Back to Journals » Journal of Pain Research » Volume 17
Treating Chronic, Intractable Pain with a Miniaturized Spinal Cord Stimulation System: 1-Year Outcomes from the AUS-nPower Study During the COVID-19 Pandemic
Authors Salmon J, Bates D, Du Toit N, Verrills P, Yu J, Taverner MG, Mohabbati V, Green M, Heit G, Levy R, Staats P, Kottalgi S , Makous J, Mitchell B
Received 25 August 2023
Accepted for publication 9 January 2024
Published 21 January 2024 Volume 2024:17 Pages 293—304
DOI https://doi.org/10.2147/JPR.S436889
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Andrea Tinnirello
John Salmon,1 Daniel Bates,2 Neels Du Toit,2 Paul Verrills,2 James Yu,3 Murray G Taverner,4 Vahid Mohabbati,5 Matthew Green,6 Gary Heit7 ,† Robert Levy,8 Peter Staats,9 Shilpa Kottalgi,10 James Makous,11 Bruce Mitchell2
1Pain Management, Pain Care Perth, Perth Cottesloe, WA, Australia; 2Pain Management, Metro Pain Group, Melbourne, VIC, Australia; 3Pain Management, Sydney Spine and Pain, Sydney, NSW, Australia; 4Pain Management, Frankston Pain Management, Frankston, VIC, Australia; 5Pain Management, Sydney Pain Management Centre, Sydney, NSW, Australia; 6Pain Management, Pain Medicine of South Australia, Adelaide, SA, Australia; 7Department of Neurosurgery, Hue University of Medicine and Pharmacy, Hue, Vietnam; 8Neurosurgery, Institute for Neuromodulation, Boca Raton, FL, USA; 9Premier Pain Centers, Shrewsbury, NJ, USA; 10Clinical Department, Nalu Medical, Inc, Carlsbad, CA, USA; 11Makous Research, LLC, Carlsbad, CA, USA
†Gary Heit passed away in February 2023
Correspondence: John Salmon, Pain Management, Pain Care Perth, 2/89 Forrest Street, Perth Cottesloe, WA, 6011, Australia, Tel +61 428 246 846, Fax +61 8 92845759, Email [email protected]
Purpose: Spinal cord stimulation (SCS) is a highly effective treatment for chronic neuropathic pain. Despite recent advances in technology, treatment gaps remain. A small SCS system with a miniaturized implantable pulse generator (micro-IPG; < 1.5 cm3 in volume) and an externally worn power source may be preferred by patients who do not want a large, implanted battery. We report here the long-term outcomes from the first-in-human study evaluating the safety and performance of a new neurostimulation system.
Patients and Methods: This was a prospective, multi-center, open-label, single-arm study to evaluate this SCS system, in the treatment of chronic, intractable leg and low-back pain. Consented subjects who passed screening continued on to the long-term phase of the study. One-year, patient-reported outcomes (PRO’s) such as pain (Numeric Rating Scale, NRS), functional disability, quality of life, and mood were captured.
Results: Twenty-six (26) evaluable subjects with permanent implants were included in this analysis. The average leg pain NRS score decreased from 6.8 ± 1.2 at baseline to 1.1 ± 1.2 at the end of the study (p < 0.001), while the average low-back pain NRS score decreased from 6.8 ± 1.2 to 1.5 ± 1.2 (p < 0.001). The responder rate (proportion with ≥ 50% pain relief) was 91% in the leg(s) and 82% in the low back. There were significant improvements in functional disability (Oswestry Disability Index) and in mood (Beck Depression Inventory), demonstrating a 46% and 62% improvement, respectively (p < 0.001). Eleven-point Likert scales demonstrated the wearable to be very comfortable and very easy to use.
Conclusion: There were considerable challenges conducting a clinical study during the COVID-19 pandemic, such as missed study programming visits. Nevertheless, subjects had significant PRO improvements through 1-year. The small size of the implanted device, along with a proprietary waveform, may allow for improved SCS outcomes and a drop in incidence of IPG-pocket pain.
Plain Language Summary: Chronic neuropathic pain of the low back and legs is a common affliction. Unfortunately, many of these cases are medically intractable and require more invasive treatments. One such treatment is spinal cord stimulation, in which very small, cylindrical electrodes (leads) are percutaneously introduced into the epidural space where they deliver mild electrical pulses to the spinal cord thereby blocking the pain signals from reaching the brain. Among the shortcomings of this general approach is the bulkiness of the implanted battery and electronics that power the electrodes, which can be as large as 44 cm3 in volume. We report here the results of a study employing a very small spinal cord stimulator implant (< 1.5 cm3 in volume) that is externally powered by a wearable device. The results of this multi-center, prospective, open-label, single-arm study are reported here. Following 1 year of spinal cord stimulation therapy, the average pain reduction was 79% in the legs and 81% in the back, both of which were statistically significant at the p< 0.001 level. At the same study endpoint, patient-reported outcomes demonstrated significant improvements in functional disability (p < 0.001) and mood (p < 0.05). These outcomes are comparable to many of the other larger, fully implantable systems.
