Back to Journals » Vascular Health and Risk Management » Volume 17
Treatment of Chinese Patients with Hypertriglyceridemia with a Pharmaceutical-Grade Preparation of Highly Purified Omega-3 Polyunsaturated Fatty Acid Ethyl Esters: Main Results of a Randomized, Double-Blind, Controlled Trial
Authors Qi L, Zhang Q, Zheng Z, Pei Z, Mao H, Jiang T, Kazei D, Kahler E, Huo Y
Received 29 June 2021
Accepted for publication 26 August 2021
Published 15 September 2021 Volume 2021:17 Pages 571—580
DOI https://doi.org/10.2147/VHRM.S325217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Konstantinos Tziomalos
Litong Qi,1 Qiuling Zhang,2 Zeqi Zheng,3 Zhaohui Pei,4 Hong Mao,5 Tingbo Jiang,6 Dmitri Kazei,7 Elke Kahler,8 Yong Huo1
1Peking University First Hospital, Beijing, 100034, People’s Republic of China; 2The Affiliated Hospital of Hangzhou Normal University, Hangzhou City, Zhejiang, 310015, People’s Republic of China; 3The First Affiliated Hospital of Nanchang University, Nanchang City, Jiangxi, 330000, People’s Republic of China; 4The Third Hospital of Nanchang, Nanchang City, Jiangxi, 330000, People’s Republic of China; 5The Central Hospital of Wuhan, Wuhan City, Hubei, 430014, People’s Republic of China; 6The First Affiliated Hospital of Soochow University, Suzhou City, Jiangsu, 215006, People’s Republic of China; 7Abbott Healthcare Products BV, Weesp, 1381 CT, The Netherlands; 8Abbott Laboratories GmbH, Hannover, 30173, Germany
Correspondence: Yong Huo
Department of Cardiology, Peking University First Hospital, No. 8 Xishiku St. Xicheng District, Beijing, 100034, People’s Republic of China
Tel +86 139 01333060
Email [email protected]
Introduction: The lipid-modifying potential of omega-3 polyunsaturated fatty acids in Chinese patients is under-researched. We conducted a multicenter, randomized, placebo-controlled, double-blind, parallel-group study of twice-daily treatment with OMACOR (OM3EE), a prescription-only formulation of highly purified ethyl esters of omega-3 polyunsaturated fatty acids in Chinese adult patients (≥ 18 years) who had elevated baseline fasting serum triglycerides (TG).
Methods: Patients were stratified according to the severity of their hypertriglyceridemia (severe HTG, with baseline TG ≥ 500 and < 1000 mg/dL or moderate HTG, with baseline TG > 200 and < 500 mg/dL) or use of statins. Patients randomized to OM3EE therapy received 2 g/day for 4 weeks, then 4 g/day for 8 weeks. The primary efficacy endpoint was the percentage change in fasting serum TG between baseline and the end of treatment in patients with severe HTG. The study was concluded after a planned interim analysis demonstrated a significant TG-lowering effect of OM3EE in that contingent (p=0.0019).
Results: The mean TG end-of-treatment effect of OM3EE was – 29.46% (standard deviation 40.60%) in the severe HTG contingent compared with +0.26% (standard deviation 54.68%) in the placebo group. Corresponding changes were – 12.12% and – 23.25% in the moderate HTG and combination cohorts (vs +55.45% and +6.24% in relevant placebo groups). A dose-dependent reduction in TG was evident in all patient contingents. Safety and tolerability of OM3EE were in line with previous experience.
Discussion: These data indicate that OMACOR therapy at a dose of 2– 4 g/day is an effective treatment for Chinese patients with raised TG levels and is well tolerated.
Keywords: omega-3 PUFAs, hypertriglyceridemia, triglycerides, clinical trial, China, statin
Introduction
OMACOR® (OM3EE; Abbott) is a pharmaceutical-grade preparation of ethyl esters of the omega-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), formulated such that each 1-g capsule contains 460 mg of EPA and 380 mg of DHA.
