3). No significant differences were observed in SAS and SDS scores one week post-treatment compared to the baseline. After that however, a consistent decline in both SAS and SDS scores was observed in all six patients starting from 4 weeks after treatment initiation. At the final observation (24 weeks post-treatment), the SAS score decreased to (30.7±5.1) and the SDS score decreased to (28.3±4.6). Furthermore, statistically significant improvement in SAS and SDS scores was found during at the T2, T3, T4, and T5 timeframes compared to the T0 period (P<0.01). Detailed information can be found in Figure 5.
Adverse Events
Following general anesthesia, one patient encountered adverse reactions manifested as nausea and vomiting. In addition, two patients experienced skin infections, which were expeditiously managed and quickly resolved under the care of the attending physician. There were no other reports of severe adverse reactions from the remaining patients.
Discussion
The pathogenesis of postherpetic neuralgia (PHN) remains uncertain. It is widely accepted that PHN is caused by sensitization of both peripheral and central neural pathways. Reske-Nielsen12 conducted autopsy studies and revealed pathological changes in the peripheral branches of the trigeminal nerve, trigeminal ganglion, and even the trigeminal spinal tract nucleus in three patients. Additionally, electrophysiological examinations confirmed the damage to trigeminal sensory function in the affected areas. Truini et al13 performed tests on 41 patients with trigeminal postherpetic neuralgia (involving the first branch), including blink reflex responses and laser-evoked potential (LEP) examinations, and confirmed functional abnormalities in Aβ, Aδ, and C nerve fibers.
Presently, therapeutic challenges persist in the clinical management of refractory postherpetic neuralgia (PHN). Trigeminal ganglion stimulation is a neuroregulatory therapy used to treat trigeminal neuropathic pain syndrome. Klein et al14 retrospectively analyzed 10 patients suffering from intractable facial pain who underwent trigeminal ganglion stimulation. Out of the 8 patients who received permanent implants, 6 reported complete pain alleviation through stimulation, while the remaining patients reported a maximum pain intensity of 3 based on the assessment using the visual analog scale (VAS). Taub et al7 reported permanent trigeminal ganglion electrical stimulation implantation in four patients with trigeminal neuralgia after herpes zoster infection. Among them, one person experienced effective pain relief in the Phase 1 trial. Recently, Gupta reported a case of postherpetic trigeminal neuralgia in which the patient experienced a 50% reduction in pain after trigeminal ganglion electrical stimulation.15 Texakalidis et al16 presented a retrospective series of patients undergoing supraorbital nerve (SON) and/or infraorbital nerve (ION) stimulation for refractory facial pain. They concluded that percutaneous nerve stimulation (PNS) treatment was effective for TPHN involving the first branch. However, most previous studies have mainly focused on postherpetic neuralgia patients involving the first branch of the trigeminal nerve, while our study specifically targeted patients with postherpetic neuralgia involving the second and third branches of the trigeminal nerve.
Most of the international cases involve long-term electrical stimulation implantation, but considering the specific circumstances of our patients, we have chosen a short-term approach of trigeminal ganglion electrical stimulation implantation. During the electrode implantation, we placed three contact points in the oval foramen to ensure a wide coverage of the patient’s painful area and effectively prevented a reduction in the coverage range caused by electrode displacement. In addition, among these six patients, five patients achieved a pain relief rate of over 50% and there were no instances of electrode dislodgement in any of the patients prior to electrode removal. In one patient, the reduction in VAS score was less than 50%, which was deemed ineffective. Initially, the patient obtained immediate and satisfactory pain relief during electrode stimulation. After the removal of the electrodes however, the patient’s symptoms recurred. Due to his advanced age and financial difficulties, the patient declined the option of long-term electrical stimulation implantation. Furthermore, after a 24-week follow-up, the VAS scores of the remaining five patients had all reduced below 3, with one patient’s VAS score being of 0. Existing clinical data17 indicated that the longer the duration of postherpetic neuralgia (PHN), the lower the rate of effective pain relief following electrical stimulation therapy, potentially stemming from the pathological and physiological mechanisms underlying PHN. Consequently, multiple research findings advocate for the early implementation of neurostimulation techniques.9,18,19
Within this retrospective study, our focus extended beyond the amelioration of patients’ pain levels to analyze variations in pregabalin dosage as one of the efficacy evaluation criteria. Furthermore, we routinely assessed improvements in sleep quality and emotional well-being among the patients. For the patient whose treatment was ineffective, there were no notable changes in sleep quality or emotional issues. In the remaining five patients however, we observed enhanced sleep quality, a substantial decrease in medication dosage, as well as reduced anxiety and depression scores (P < 0.01).All published case series on trigeminal ganglion electrical stimulation (TGES) have reported incidents of skin infections. The electrodes and extenders used were not specifically designed for subcutaneous application on the face, unlike their counterparts intended for subcutaneous tissue in the lower back. Therefore, it is vital to reduce the lead diameter during the manufacturing process of neurostimulation devices to accommodate thinner tissue layers.
The complexity of the TGES procedure has impeded its extensive implementation in clinical practice. This study is obviously not perfect considering its small sample size and the lack of a control group. Consequently, future research endeavors should involve larger sample sizes and incorporate controlled studies to substantiate the effectiveness and safety of TGES as a treatment for postherpetic neuralgia (PHN) involving the second and third divisions of the trigeminal nerve.