Keywords: implantable pulse generator, IPG, battery free, neuropathic pain, pulsed stimulation pattern, micro-IPG, persistent spinal pain syndrome, failed back surgery syndrome, FBSS
Introduction
Spinal cord stimulation (SCS) is a highly effective treatment for chronic neuropathic pain that is not adequately managed with medical treatments. The most common indications for SCS are Persistent Spinal Pain Syndrome (PSPS) and complex regional pain syndrome (CRPS),1–4 although a wide range of pain etiologies are amenable to treatment.5,6 A search for SCS case reports in the National Library of Medicine’s PubMed yielded nearly 1500 titles. Current estimates of yearly implantation may exceed 36,000 in the Medicare population alone.7 Compared to other therapeutic options such as neurosurgical ablative procedures and implanted drug pumps, SCS may be preferable due to its minimally invasive, reversible, and drug-free nature.8
SCS exerts its neuromodulatory effects by delivering mild electrical pulses in the epidural space above the dorsal column, of the spinal cord, to create an energy field that can alter the transmission of pain signals to the brain.9 Traditional SCS systems typically deliver a specific stimulation pattern or waveform to tap into a hypothetical mechanism of action (MOA).9 Recent estimates suggest that 50% to 80% of patients may respond well to SCS therapy, defined as a 50% reduction in pain compared to baseline, prior to intervention.10–12 Even so, therapy gaps remain. A lack of therapeutic benefit is the motivation for device removal in 44% to 65% of explants, according to three studies.13–15 In addition, adverse events (AEs) such as infection and IPG-pocket pain may also necessitate surgical revision or removal.16–20
This micro-IPG system supports a variety of lead configurations that support SCS and peripheral nerve stimulation applications. For this study, standard 8-contact leads were used. The system uses a unique, proprietary pulsed stimulation pattern (PSP), which delivers therapy in a paresthesia-independent manner. PSP is a unique, layered therapeutic approach that allows each of three stimulation layers (pulse patterns, trains, and dosages) to be adjusted in a hierarchical structure to individualize therapy for each patient. Each of these layers may simultaneously access different potential MOAs, providing multiple pathways of relief.21 The system also provides advanced programming features such as scheduling, layering, and current steering as well as standard Tonic waveforms.
Ninety-day (90-day) results from this population of subjects have previously been published,22 which showed that PSP treatment relieved both low-back and leg pain, with comparable efficacy to other commercially available SCS treatment options. This report documents the long-term safety and efficacy of this SCS system, delivering PSP, in the same population of subjects.
Materials and Methods
This was a prospective, multi-center, open-label, trial conducted in accordance with Good Clinical Practice guidelines, ISO 14155 and the Declaration of Helsinki. The study was approved by an independent Ethics Committee (Bellberry Ltd, SA, Australia) and registered publicly on the Australian New Zealand Clinical Trials Registry (ANZCTR); registration number ACTRN12618001862235. Subjects were recruited from 6 pain-management practices across Australia and gave their written informed consent prior to any study activities. A previous publication described the initial 90-day outcomes for these subjects.22 This study involved a miniaturized implantable pulse generator (micro-IPG) with a volume of less than 1.5 cm3. The micro-IPG is powered by an externally worn battery known as a Therapy Disc (TD; Nalu Neurostimulation System, Carlsbad, CA, USA). The TD can store up to 8 stimulation programs and provides two-way communication with the micro-IPG via a radiofrequency (RF) link. The two-way communication allows impedance checks and verification that treatment is indeed being delivered. The TD is placed over the micro-IPG site and affixed to the skin with an adhesive clip or a custom belt, provided by the manufacturer (See Malinowski et al23 for device details).
Eligible subjects had predominantly neuropathic pain, of the legs and/or low back, with a diagnosis of PSPS. To meet the inclusion criteria, the pain had to be chronic (defined as more than a 6-month duration), with an intensity of ≥6 on a 0–10 numeric rating scale (NRS; 0 = no pain, 10 = worst pain imaginable), collected via a pain diary. Subjects were unresponsive to conservative treatment and were able to appropriately place an adhesive clip on their back, to hold the TD in place. Subjects were excluded from participation in the study if they had previously failed SCS, were sensitive to adhesives worn on the skin, were taking greater than 120 mg-morphine equivalents per 24 hours, had mechanical spine instability, or had comorbidities or circumstances that could interfere with study participation.