This preparation has been extensively evaluated in ethnic European populations and shown, at doses of 2–4 g/day, to lower serum triglyceride (TG) levels.1 PUFAs have been associated in meta-analysis with significantly lower risks of myocardial infarction (MI), coronary heart disease (CHD) death, cardiovascular disease (CVD) death and total CVD events. Relationships between dose and degree of risk reduction were found for several outcomes: for example, every 1 g/day increment in EPA+DHA corresponded to 9% and 7% lower risks of MI and total CHD,2 respectively.
The rapid economic advances of China in recent decades have been characterized, among other things, by significant alterations in dietary habits, which have increased the number of people in China affected by hypertriglyceridemia (HTG) and substantially increased the burden of atherosclerotic disease.3 The data outlined above suggest that use of pharmaceutical-grade PUFAs to moderate TG levels is a logical response to these emerging trends,4 but it is desirable to have experience with these medications in Chinese patients before introducing them on a wide scale. As a contribution to this requirement, we undertook a 12-week, placebo-controlled, randomized, controlled trial designed to assess the efficacy and safety of OM3EE, used alone or in combination with a statin, in Chinese patients with HTG.
Methods
Study Design
This was a multicenter, randomized, double-blind, parallel-group study of twice-daily treatment with OM3EE or matching placebo (containing 1 g olive oil) in adult patients (≥18 years) who had baseline fasting serum TG levels in the range >200 to <1000 mg/dL despite adherence to diet and exercise interventions anticipated to normalize TG levels and the use of other measures, such as review of medications associated with the development of HTG. Dietary advice was based on the 2007 Guideline for the Prevention and Treatment of Dyslipidemia in Chinese Adults and the Consensus of Chinese Experts on Prevention and Treatment of Hypertriglyceridemia and included a recommended daily cholesterol intake <200 mg/day, saturated fatty acids <7% of total energy, increased intake of vegetables and high-quality protein, and restriction of alcohol intake.5
Patients already using a statin could continue medication if they had been on a stable dose for ≥3 months prior to enrolment. All other lipid-lowering drugs were withdrawn during a 4-week, single-blind, run-in period. Patients who satisfied all entry criteria at the end of the run-in period were randomized to OM3EE or placebo in one of three groups:
- OM3EE monotherapy in severe HTG (patients with baseline TG ≥500 and <1000 mg/dL; the sHTG cohort)
- OM3EE monotherapy in moderate HTG (patients with baseline TG ≥200 and <500 mg/dL; the mHTG cohort)
- Co-administration of OM3EE with existing statin therapy (the combination cohort).
Within each group, patients were randomized to OM3EE or placebo in a 1:1 ratio.
Following the placebo run-in period, all patients were randomly assigned to receive either OM3EE (2 g/day for 4 weeks, then 4 g/day for 8 weeks) or matching placebo. Lifestyle measures endorsed in Chinese guidelines on the management of dyslipidemia were maintained throughout the study.
The timetable of the study is shown in Figure 1.
 |
Figure 1 Study timeline. |
OM3EE and corresponding placebo capsules were supplied by Pronova Biopharma Norge AS (Oslo, Norway). Blinded and packaged medication was provided to each investigational site and dispensed to the patients. Randomization of patients to treatment group was performed using a centralized electronic system (WebEz). Stratified randomization was applied by site, treatment, and cohort to ensure equal balance in the 2 groups for the respective subgroups. The randomization scheme consisted of a 4-digit randomization number and the randomly allocated treatment group.
The study investigators were responsible for the conduct of the study and for the interpretation and reporting of its findings.
Endpoints
The primary efficacy endpoint was the percentage change in fasting serum TG between baseline and the end of treatment, assessed by analysis of covariance (ANCOVA). The model included treatment group and center as fixed effects and TG at baseline as covariate.
Secondary efficacy endpoints included changes in total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), non-HDL-cholesterol, change in the LDL:HDL ratio between baseline and end of treatment and the absolute change in TG between baseline and the end of treatment.
