Back to Journals » Journal of Inflammation Research » Volume 14
TRAP1 Shows Clinical Significance in the Early Diagnosis of Small Cell Lung Cancer
Authors Li X , Li X, Chen S, Wu Y, Liu Y, Hu T, Huang J, Yu J, Pei Z, Zeng T, Tan L
Received 1 April 2021
Accepted for publication 28 May 2021
Published 14 June 2021 Volume 2021:14 Pages 2507—2514
DOI https://doi.org/10.2147/JIR.S313440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Xiaohang Li,1 Xu Li,1 Simei Chen,1 Yang Wu,1 Yuhan Liu,1 Tingting Hu,1 Jiayi Huang,1 Jianlin Yu,1 Zihuan Pei,1 Tingting Zeng,2 Liming Tan1
1Department of Clinical Laboratory, The Second Affiliated Hospital of Nanchang University, Jiangxi Province Key Laboratory of Laboratory Medicine, Nanchang, Jiangxi, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, People’s Republic of China
Correspondence: Liming Tan
Department of Clinical Laboratory, The Second Affiliated Hospital of Nanchang University, Jiangxi Province Key Laboratory of Laboratory Medicine, No. 1 Minde Road, Donghu District, Nanchang, Jiangxi, People’s Republic of China
Tel +86 791-86300410
Email [email protected]
Purpose: To explore the clinical significance of tumor necrosis factor receptor-associated protein (TRAP1), mitotic arrest deficient 2 (mad2) and anti-nuclear mitotic spindle apparatus antibody (MSA) in the diagnosis of small cell lung cancer (SCLC).
Patients and Methods: Serum concentrations of TRAP1 and MSA were determined by enzyme-linked immunosorbent assay (ELISA), including SCLC group (Num.=86), non-small cell lung cancer (NSCLC) group (Num.=105), pulmonary nodules (PN) group (Num.=94), and 60 healthy subjects as control group (Num.=60). Whereas fluorescence quantitative PCR (qt-PCR) method was used to detect the expression of mad2.
Results: The expression of TRAP1 was low in SCLC and NSCLC compared with the other two groups, and was the lowest in SCLC, which was negatively correlated with the occurrence of the disease (P< 0.05); the sensitivity and specificity of TRAP1 for SCLC were 75.29%, 93.33%, and the area under SCLC curve was 0.903; compared with the other three groups, the level of MSA was the highest in the SCLC, and the results were significantly different (P< 0.05), while the area under the SCLC curve was 0.856, and the sensitivity and specificity were 62.78% and 95.24%, respectively. Mad2 is overexpressed in SCLC, but not in PN. The area under the SCLC curve is 0.835, and the sensitivity and specificity are 56.98% and 92.38%; TRAP1 levels are negatively correlated with SCLC tumor stage, the level of TRAP1 was significantly lower in stage III–IV than in stage I–II (P< 0.05); combined analysis of TRAP1 and MAD2 and MSA showed that the sensitivity and specificity for SCLS were 95.35% and 99.05%, respectively.
Conclusion: TRAP1 is of great value in the early diagnosis of SCLC as well as differential diagnosis with NSCLC. TRAP1 combined with MAD2 and MSA improved the sensitivity and specificity and provided a new idea for the clinical diagnosis of SCLC.
Keywords: TRAP1, diagnosis, small cell lung cancer, clinical stage, metastasis
Introduction
Small cell lung cancer (SCLC) accounts for about 14% of all types of lung cancer (LC).1 At present, the pathogenesis and origin of SCLC are still unclear. But it is an aggressive disease with high metastatic potential, leading to poor clinical prognosis, and one of the most deadly malignant tumors.2 The current gold standard for diagnosis of SCLC is still pathological examination, which belongs to invasive examination, usually comes with high costs and long waiting times for results. Therefore, noninvasive indicators are extremely important for the diagnosis of early SCLC. Tumor necrosis factor receptor-associated protein (TRAP1) is a mitochondrial protein, belongs to the heat shock protein 90 family, and is mainly located in the mitochondria of tumor cells.3 HSP90 plays a major role in the apoptosis of SCLC cells. Its inactivation may effectively induce tumor cell apoptosis. Restall4 found that TRAP1 may be a candidate target for HSP90 inhibitors, and it plays a more important role than other subtypes in the anti-apoptotic survival of SCLC cells. Lee et al5 found that TRAP1 is strongly expressed in SCLC tissues. And the positive rate is 100%, which is much higher than other subtypes of the HSP90 family. But there are few reports about serum TRAP1 levels in the diagnosis of SCLC. Here, we examined the serum levels of TRAP1, mad2 and MSA in patients diagnosed with SCLC, non-small cell lung cancer (NSCLC), pulmonary nodules (PN) and healthy controls in the Second Affiliated Hospital of Nanchang University to explore their clinical significance in the diagnosis of SCLC. The report is analyzed as follows.