Study assessments were completed prior to implant (baseline) and at multiple points following the activation of the permanent system. Follow-up visits were completed at protocol-specified time points, including 90 days, 185 days, and 1 year, reported here. The widespread healthcare disruptions caused by the COVID-19 pandemic24 made it exceptionally challenging for the subjects to attend follow-up visits in the clinic. Consequently, many data collection points were missing, and a Last Observation Carried Forward (LOCF) analysis was completed to allow for all evaluable subjects to be included in the 1-year endpoint.
Prior to the SCS trial phase, subjects were asked to undergo a wearability assessment (for a minimum of 7 days) in which an inactive Therapy Disc (TD), and affiliated adhesive clip, were worn to identify the ideal location of the micro-IPG (<1.5 cm3). Based upon TD comfort and ease of placement, the preferred position of the adhesive clip and disc on the torso was documented in collaboration with the implanting physician.
Subjects underwent a trial phase in one of two ways: 1) temporary leads were placed and then removed at the end of the trial (temporary trial), or 2) the full micro-IPG system was implanted to be left in after a successful trial (IPG trial; see Salmon et al22 for details). Subjects returned to the clinic after a 7–21 day trial period. If a subject reported a 50% or greater pain relief relative to their baseline diary, they were considered a responder and moved into the long-term phase of the study, which included a micro-IPG implant in the case of the temporary trial. In the case of a successful IPG-trial, the subject kept the micro-IPG. Non-responders from a temporary trial were exited from the study, whereas non-responders after an IPG trial were followed for safety, as they still kept the device.
In all study subjects, two eight-contact leads were implanted. Each lead was inserted into the epidural space and advanced such that the contacts of both leads spanned the T9 vertebral body. Lead placement was typically completed using anatomic landmarks and fluoroscopy, rather than intra-operative paresthesia mapping to guide placement. At the time of micro-IPG implant, a small, 1.5-cm-long incision was made to create a miniature IPG pocket, to accommodate the size of the micro-IPG (See Malinowski et al23 and Salmon et al22 for a detailed description of the implant procedure). Impedance measurements were always taken prior to closing to ensure communication and proper device function.
By modulating a fundamental carrier frequency with multiple patterning envelopes, stimulation was optimized for each subject with this paresthesia-independent waveform (See Desai et al21 for a detailed description of the PSP waveform). Subjects were able to choose among customized, pre-specified PSP programs with a remote-control application loaded on their smart phone.
Subjects underwent multi-modal screening assessments to verify their suitability for treatment with the SCS system and for inclusion in the study (see above for eligibility criteria). At pre-implant baseline, subjects completed the in-office assessments regarding pain (100-mm visual analog scale [VAS], 0–10 numeric rating scale [NRS],25 Brief Pain Inventory [BPI]),26 functional disability (Oswestry Disability Index [ODI]),27 quality of life (EuroQol questionnaire [EQ-5D-5L])28 mood (Beck Depression Inventory [BDI]).29 At post-implantation follow-up visits, these PROs were repeated, along with device-comfort and ease-of-use questionnaires. A 7-point Likert scale (Patient Global Impression of Change [PGIC])30 was also completed. An NRS pain diary was also completed during the 7 days prior to each study visit, including baseline. Adverse events were collected at all follow-up visits.
Statistical Analysis
Study data were recorded in an electronic data capture system, which was periodically monitored by Sponsor representatives. As this was a first-in-human trial, no a priori sample size calculations were performed. Study data were analyzed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA). Unless otherwise stated, descriptive statistics were expressed as means ± 1 standard deviation (SD) and percentages. Hypothesis testing was conducted using t-tests based on paired data accepting significance as p<0.05. The Australian COVID-19 restrictions wrought havoc with the data collection, primarily at the 1-year follow-up visits, where only 12 subjects had available data at that endpoint. This, in turn, led us to add the Last Observation Carried Forward (LOCF) strategy to the outcomes, allowing a direct comparison between both the 1-year and LOCF endpoints. The last collected data point was carried forward to 1-year, for subjects who had missed the protocol specified 1-year visit, as long as they still had an implant. Hence, outcomes of one patient who had their device explanted, due to infection were not included in the LOCF analysis.
Results
A total of 35 subjects were enrolled across 6 comprehensive pain centers in Australia. Eighty-nine percent (89%) of subjects (31/35) had successful trials with ≥50% pain relief compared to baseline. Three additional subjects were withdrawn for study non-compliance post-trial and 1 subject was excluded post-trial due to COVID-19 restrictions (Figure 1). During the study, 1 subject was excluded from analysis due to lack of adherence to study requirements, thus leaving a total of 26 evaluable subjects. The long-term visits (>90 days post-device activation) saw the most missing data, largely due to pandemic-related issues (see Figure 1). The group was made up of 46% male subjects, and the average age was 61.0 years (range: 36–82; see Table 1).