Safety was assessed based on treatment-emergent adverse events (TEAEs), laboratory measurements (hematology, biochemistry) and vital signs.
Statistical Analysis
Percent changes were first tested for normality and, if departure from normality was demonstrated, log-transformed data was used for statistical analysis. The primary cohort for assessment of this endpoint was the sHTG cohort, which is the main focus of this report. Comparisons were based on a two-sided (overall) significance level of 5%. A supportive efficacy and safety analysis was performed for the other two cohorts.
One interim analysis was performed by an independent unblinded statistical team to compare responses to treatment in the sHTG cohort. For that analysis, the O’Brien–Fleming α-spending function was used to control the overall type-one error at a level of 5% two-sided, with a nominal α-level of 0.00328 at the interim stage.
Ethics
The design and conduct of the study conformed to Chinese national regulations consistent with the principles of the Declaration of Helsinki and the provisions of Good Clinical Practice. The study protocol and all amendments were approved by appropriate local or regional independent ethics committees (N=18); written approval was also obtained from the provincial Office of Human Genetic Resources Administration. Written informed consent was obtained from each participating patient. All prospective participants were advised that they could leave the study at any time without explaining their reasons for doing so and without prejudice to their ongoing treatment.
This trial was registered prospectively on the Chinese clinical studies registry www.chinadrugtrials.org.cn (ID CTR20160569; in Chinese) and subsequently registered at www.clinicaltrials.gov (NCT04756180; in English).
Results
Clinical trial approval was granted by the Chinese Food and Drug Administration on 27 May 2015. The study was subsequently carried out between 14 October 2016 and 23 April 2019 in 10 provinces of China (Fujian, Guangdong, Hebei, Jiangsu, Jiangxi, Jilin, Shandong, Sichuan, Yunnan and Zhejiang), plus the municipalities of Beijing and Tianjin. Patients were recruited at 21 centers, details of which appear in Appendix 1.
A total of 728 patients initially consented to participate but 480 were excluded during screening. The assignment of patients to each of the three treatment cohorts is shown in Figure 2.
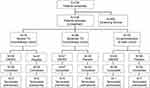 |
Figure 2 CONSORT diagram of the patient population of the study. |
The interim analysis was performed when 248 of the planned 324 patients had been enrolled. At that point, there were 76 patients in the sHTG cohort, 96 in the mHTG cohort and 76 in the combination cohort. Prior to this analysis, inspection of data identified a clear departure from normality for percentage change in TG. Accordingly, the absolute change in log-transformed TG values (ANCOVA) was used for the primary efficacy analysis. The ANCOVA analysis of percentage change was performed as a supplementary exploratory analysis.
The interim analysis demonstrated a significant TG-lowering effect of OM3EE compared with placebo in the sHTG cohort (p=0.0019; O’Brien–Fleming threshold p=0.00328). The study was concluded at that point and no further patients were enrolled.
Extended demographic data for the sHTG contingent are presented in Table 1. Corresponding lipid data for the mHTG and combination cohorts are shown in Supplementary Table 1. The pattern of concomitant medication use in the sHTG and mHTG cohorts was similar but was dissimilar to that in the combined therapy group, particularly in respect of antithrombotic agents, traditional Chinese medicines (TCM) and various forms of cardiac and antihypertensive medications (Supplementary Table 2).
 |
Table 1 Baseline Demographic, Lipid/Lipoprotein and Concomitant Medication Data for the sHTG Cohort |
All 76 patients in the sHTG cohort contributed to the efficacy analysis. These patients had a mean age of 50.9±11.4 years and the majority (n=66) were aged <65 years. Forty of these patients (52.6%) were male and all were classified as Asian. Mean baseline TG level was 632.7±111.0 mg/dL, mean body mass index was 25.9±3.1 kg/m2 and mean waist circumference was 90.3±7.7 cm. Forty of the sHTG patients (52.6%) had a history of hypertension, 20 (26.3%) had a history of hepatic steatosis and 12 (15.8%) had a history of diabetes mellitus. Use of concomitant allopathic medications reflected these pathologies (Table 1).