Patients and Methods
Subjects Source
A total of 86 patients with SCLC, 105 patients with NSCLC and 94 patients with PN were enrolled from the inpatients and outpatients of the Second Affiliated Hospital of Nanchang University from December 2019 to December 2020. The diagnostic criteria were based on the 2015 WHO diagnostic criteria for lung tumor classification.6 Whereas, 60 healthy controls were from the Physical Examination Center of the Second Affiliated Hospital of Nanchang University. All participants have been informed and signed informed consent. This research has approved by the ethics committee of the Second Affiliated Hospital of Nanchang University. And this study was conducted in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: 1) Informed and voluntary participation; 2) Clear diagnosis, completing clinical, imaging, and histopathological data; 3) Re-evaluate the data of selected patients, deny other autoimmune diseases and other cancers; 4) The blood routine, liver and kidney function, blood pressure, chest radiograph, electrocardiogram and hepatobiliary and pancreatic examinations of the healthy volunteers control group.
Exclusion criteria were as follows: 1) Unclear tumor type or non-primary tumor; 2) Combined with serious heart, lung, liver and other system diseases, thyroid disease, diabetes; 3) pregnancy or lactation.
Specimen Collection
1) The TRAP1 and MSA test samples were collected by vacuum blood collection containing separation gel and coagulant. The specific procedure included fasting venous blood collection of 3.0mL, centrifugation at 1026×g/15min, serum collection, and kept at −80°C for storage. 2) The mad2 test specimens were collected by vacuum blood collection containing EDTA K2 anticoagulant. The simple procedure included 2.0 mL of fasting static blood collection, RNA extraction with RNA Trizol kit, and kept at −80°C for storage.
Indicator Detection
TRAP1 (Bohu Biotechnology Co., Ltd. Shanghai, China) and MSA (R&D Company, Lorton, Virginia, USA) were detected by enzyme-linked immunosorbent assay (ELISA). All operations were carried out strictly in accordance with reagent instructions, and blank controls and standard controls were established. Use fluorescence quantitative PCR (qt-PCR) to detect mad2, The reagents used in the experiment include RNA extraction kits (Takara Bio, Inc., Otsu, Japan). And RNA reverse transcription kits (Takara Bio, Inc., Otsu, Japan). The length of mad2 mRNA is 163 bp, and GAPDH is used as an internal control. The DNA Ladder is 146 bp in length. 2) Primer sequence: mad2 mRNA upstream primer sequence: 5ʹ-AGCTCCTTTTGACCTTCATTTC-3ʹ; downstream primer sequence: 5ʹ-TCCATTGCTTCATAGGTTCAAG-3ʹ; GAPDH upstream primer sequence: 5ʹ-ACGGTGACATTTCTGCCACT-3ʹ; downstream primer sequence 5ʹ- TGGTCCCGACTCTTCCCATT-3ʹ.
Statistical Analysis
Statistical analysis used SPSS 22.0 software, measurement data were expressed as mean ± standard deviation. The experimental sample size was calculated using PASS 11. Enumeration data were described in percentage, and the differences between the groups were analyzed by χ2 test. The data of TRAP1 and MSA used one-way analysis of variance. For mad2, Wilcoxon rank sum test was used; The diagnostic efficacy of TRAP1, mad2 and MSA for SCLC was assessed by receiver operating characteristics (ROC) analysis. P<0.05 was considered to be statistically significant.
Results
Clinical Characteristics
After χ2 test analysis, there is no difference in age and sex between the disease group and the control group (P>0.05, Table 1).