 |
Table 1 Patient Baseline Demographics and Clinical Characteristics |
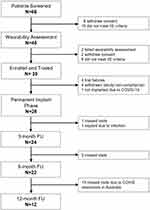 |
Figure 1 Study subject flow. |
Analysis of the NRS pain outcomes captured in each subject’s pain diary is shown in Figure 2 as a function of follow-up visits from the low back and the leg. Both leg and low-back average NRS-diary-pain scores were 6.8 at baseline (11-point, NRS scale) prior to intervention, and they dropped (improved) to average scores of 1.4 in the leg and 1.7 in the low back, at 90 days (p < 0.001). This significant pain relief was maintained through all time points (p < 0.001) with the following NRS averages in the leg: 1.3 at 185 days, 1.1 at 1 year, and 1.5 in the LOCF analysis. In the low back, the NRS averages were as follows: 1.5 at 185 days, 1.5 at 1 year and 1.6 in the LOCF analysis. Each pair-wise comparison between baseline scores and the scores at each of the follow-up visits was statistically significant (p < 0.001).
In terms of NRS responder rates (≥50% NRS improvement relative to baseline), outcomes at each time point, in both the leg and back, were at or above 78%. Responder rates in the leg were as follows: 83% at 90 days, 86% at 185 days, 91% at 1 year and 80% for the LOCF analysis. Responder rates in the low back were as follows: 78% at 90 days, 86% at 185 days, 82% at 1 year, and 83% for the LOCF analysis. High responder rates (≥80% improvement) at 1 year were 55% and 45% in the legs and back, respectively, and high responder rates, based upon the LOCF analysis, were 52% in the leg and 50% in the low back. The percent drop in NRS pain scores relative to baseline ranged from 78% to 82% in the leg and from 73% to 76% in the back, looking across all follow-up visits including the LOCF analysis. The percent drop in leg NRS pain scores were as follows: 79% at 90 days, 81% at 185 days, 82% at 1 year, and 78% for the LOCF analysis. The percent drop in low-back NRS pain scores were as follows: 73% at 90 days, 76% at 185 days, 76% at 1 year, and 74% for the LOCF analysis.
BPI scores and the 24-hour VAS scores are captured in Table 2. The BPI severity scores and interference scores (which are “overall” pain scores) were both 6.6 and 6.6 at baseline, compared to 1.6 and 1.9, respectively, at 1 year. In-office 24-hr VAS scores were also captured. At the baseline, the average 24-hr VAS was 78.1 for the leg and 64.2 for the low back. At 90 days, these VAS scores fell to 14.6 in the leg and 15.5 in the low back, whereas they dropped to 9.3 in leg and 6.3 in low back at 1 year (p < 0.001). The 1-year LOCF analysis yielded VAS scores of 16.4 in the leg and 12.4 in the low back. With respect to VAS responder rates, for the leg these ranged from 88% to 100%, and for the low back, responder rates ranged from 83% to 100%. VAS high responder rates (≥80% improvement) were 75% and 83%, in the leg and the low back, respectively, at 1 year.
 |
Table 2 Pain Scores Captured with BPI and VAS |
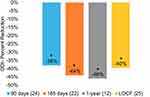
Findings with respect to functional disabilities (ODI) and mood (BDI) also showed strong and statistically significant improvements. Improvements in ODI ranged from 38% at 90 days to 46% at 1 year, whereas the LOCF analysis showed a 40% improvement (p < 0.001; Figure 3). When looking at Table 3, 62% (16/26) of subjects reported severe or crippling disability at baseline. However, by 1 year 0% of subjects reported severe or crippling disability. In the case of the LOCF analysis, 12% (3/25) of the subjects remained either severely disabled or crippled. The Minimal Clinically Important Difference (MCID) shown for ODI is a 10-point change.31 Similarly, the BDI improvements in mood ranged from 48% at 90 days to 62% at 1 year, and the LOCF analysis showed a 53% improvement (Figure 4). The BDI MCID (dashed line) displayed in Figure 4 is 17.5%.32 Table 4 shows that 52% (13/25) of subjects were mildly, moderately or severely depressed at baseline. By 1 year only 16% (2/12) fell into these three categories, and the LOCF analysis found only 12% (3/25) in these three categories.
 |
Table 3 Oswestry Disability Index |
 |
Table 4 Beck Depression Inventory |
EQ-5D-5L was used to record quality of life (QOL); change in QOL was measured on a VAS scale (0–100 mm; 0 = worst imaginable health, 100 = best imaginable health). QOL VAS scores improved by 70% at 90 days (p < 0.05) and 77% (p < 0.001) at 1-year with the LOCF analysis yielding a 67% (p < 0.001) improvement from baseline. A combination of individual dimension scores of the EQ-5D-5L were converted into an index value for health status (0 = worst possible health, 1 = best possible health), which reflects the health state according to the preferences of the general population of a country/region. Index values were calculated based on general population valuation surveys, in various countries. Index values are not available for the general population in Australia; hence, the US value set was used as a comparison. The EQ-5D-5L mean index value at baseline was 0.56 ± 0.15 and increased to 0.77 ± 0.10 at 90 days (p < 0.001); this further increased at 1-year to 0.84 ± 0.09 at 1-year (p < 0.001). The LOCF analysis reported the mean index value at 0.78 ± 0.14, which was also statistically significant at p < 0.001. The closer the mean index value is to 1.0, the better the QOL of the population.