Among patients for whom OM3EE was co-administered with statin, hypertension (69.3%), coronary artery disease (34.7%) and diabetes mellitus (29.3%) were recorded more often than in other cohorts, with correspondingly higher rates of prescription of relevant drug therapies. The most frequently administered statins were atorvastatin (n=38) and rosuvastatin (n=23).
Eighteen sHTG patients (23.7% of the cohort) were using herbal remedies or TCMs. Use of TCMs was more widespread in the combination cohort (n=31 [41.3%], of which OM3EE, n=21).
Adequate compliance with study medication (defined as a value of 80–120% for the number of capsules that were actually taken relative to the number of capsules that should have been taken) was recorded for 93.3% of patients in the sHTG cohort, 86.2% in the mHTG cohort and 90.7% in the combination cohort.
Descriptive summary statistics of TG levels at baseline and end of treatment and of corresponding absolute and percentage changes from baseline for the sHTG cohort are shown in Table 2 and Figure 3. For the primary efficacy endpoint of absolute change in mean TG levels from baseline, the reduction in the OM3EE-treated patients was 180 mg/dL (–29.5%). No meaningful alteration in mean TG level was noted in the placebo group (+0.26% from baseline). OM3EE was associated with a statistically robust reduction in end-of-study TG levels (OM3EE:placebo ratio 0.68 [95% confidence interval 0.54–0.86]; p=0.0019 by ANCOVA). Other factors in the ANCOVA model (center and baseline TG levels) showed non-significant p-values (p>0.05). During the first 4 weeks of the study, when OM3EE was administered at a dose of 2 g/day, the mean percentage reduction in TG levels was 15.36% (mean change in placebo group +10.7%). Thus, doubling of the OM3EE dose after Week 4 was associated with a near doubling in the percentage reduction in TG. A similar dose-related increment in response to OM3EE was observed in the other two cohorts.
 |
Table 2 TG Responses in the sHTG Cohort. All Data are Presented in the Form Mean (Standard Deviation) |
The mean TG end-of-treatment effect of OM3EE was –12.12% and –23.25% in the mHTG and combination cohorts, respectively, compared with changes of +55.45% and +6.24% in the corresponding placebo groups. (For further details see Supplementary Table 3.) The comparison of mean changes in TG between active treatment and placebo in the mHTG group was inflated by very large (>200%) increases in TG in four placebo-treated patients but was still evident when those patients were excluded.
Among secondary endpoints, the placebo-adjusted reduction in mean TG during treatment was –32.48% in the sHTG cohort (p=0.0072), –61.96% in the mHTG cohort (p=0.0048) and –32.73% in the combination cohort (p=0.0094).
Analysis of effects of OM3EE therapy on non-TG lipid and lipoprotein indices in the primary analysis contingent of sHTG patients identified placebo-corrected statistically significant reductions in the levels of total cholesterol (p=0.0163 by ANCOVA, with center and treatment as fixed effects and level at baseline as covariate) and non-HDL cholesterol (p=0.0243 by same ANCOVA methodology), with no numerically large or statistically significant effects on other indices (Table 3). Corresponding data for the mHTG and OM3EE/statin cohorts are presented in Supplementary Table 4. None of the covariates had a significant effect on changes in TG in the two monotherapy cohorts but, in the combination cohort, patients with larger baseline values had higher reductions from baseline.
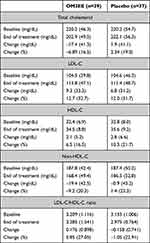 |
Table 3 Non-TG Lipid and Lipoprotein Responses in the sHTG Contingent. All Data are Presented in the Form Mean (Standard Deviation) |
Plans to explore responses according to the intensity of statin therapy (low, moderate or high) proved to be infeasible because almost all enrolled patients received therapy of moderate intensity (n=90).