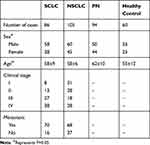 |
Table 1 Clinical Characteristics |
Serum Levels of TRAP1 and MSA
The level of TRAP1 in SCLC group was the lowest than that in other three groups, which showing a negative correlation between TRAP1 and SCLC (SCLC is 152.52±84.26 pg/mL, NSCLC is 320.39±141.68 pg/mL, PN is 416.05 ±87.22 pg/mL, control is 740.80±302.56 pg/mL). The level of MSA in SCLC was significantly higher compared with NSCLC group, PN group and control group (SCLC is 112±36.48pg/mL, NSCLC is 58.26±18.22pg/mL, PN is 28.78±14.64 pg/mL, control is 25.40±13.15 pg/mL). The difference of TRAP1 and MSA in SCLC and other groups was statistically significant by one-way analysis of variance (P<0.05, Figure 1A and B).
 |
Figure 1 Serum concentration of TRAP1 (A) and MSA (B) in different disease groups and control groups. Note: *Represents the difference is statistically significant, P<0.05. |
Expression of Mad2
Through qt-PCR quantitative expression analysis, mad2 is highly expressed in SCLC. The expression of NSCLC is lower than that of SCLC (P<0.05, Figure 2).
 |
Figure 2 The expression of mad2 in different disease groups. Note: *Represents the difference is statistically significant, P<0.05. |
ROC Curve of TRAP1, Mad2 and MSA in Diagnosing SCLC
The AUC of TRAP1 for the diagnosis of SCLC is 0.903, the cut-off value is 209.26pg/mL, the diagnostic sensitivity is 75.29%, the specificity is 93.33%, and the Youden index is 0.686. When the MSA marginal value is 97.10pg/mL, the AUC for diagnosis of SCLC is 0.919, the diagnostic sensitivity is 61.63%, the specificity is 98.10%, and the Youden index is 0.656. The AUC of mad2 for the diagnosis of SCLC is 0.835, the diagnostic sensitivity is 56.98%, the specificity is 92.38%, and the Youden index is 0.533. The ROC curves were shown in Figure 3.
 |
Figure 3 ROC curve of TRAP1, mad2 and MSA in the diagnosis of SCLC. Notes: MSA has the highest area under the curve, but the sensitivity is too low, TRAP1 has better diagnostic performance. |
Results of the Combined Analysis of TRAP1, Mad2 and MSA
By binary logistic analysis, the combination of TRAP1, mad2 and MSA has a sensitivity and specificity of 95.35% and 99.05%. TRAP1 combined with mad2, AUC was 0.954, sensitivity and specificity were 77.91% and 98.10%, TRAP1 combined with MSA, AUC was 0.988, sensitivity and specificity were 93.02% and 98.10%, respectively. The ROC curves were shown in Figure 4.
 |
Figure 4 TRAP1, mad2, MSA joint analysis results. Note: The AUC, sensitivity, and 1-specificity of the combined diagnosis of the three are the highest. |
The Relationship Between TRAP1 Serum Level and SCLC, Including Staging and Metastasis
The clinical stage of SCLC was negatively correlated with the concentration of TRAP1, which was significantly lower in stage III–IV than in stage I–II (stage III–IV is 113.27±51.11 pg/mL, I–II is 274.01±36.21 pg/mL); At the same time, the concentration of TRAP1 in the SCLC metastatic group was significantly lower than that in the non-metastatic group (130.00±67.07pg/mL in the metastatic group, 251.06±82.54 pg/mL in the non-metastatic group). The result was shown in Figure 5.
 |
Figure 5 TRAP1 serum level and SCLC, including staging and metastasis. Note: *Represents the difference is statistically significant, P<0.05. |
Discussion
SCLC is the most malignant type of lung cancer and is most prone to early metastasis. More than 70% of SCLC patients have metastasized at the time of diagnosis, and the 5-year survival rate of metastatic patients is less than 1%.7 TRAP1 can interact with TNF receptors and participate in cell signal transduction by helping protein folding, thereby promoting the occurrence and development of tumors.8 In addition, TRAP1 can protect mitochondria from oxidative stress and reactive oxygen species (ROS). It reduces the level of ROS and inhibits the opening of mitochondrial permeability transition pores, both of which play an important role in the process of apoptosis.9,10 This is closely related to the role of TRAP1 in metabolism, which can control the flow of electrons in the respiratory complex of the electron transport chain.11 Through this process, TRAP1 can reshape metabolism by down-regulating mitochondrial oxidative phosphorylation and promoting glycolysis, which is the main metabolic mode of tumor cells. Lee et al5 found that TRAP1 was up-regulated in lung cancer patient tissues, showing 100% positive. However, the acquisition of tissue specimens and the cumbersome diagnostic methods limit the diagnostic value. In this study, the detection of serum TRAP1 level was the first to explore its diagnostic value for SCLC.