The PGIC LOCF analysis (Figure 5) showed that 96% of subjects found themselves as feeling very much improved (60%), much improved (20%) or minimally improved (16%) at 1 year. Hence, 96% of subjects demonstrated a favorable MCID impression of change.33 The remainder of the subjects demonstrated no change (4%). No subjects (0%) reported a decline in their global impression.
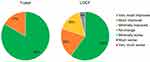 |
Figure 5 Patient Global Impression of Change (PGIC). Left) PGIC at 1 year following device activation. Right) analysis at 1 year. Abbreviation: LOCF, Last Observation Carried Forward. |
Subjects were asked to rate the ease of use and comfort of the wearable aspects (adhesive clip and TD) of the device on an 11-point Likert scale, where 0 was very comfortable/very easy to use, and 10 was very uncomfortable/very difficult to use. At 90 days and at 1 year, 75% (18/24) and 73% (8/11), respectively, found the device to be very comfortable (Likert scores of 0). At 90 days, 88% (21/24) found the device very easy to use, whereas at 1 year, 92% (11/12) found it very easy to use (Likert scores of 0).
Since the 3-months results were published,22 11 additional (9 subjects) non-serious adverse device effects were reported, which were typical of SCS systems.34 One of these remained unresolved at the time of publication. This subject reported mild, tender lumps (lead anchors) around the midline scar and was prescribed a topical cream. There continued to be no reports of pocket pain associated with the micro-IPG. Four (4) additional SAEs were reported, none of which were device and/or procedure related.
Discussion
This single-arm, prospective, observational, study followed subjects implanted with the micro-IPG system for 1 year. All subjects were given the option of multiple therapeutic waveforms including tonic and the novel, paresthesia-independent PSP waveform, with the amplitude optimized for each subject. All subjects preferred the PSP waveform over the more traditional Tonic therapy, during the long-term follow-up stage of this study.
The 1-year follow-up portion of this study was, in large part, managed during the peak of the COVID-19 pandemic. As such, the study subjects had limited contact with the clinicians and limited access to device/programming support, due to quarantine and travel restrictions. This, in turn, led to a paucity of long-term outcomes, although attempts were made to collect missing data from all subjects. Given what appeared to be a therapeutic carry-over effect of PSP stimulation, many subjects choose to self-dose their therapy, only using the system when they felt a return of pain, sometimes lasting up to two weeks or more. In these instances, subjects often failed to complete a pain diary since they may not have been using the system at the study-dictated time. It may well be the case that these patients who are only applying the minimal amount of stimulation to maintain therapeutic effect are thereby avoiding stimulation habituation issues that can sabotage long-term outcomes in fully implanted patients.
In this study, 12 of 22 subjects were followed through 1 year and overall they demonstrated statistically significant improvements in pain, functional disability, quality of life, and depression at all study follow-up visits. In addition to being robust and consistent with previous reports,21,22 the effects were durable and maintained through the duration of the study. The confluence of similar trends across multiple domains of patient-reported outcomes suggests that the treatment effects are real and important.
The 1-year results captured here were comparable to recent Randomized Controlled Trials (RCTs) with other SCS devices12,35,36 (Table 5). All of the novel waveforms and newer therapies appear to yield similar results in terms of pain reduction and responder rates. In the current study, the responder rates ranged from 78% (low back at 90 days) to 91% (leg at 1 year) when looking at diary-based NRS (Figure 2) across all study time points. When considering the VAS-based responder rates at 1 year (Table 2 and Table 5), the LOCF analysis showed 88% both in the leg and the low back. The average percent pain reduction (VAS) at 1 year (LOCF analysis) was 81% in the back and 79% in the leg (Table 5). Responder rates among the 3 RCTs, shown in Table 5, were similar to the current findings ranging from 79 to 83% in the leg and 79 to 84% in the back; whereas, percent pain reduction in the leg ranged from 70 to 76% and in the low back ranged from 67 to 76%. In general, the outcomes reported here appeared to be slightly better than the RCTs cited in Table 5. These differences were on the order of a few to several percentage points; however, these differences are unlikely to be clinically meaningful.