Safety and Adverse Events
Principal safety findings for the safety population of 248 patients are summarized in Table 4. No substantial variations in major safety findings were identified between study cohorts (Supplementary Table 5).
The principal categories of TEAEs recorded in the pooled safety cohort were upper respiratory tract infection (n=8 [6.3%] in OM3EE-treated patients; n=13 [10.7%] in placebo-treated patients) and increased TG levels (n=4 [3.2%] in OM3EE-treated patients; n=12 [9.8%] in placebo-treated patients) (Table 4). Effects noted in single cohorts included dizziness, liver injury (predominantly elevated serum transaminases and/or gamma glutamyl transferase) and pharyngitis (n=2 each [5.1%]) in the sHTG cohort, and impaired fasting glucose or herpes zoster infection in the OM3EE/statin cohort (n=2 each [5.6%]) in the placebo group. Fewer TEAEs leading to study termination were recorded among OM3EE-treated patients than in the placebo group (n=2 [1.6%] vs n=4 [3.3%]). In all categories, the majority of TEAEs were regarded as being mild in severity.
The number of OM3EE-treated patients experiencing one or more TEAEs judged to have a reasonable likelihood of a causal relationship to study treatment was lower than with placebo (n=8 [6.3%] vs n=15 [12.3%]). Treatment-emergent serious adverse events (TESAEs) were recorded in nine patients treated with OM3EE (14 events, including one case each of acute MI and angina pectoris) and four treated with placebo (five events). None of these events were judged to be treatment-related.
In the whole study population, more patients taking concomitant antithrombotic drugs plus OM3EE (n=71) experienced SAEs (25.0% vs 14.3%), TESAEs (19.4% vs 8.6%) or severe TEAEs (13.9% vs 5.7%) than patients taking placebo. No comparable differences were evident in patients not using concomitant antithrombotics (n=177). No TEAEs with the primary classification of ‘Blood and lymphatic system disorders’ were recorded during the study. The international normalized ratio was within the normal range in >94% of patients throughout the study.
No patients died during the study.
Discussion
Severe HTG (≥500 mg/dL) requires an effective medical response because it increases the risks of acute pancreatitis and atherosclerotic CVD. Zhang et al identified TG >500 mg/dL as a significant risk factor for acute pancreatitis in a retrospective survey of seven Beijing hospitals6 and reported separately that all major deleterious clinical outcomes, including mortality, were related to TG levels on admission6 in a cohort of 1233 patients with acute pancreatitis. Deng et al have reported that, among Chinese patients hospitalized with pancreatitis (n=176), the presence of HTG at admission was associated with greater symptomatic severity.7
The risk of acute pancreatitis is most appreciable at TG levels >500 mg/dL. Cardiovascular risk, by contrast, is discernibly affected at lower TG levels (>250 mg/dL), which have been associated with increased risk of atherosclerotic CVD, even after adjustment for LDL-cholesterol levels.8
TG are regarded as an important secondary target in both assessment and treatment of cardiovascular risk, and a target level of <150 mg/dL was proposed in the 2001 US National Cholesterol Education Program on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) report. The 2004 update of those guidelines advocated the addition of TG-lowering therapy to a statin in patients deemed to be at high risk of CVD and who had persistently high TG levels after statin monotherapy. Similar principles have been incorporated into the latest Chinese expert guidelines9 and European recommendations.10 Our data indicate that OM3EE may be used for this purpose in Chinese patients, with expectations of similar effectiveness as in non-Chinese populations. This conclusion applies across the three cohorts of patients we studied.
Some attention should be given to clinical metabolic conditions that often accompany elevated TG. About a quarter of our patients had baseline diagnoses of diabetes mellitus. Such patients may derive particular benefit from control of HTG, as evidenced in the 23-year follow-up of the Daqing Diabetes Study,11 in which HTG in combination with diabetes (or impaired glucose tolerance) was associated with a 28% higher risk of a cardiovascular event than in normotriglyceridemic peers. The same study highlighted the interplay between hypertension and long-term cardiovascular risk,12 also a noteworthy finding given that half of our patients were classified as hypertensive at baseline.