Conclusion
The trigeminal ganglion electrical stimulation for postherpetic neuralgia has demonstrated effectiveness in reducing pain intensity, improving sleep quality, and alleviating anxiety and depression in patients with refractory trigeminal postherpetic neuralgia (TPHN) affecting the second and third branches of the trigeminal nerve.
Funding
Funding Project: Natural Science Foundation of Shanxi Provincial Science and Technology Department. Project Name: Study on the effects of leukocyte-rich and leukocyte-poor PRP on the proliferation ability of nucleus pulposus cells and inflammatory factors. Project No.: 201801D121346.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Sinofsky A, Sharma T, Wright T. Stellate Ganglion Block for Debilitating Photophobia Secondary to Trigeminal, Postherpetic Neuralgia. Pain Practice. 2016;16(7):E99–E102. doi:10.1111/papr.12471
2. Gan EY, Tian EAL, Tey HL. Management of Herpes Zoster and Post-Herpetic Neuralgia. Am J Clin Dermatol. 2013;14(2):77–85. doi:10.1007/s40257-013-0011-2
3. O’Neill F, Nurmikko T, Sommer C. Other facial neuralgias. Cephalalgia. 2017;37(7):658–669. doi:10.1177/0333102417689995
4. Bouhassira D, Chassany O, Gaillat J, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. PAIN. 2012;153(2):342. doi:10.1016/j.pain.2011.10.026
5. Johnson RW, Rice ASC. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi:10.1056/NEJMcp1403062
6. Liu B, Yang Y, Zhang Z, Wang H, Fan B, Sima L. Clinical Study of Spinal Cord Stimulation and Pulsed Radiofrequency for Management of Herpes Zoster-Related Pain Persisting Beyond Acute Phase in Elderly Patients. Pain Physician. 2020; 23(3):263–270.
7. Taub E, Munz M, Tasker RR. Chronic electrical stimulation of the gasserian ganglion for the relief of pain in a series of 34 patients. J Neurosurg. 1997;86(2):197–202. doi:10.3171/jns.1997.86.2.0197
8. Zhao L, Song T. Case Report: short-Term Spinal Cord Stimulation and Peripheral Nerve Stimulation for the Treatment of Trigeminal Postherpetic Neuralgia in Elderly Patients. Front Neurol. 2021;12:713366. doi:10.3389/fneur.2021.713366
9. Wan CF, Song T. Efficacy of Pulsed Radiofrequency or Short-Term Spinal Cord Stimulation for Acute/Subacute Zoster-Related Pain: a Randomized, Double-Blinded, Controlled Trial. Pain Physician. 2021;24(3):215–222.
10. Han R, Guo G, Ni Y, et al. Clinical Efficacy of Short-Term Peripheral Nerve Stimulation in Management of Facial Pain Associated With Herpes Zoster Ophthalmicus. Front Neurosci. 2020;14:574713. doi:10.3389/fnins.2020.574713
11. García-González AI, Rosas-Carrasco O. Herpes zoster and post-herpetic neuralgia in the elderly: particularities in prevention, diagnosis, and treatment. Gaceta Médica de México. 2017;153(1):92–101.
12. Reske-Nielsen E, Oster S, Pedersen B. HERPES ZOSTER OPHTHALMICUS AND THE MESENCEPHALIC NUCLEUS: a Neuropathological Study. Acta Pathologica Microbiologica Scandinavica Series A. 2009;94A(1–6):263–269. doi:10.1111/j.1699-0463.1986.tb02993.x
13. Truini A, Galeotti F, Haanpaa M, et al. Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study. Pain. 2008;140(3):405–410. doi:10.1016/j.pain.2008.08.018
14. Klein J, Sandi-Gahun S, Schackert G, Juratli TA. Peripheral nerve field stimulation for trigeminal neuralgia, trigeminal neuropathic pain, and persistent idiopathic facial pain. Cephalalgia. 2016;36(5):445–453. doi:10.1177/0333102415597526
15. Gupta K. Case Report: novel Anchoring Technique and Surgical Nuances for Trigeminal Ganglion Stimulation in the Treatment of Post-Herpetic Trigeminal Neuropathic Facial Pain. Front Pain Res. 2022;3:835471. doi:10.3389/fpain.2022.835471
16. Texakalidis P, Tora MS, Anthony CL, et al. Peripheral trigeminal branch stimulation for refractory facial pain: a single-center experience. Clin Neurol Neurosurg. 2020;194:105819. doi:10.1016/j.clineuro.2020.105819
17. Woolf CJ, Max MB. Mechanism-based Pain Diagnosis. Anesthesiology. 2001;95(1):241–249. doi:10.1097/00000542-200107000-00034
18. Yanamoto F, Murakawa K. The Effects of Temporary Spinal Cord Stimulation (or Spinal Nerve Root Stimulation) on the Management of Early Postherpetic Neuralgia from One to Six Months of Its Onset. Neuromodulation. 2012;4:151.
19. Xiao L. Early Treatment with Temporary Spinal CordStimulation Effectively Prevents Development of Postherpetic Neuralgia. Pain Phys. 2020;23(4;2):E219–E230. doi:10.36076/ppj.2020/23/E219
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.