 |
Table 5 1-Year VAS Pain Outcomes Compared to Published RCTs |
Despite considerable advances in lead anchoring methods, rechargeable battery technology and novel stimulation waveforms/patterns, typical IPGs remain relatively large in volume ranging from 1437 to 4038 cm3. These large devices implanted just below the skin can lead to pocket pain and infection via erosion, which in turn can lead to explant or revision surgery.39 For example, reports of pocket pain can be as high as 64% according to one report.40 The small size of the micro-IPG may reduce the incidence of pocket pain, as reported in one case41 and corroborated in the current study, in which there were no reports of micro-IPG pocket pain. At the time of the writing of this manuscript there were no reports of pocket pain associated with this micro-IPG in the peer-reviewed literature. The risk of infection is also considered to have a significant relationship to the size of the IPG, and although the sample size in this study was too small to demonstrate an advantage it can be anticipated that infection risk will be markedly reduced when utilizing the micro-IPG.
This neurostimulation system is composed of a microstimulator devoid of an implanted power source; rather, it is powered by an externally worn TD, which is radio frequency (RF) coupled to the micro-IPG. The micro-IPG contains microchip technology, which allows the generation of a family of complex pulsed stimulation patterns (PSP). The micro-IPG generates the electrical stimulation pulses inside the implant, unlike implants that are simple passive receivers.
There are additional reasons for explantation of traditionally sized IPGs beyond those mentioned above. For example, 22% of reportable events related to traditionally sized IPGs, in the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database,42 arise from battery issues. Even in the world of rechargeable IPGs, there remains a limited life of 7.2 years,43 whereas primary-cell IPGs last only 3.7 years.43 However, in the case of the micro-IPG, there is an 18-year service life,44 facilitated by the fact that there is no implanted battery – the battery resides in the TD worn on the skin, over the micro-IPG. Additional explants of traditionally sized IPGs arise from a need to upgrade those traditional SCS systems with newer software and/or newer technology. In the case of the micro-IPG system, the software can be upgraded via the TD, removing the need for surgery.
The bulkiness of the traditional IPGs can also limit the options in terms of implant location. This micro-IPG, with a volume of up to 27 times smaller than conventional IPGs, at <1.5 cm3, provides many more options for implant location. In fact, prior to surgery, the subjects and implanting physician choose the best micro-IPG location based upon the comfort of the subject and the surgical considerations of the implanter. Subjects in this study rated the device, including the externally worn TD, to be very comfortable. This remained consistent throughout the 1-year reporting period of this study.
Despite its small size, this micro-IPG system can deliver multiple-complex waveforms, including a family of novel PSPs. The PSP is paresthesia independent and is postulated to operate under multiple mechanisms of action (MOA). PSP is a layered waveform that allows each of three stimulation layers (pulse patterns, trains, and dosages) to be adjusted in a hierarchical structure to individualize therapy for each patient (see Desai et al21 for a detailed description of the PSP waveforms).
Limitations of this study include its relatively small sample size and instances of missing data, which was unavoidable due to the COVID-19 pandemic. Last observation carried forward (employed here) is a common statistical approach used in the analysis of longitudinal repeated measures data where some follow-up observations may be missing. In this analysis, a missing follow-up visit value was replaced by a subject’s previously (most recently) observed value. The combination of the observed and imputed data was then analyzed as though there were no missing data. Unfortunately, an inherent assumption of the LOCF approach is that all the samples (actual and imputed) are drawn from the same distribution, which may not be the case. Therefore, caution should be exercised when interpreting the results of the LOCF analysis.
Despite the global pandemic, the favorable outcomes reflect the flexibility that many pain physicians were obliged to adopt to maintain good patient care.24 Importantly, the fact that excellent clinical outcomes were observed despite massive worldwide disruptions in healthcare, including the inability to provide additional re-programming visits, provides empirical validation of this therapy through 1 year of treatment.
Conclusions
The favorable results reported here demonstrate the long-term sustainability of this therapy. Outcomes are comparable to recent large RCTs, showing greater than 80% responder rates and greater than 70% pain reduction at 1 year. Alternate PROs such as functional disability and mood also displayed impressive improvements out to 1 year, in the range of ~45% to ~60%. Device comfort and ease of use were both highly rated. These outcomes show that SCS therapy with a micro-IPG, an external TD, and the PSP waveform may represent an important new option for patients with intractable pain.
Data Sharing Statement
The authors do not intend to share any data beyond what is included in the article.
Acknowledgments
Third-party writing assistance was provided by Allison Foster, PhD, of Foster Medical Communications.
Funding
This study was sponsored by Nalu Medical, Inc.