With elevated TG, widespread concomitant hypertension and diabetes mellitus and a median waist circumference of 90 cm, many of our patients met the criteria for a diagnosis of metabolic syndrome (MetS), a condition estimated to affect >400 million adults in China13 and one associated with a higher 10-year risk of developing CHD. Studies such as SPECT-China14 indicate that, in women, the prevalence of MetS rises continuously throughout the adult years. Further investigation of the effects of OM3EE in older adults with MetS might therefore prioritize women, in whom TG levels continue to be a strong contributor to MetS-related cardiovascular risk. This would be consistent with proposals for a greater emphasis on the identification and management of dyslipidemia-related risk in post-menopausal Chinese women.15,16 Shen and Ge have recently set out ambitious proposals for a nationwide program to address the challenges posed to the health of people in China by the rising trajectory of CVD,3 and the government has recognized the importance of a comprehensive response in its Healthy China 2030 strategy. Our study may be regarded as a contribution towards those goals.
Use of PUFAs in combination with statins has been evaluated in meta-analysis to exert a range of nominally favorable effects on non-LDL lipid and lipoprotein indices, at the cost of some increase (versus statin alone) in gastrointestinal (GI) AEs. Experience in our own small contingent of patients treated with both OM3EE plus statin supports those effects on lipids and lipoproteins. No specific increase in GI-related AEs was seen but our sample size was small; any such effect would be consistent with the known profile of omega-3 PUFAs. Our overall findings for this patient group are congruent with the results of the ROMANTIC study, which evaluated lipid and lipoprotein responses in 201 patients with residual HTG treated with OM3EE plus rosuvastatin17 (although our findings on non-HDL-cholesterol did not pass the threshold for statistical significance), and with earlier research.18
The safety profile of OM3EE in this study was reassuring, and consistent with earlier experience in non-Chinese patients. OM3EE at a dose of 2 g/day has been reported to be safe and effective when administered for up to 1 year to patients with CHD.
One limitation of our methodology is that, while lifestyle modifications focused on diet and exercise were implemented in our study, compliance was not formally assessed, and so we are unable to comment on their contribution to the results. We nevertheless endorse the view that an emphasis on these measures should form part of the real-world response to HTG in the Chinese population.15 Ours was also a small study of short duration and patient numbers were further reduced by the early completion of the trial following the clear demonstration of a statistical superiority of OM3EE over placebo in our designated primary analysis cohort at a scheduled interim analysis. We have, however, no reason to doubt the statistical resilience of our analysis and are confident that these findings represent a definitive demonstration of the TG-lowering efficacy of OM3EE, at doses of 2–4 g/day, in Chinese patients.
Conclusion
The results of our study indicate that OM3EE therapy at a dose of 2–4 g/day is an effective treatment for Chinese patients with raised TG levels and is well tolerated. EPA and DHA have complementary effects on TG levels and on the wider lipoprotein profile and atherogenic milieu19,20 and our efficacy data, including from the patients treated with OM3EE plus statins, are consistent with the assessment of the American Heart Association that EPA and DHA represent “an effective and safe option for reducing triglycerides as monotherapy or as an adjunct to other lipid-lowering agents”.21
Data Sharing Statement
All relevant data are within the paper and its Supplementary Materials.
Acknowledgments
The investigators express their appreciation to the patients who agreed to participate in this study, which was supported by Abbott Products Operations AG, Allschwil, Switzerland. Hughes associates, Oxford, UK, provided editorial assistance in the preparation of this report.
Disclosure
L.Q., Q.Z., Z.Z., Z.P., H.M., T.J. and Y.H. report no conflicts of interest in this work. E.K. is an employee of Abbott Laboratories GmbH, Hannover, Germany and owns shares of Abbott. D.K. is an employee of Abbott Healthcare Products B.V., Weesp, The Netherlands and owns shares of Abbott. The authors report no other conflicts of interest in this work.
References
1. Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016;15(1):118. doi:10.1186/s12944-016-0286-4
2. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19):e013543. doi:10.1161/JAHA.119.013543
3. Shen C, Ge J. Epidemic of cardiovascular disease in China: current perspective and prospects for the future. Circulation. 2018;138(4):342–344. doi:10.1161/CIRCULATIONAHA.118.033484
4. Kris-Etherton PM, Richter CK, Bowen KJ, et al. Recent clinical trials shed new light on the cardiovascular benefits of omega-3 fatty acids. Methodist Debakey Cardiovasc J. 2019;15(3):171–178.
5. Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(4):390–419. Chinese.
6. Zhang XL, Li F, Zhen YM, Li A, Fang Y. Clinical study of 224 patients with hypertriglyceridemia pancreatitis. Chin Med J (Engl). 2015;128(15):2045–2049. doi:10.4103/0366-6999.161361
7. Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14(28):4558–4561. doi:10.3748/wjg.14.4558
8. Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–2275.
9. China Cholesterol Education Program. [Chinese expert consensus on hypertriglyceridemia and hypertriglyceridemia-related cardiovascular risk management]. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;45(2):108–115. Chinese. doi:10.3760/cma.j.issn.0253-3758.2017.02.008
10. Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi:10.1016/j.atherosclerosis.2019.08.014
11. Wang J, Shen X, He S, et al. Hypertriglyceridaemia predicts subsequent long-term risk of cardiovascular events in Chinese adults: 23-year follow-up of the Daqing Diabetes Study. Diabetes Metab Res Rev. 2019;35(6):e3163. doi:10.1002/dmrr.3163
12. Li X, Wang J, Shen X, et al. Higher blood pressure predicts diabetes and enhances long-term risk of cardiovascular disease events in individuals with impaired glucose tolerance: twenty-three-year follow-up of the Daqing diabetes prevention study. J Diabetes. 2019;11(7):593–598. doi:10.1111/1753-0407.12887
13. Lu J, Wang L, Li M, et al. 2010 China Noncommunicable Disease Surveillance Group. Metabolic syndrome among adults in China: the 2010 China noncommunicable disease surveillance. J Clin Endocrinol Metab. 2017;102(2):507–515. doi:10.1210/jc.2016-2477
14. Jiang B, Zheng Y, Chen Y, et al. Age and gender-specific distribution of metabolic syndrome components in East China: role of hypertriglyceridemia in the SPECT-China study. Lipids Health Dis. 2018;17(1):92. doi:10.1186/s12944-018-0747-z
15. Pan L, Yang Z, Wu Y, et al. China National Survey of Chronic Kidney Disease Working Group. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. 2016;248:2–9. doi:10.1016/j.atherosclerosis.2016.02.006
16. Xia S, Du X, Guo L, et al. Sex differences in primary and secondary prevention of cardiovascular disease in China. Circulation. 2020;141(7):530–539. doi:10.1161/CIRCULATIONAHA.119.043731
17. Kim CH, Han KA, Yu J, et al. Efficacy and safety of adding omega-3 fatty acids in statin-treated patients with residual hypertriglyceridemia: ROMANTIC (Rosuvastatin-OMAcor iN residual hyperTrIglyCeridemia), a randomized, double-blind, and placebo-controlled trial. Clin Ther. 2018;40(1):83–94. doi:10.1016/j.clinthera.2017.11.007
18. Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega-3-acid ethyl esters on non–high-density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. 2010;85(2):122–128. doi:10.4065/mcp.2009.0397
19. Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13(6):474–483. doi:10.1007/s11883-011-0210-3
20. Asztalos IB, Gleason JA, Sever S, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: a randomized clinical trial. Metabolism. 2016;65(11):1636–1645. doi:10.1016/j.metabol.2016.07.010
21. Skulas-Ray AC, Wilson PWF; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140(12):e673–e691. doi:10.1161/CIR.0000000000000709
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