Disclosure
Drs. Salmon and Vahid Mohabbati have received financial assistance and fees for clinical trial support and costs associated with the implantation of Nalu medical SCS device. Dr. Salmon also has Nevro stock options and reports grants from PainCare Perth, during the conduct of the study. Dr. Makous is a consultant at Nalu Medical, BackStop Medical, Sella Therapies and ABVF, he owns stock in Nalu Medical and in addition has a patent US11090491B2 issued to Nalu Medical. Shilpa Kottalgi is an employee at Nalu Medical. Dr. Taverner is a consultant and lab instructor for Nevro, and he also is an unpaid board member of ANZCA and NSANZ. Dr. Bates was granted Nalu stock options, was compensated for flights and accommodations for speaking at NANS and is a consultant for Nalu and Abbott. Dr. Verrills was issued Nalu stock options. He sits on the DSMB and is a consultant for Saluda and Presidio. He also is paid honoraria for lectures on behalf of Saluda. Dr. Levy is an unpaid consultant for Biotronik, Nalu, Saluda and Abbott. He is INS past president and editor-in-chief of Neuromodulation. He has received stock option grants from Nalu and Saluda. Dr. Staats received royalties from Averitas and Qutenza Patch – he receives consulting fees from Saluda, Nalu, Vertos and Biotronik and honoraria from Medtronic. He is CMO for NSPC, sits on the DSMB of Nalu and electroCore and holds stock options in SPR, Nalu and Saluda. Dr. Heit is deceased. Drs. Mitchell, Du Toit, Yu and Green certified that they have no conflicting affiliations or involvements.
References
1. Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Supplement):S13–S19. doi:10.1016/j.jpainsymman.2005.12.010
2. Labaran L, Jain N, Puvanesarajah V, Jain A, Buchholz AL, Hassanzadeh H. A retrospective database review of the indications, complications, and incidence of subsequent spine surgery in 12,297 spinal cord stimulator patients. Neuromodulation. 2020;23(5):634–638. doi:10.1111/ner.12952
3. Orhurhu V, Gao C, Agudile E, et al. Socioeconomic disparities in the utilization of spinal cord stimulation therapy in patients with chronic pain. Pain Pract. 2021;21(1):75–82. doi:10.1111/papr.12936
4. Harmsen IE, Hasanova D, Elias GJB, et al. Trends in clinical trials for spinal cord stimulation. Stereotact Funct Neurosurg. 2020;27:1–12.
5. Karri J, Joshi M, Polson G, et al. Spinal cord stimulation for chronic pain syndromes: a review of considerations in practice management. Pain Physician. 2020;23(6):599–616.
6. Thomson S, Huygen F, Prangnell S, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. Eur J Pain. 2020;24(6):1169–1181. doi:10.1002/ejp.1562
7. Manchikanti L, Pampati V, Vangala BP, et al. Spinal Cord Stimulation Trends of Utilization and Expenditures in Fee-For-Service (FFS) Medicare Population from 2009 to 2018. Pain Physician. 2021;24(5):293–308. doi:10.36076/ppj.2021.24.401
8. Verrills P, Sinclair C, Barnard A. A review of spinal cord stimulation systems for chronic pain. J Pain Res. 2016;9:481–492. doi:10.2147/JPR.S108884
9. London D, Mogilner A. Spinal cord stimulation: new waveforms and technology. Neurosurg Clin N Am. 2022;33(3):287–295. doi:10.1016/j.nec.2022.02.006
10. Pope JE, Falowski S, Deer TR. Advanced waveforms and frequency with spinal cord stimulation: burst and high-frequency energy delivery. Expert Rev Med Devices. 2015;12(4):431–437. doi:10.1586/17434440.2015.1026805
11. Ghosh PE, Gill JS, Simopoulos T. The evolving role of high-frequency spinal cord stimulation as salvage therapy in neurostimulation. Pain Pract off J World Inst Pain. 2020;20(7):706–713. doi:10.1111/papr.12898
12. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134. doi:10.1016/S1474-4422(19)30414-4
13. Al-Kaisy A, Royds J, Al-Kaisy O, et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg Anesth Pain Med. 2020;45(11):883–890. doi:10.1136/rapm-2020-101681
14. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543–552. PMID: 28714533. doi:10.1111/ner.12634
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642–649. PMID: 28834092; PMCID: PMC5656934. doi:10.1111/ner.12642
16. Patel SK, Gozal YM, Saleh MS, Gibson JL, Karsy M, Mandybur GT. Spinal cord stimulation failure: evaluation of factors underlying hardware explantation. J Neurosurg Spine. 2019;32(1):133–138. doi:10.3171/2019.6.SPINE181099
17. Reddy RD, Moheimani R, Yu GG, Chakravarthy KV. A review of clinical data on salvage therapy in spinal cord stimulation. Neuromodulation. 2020;23(5):562–571. doi:10.1111/ner.13067
18. Dougherty MC, Woodroffe RW, Wilson S, Gillies GT, Howard MA, Carnahan RM. Risk factors and survival analysis of spinal cord stimulator explantation. Neuromodulation. 2021;24(1):61–67. doi:10.1111/ner.13173
19. Simopoulos T, Aner M, Sharma S, Ghosh P, Gill JS. Explantation of Percutaneous spinal cord stimulator devices: a retrospective descriptive analysis of a single-center 15-year experience. Pain Med Malden Mass. 2019;20(7):1355–1361.
20. Gill JS, Kohan LR, Hasoon J, et al. A survey on the choice of spinal cord stimulation parameters and implantable pulse generators and on reasons for explantation. Orthop Rev. 2022;14(4):39648. doi:10.52965/001c.39648
21. Desai MJ, Salmon J, Verrills P, et al. A novel pulsed stimulation pattern in spinal cord stimulation: clinical results and postulated mechanisms of action in the treatment of chronic low back and leg pain. Neuromodulation. 2023;26(1):182–191. doi:10.1016/j.neurom.2022.10.053
22. Salmon J, Bates D, Du Toit N, et al. Early experience with a novel micro Spinal Cord Stimulation (SCS) system for the management of chronic intractable pain of the back and legs. Neuromodulation. 2023;26(1):172–181. doi:10.1016/j.neurom.2022.11.002
23. Malinowski MN, Heit G, Poree LR, et al. Novel spinal cord stimulation system with a battery-free micro-implantable pulse generator. Pain Pract. 2022;22(6):592–599. PMID: 35509116. doi:10.1111/papr.13124
24. Deer TR, Sayed D, Pope JE, et al. Emergence from the COVID-19 pandemic and the care of chronic pain: guidance for the interventionalist. Anesth Analg. 2020;131(2):387–394. doi:10.1213/ANE.0000000000005000
25. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi:10.1016/j.pain.2011.07.005
26. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain off J Am Pain Soc. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
27. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):
28. EuroQol Group. EuroQol--A new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9
29. Beck AT, Steer RA, Carbin MG. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. doi:10.1016/0272-7358(88)90050-5
30. Guy W; National Institute of Mental Health (U.S.). Psychopharmacology Research Branch. Division of Extramural Research Programs. ECDEU assessment manual for psychopharmacology [Internet]. Rockville, Md: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976 [cited May 25, 2021]: 616. Available from: http://archive.org/details/ecdeuassessmentm1933guyw.
31. Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):593–607. PMID: 15949778. doi:10.1016/j.berh.2005.03.003
32. Button KS, Kounali D, Thomas L, et al. Minimal clinically important difference on the beck depression inventory--II according to the patient’s perspective. Psychol Med. 2015;45(15):3269–3279. PMID: 26165748; PMCID: PMC4611356. doi:10.1017/S0033291715001270
33. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. PMID: 18055266. doi:10.1016/j.jpain.2007.09.005
34. Deer T, Skaribas I, McJunkin T, et al. Results from the partnership for advancement in neuromodulation registry: a 24-month follow-up. Neuromodulation. 2016;19(2):179–187. PMID: 26890015. doi:10.1111/ner.12378
35. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. PMID: 26218762. doi:10.1097/ALN.0000000000000774
36. Fishman M, Cordner H, Justiz R, et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021;21(8):912–923. PMID: 34363307; PMCID: PMC9290817. doi:10.1111/papr.13066
37. Medtronic, Inc. Implant manual for IntellisTM with AdaptiveStimTM technology (97715) and IntellisTM (97716) rechargeable neurostimulators. Available from: https://www.manualslib.com/manual/1760170/Medtronic-Intellis.html.
38. Summary of Safety and Effectiveness Data (SSED). Nevro. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130022B.pdf.
39. Baranidharan G, Bretherton B, Richert G, et al. Pocket pain, does location matter: a single-centre retrospective study of patients implanted with a spinal cord stimulator. Reg Anesth Pain Med. 2020;45(11):891–897. doi:10.1136/rapm-2020-101752
40. Dietvorst S, Decramer T, Lemmens R, et al. Pocket pain and neuromodulation: negligible or neglected? Neuromodulation. 2017;20(6):600–605. PMID: 28699685. doi:10.1111/ner.12637
41. Hasoon J, Urits I, Mahmood S, Kaye AD. Restoring successful spinal cord stimulation therapy for a patient with severe pocket pain utilizing nalu micro-implantable pulse generator. Orthop Rev. 2022;14(3):35326.
42. MAUDE - Manufacturer and User Facility Device Experience. Available from: https://www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers.
43. Costandi S, Mekhail N, Azer G, et al. Longevity and utilization cost of rechargeable and non-rechargeable spinal cord stimulation implants: a comparative study. Pain Pract. 2020;20(8):937–945. PMID: 32543118. doi:10.1111/papr.12926
44. Nalu medical implant manual for the nalu neurostimulation system. Available from: https://nalumed.com/wp-content/uploads/2021/12/MA-000007-Rev-J.pdf.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.