The results showed that TRAP1 was detected in SCLC, NSCLC, PN, and healthy controls. Among them, the average level of healthy controls was the highest, PN, NSCLC, and SCLC decreased successively, and the expression of SCLC was the lowest, showing a negative correlation (P<0.05). Interestingly, this is contrary to the results of TRAP1 expression in lung cancer tissues by Lee et al,5 and may be related to its dual effects in tumors. Matassa12 found that TRAP1 exhibits dual effects in tumors. On the one hand, it is related to the occurrence and development of tumors, on the other hand, it exerts a tumor suppressor effect. TRAP1 in tumor tissues promotes and protects tumorigenesis. The literature also reported that the high expression of TRAP1 tissue is associated with lung cancer resistance to cisplatin.13 It is speculated that TRAP1 secreted into the serum may play a different role.
Spindle tubulin may be denatured during degradation and induce the body to produce MSA. During mitosis, spindle microtubules are emitted from the poles to connect with sister chromatids and kinetochores, pulling the two sets of sister chromatids into offspring cells. This process is regulated by a highly conserved signaling mechanism. The relevant regulatory gene is the mitotic spindle checkpoint gene (SAC), and mad2 is one of the SAC family. The process of spindle-guided chromosome separation and abnormalities in SAC can lead to chromosomal instability (CIN), which is considered to be closely related to tumors. This study found that MSA has the highest concentration in SCLC group, which is 112±36.48pg/mL. And the concentration in the control group was the lowest. Compared with NSCLC, PN and the control groups, the difference was statistically significant (P<0.05). This is consistent with the results of Tan14 What’s more, the results of qt-PCR showed that Mad2 was overexpressed in SCLC, and the expression of NSCLC and PN was significantly lower than that of SCLC, which indicates that MSA and mad2 have a promoting effect on the occurrence of SCLC, and are positively correlated with SCLC. The study by Wu15 also showed this result, which also shows that the biological effect of serum TRAP1 is different from that of serum MSA and mad2. The former may play a role as a tumor suppressor.
The advantage of TRAP1 over mad2 and MSA is that, compared with healthy controls, the concentration of SCLC can be reduced by more than 4 times. In this research, the differences between TRAP1, mad2 and MSA in SCLC, NSCLC and PN are all statistically significant. Therefore, the three can be distinguished by the difference in expression. Studies have found that PN is closely related to the occurrence of lung cancer.16 The variety between the PN group and the healthy control group was statistically significant in TRAP1, while there was no difference or no difference between MAD2 and MSA. Therefore, TRAP1 has potential value for early detection of PN and early prevention of lung cancer. Moreover, the expression level of TRAP1 was negatively correlated with the clinical stage, stage I~II was significantly higher than stage III~IV (P<0.05). The level of TRAP1 is also significantly different between metastasis and non-metastatic, non-metastatic group is significantly higher than that of metastatic group (P<0.05). This is of great significance for the early diagnosis of SCLC.
When the TRAP1 cut-off value is 209.26pg/mL, the AUC is 0.903, the sensitivity for diagnosing SCLC is 93.33%, and the specificity is 75.29%, showing good diagnostic value. When the MSA cut-off value is 97.10pg/mL, the AUC is 0.919, the sensitivity is 61.63%, the specificity is 98.10%. Although AUC is higher than TRAP1, it is less sensitive, and the diagnostic value is moderate. The AUC of mad2 is 0.835, the sensitivity is 56.98%, the specificity is 92.38%, the diagnostic value is moderate, and it is comparable to MSA. From the results, it can be seen that TRAP1 has significantly better diagnostic value for SCLC than the other two indicators. However, combined detection can achieve higher sensitivity and specificity, so it is a better choice. Through binary logistic analysis, TRAP1 combined with mad2 has an AUC of 0.954, and the sensitivity and specificity can reach 77.91% and 98.10%. Compared with the detection of TRAP1 alone, the diagnostic value is not much improved; TRAP1 combined with MSA has an AUC of 0.988, which has better sensitivity and specificity, reaching 93.02% and 98.10%, which is significantly higher than detecting TRAP1 alone; the combination of TRAP1, mad2 and MSA has the best sensitivity and specificity, which is 95.35% and 99.05% and much higher than the three separate tests. This provides a valuable new method for the diagnosis of SCLC.
Conclusion
In summary, TRAP1 has good early diagnosis and differential diagnosis significance for SCLC, NSCLC and PN. It is negatively correlated with the clinical staging of SCLC. Additionally, TRAP1 is better than MSA and mad2 in diagnosing SCLC; TRAP1 combined with mad2 and MSA can significantly improve the specificity and sensitivity of diagnosis, providing a new clinical diagnosis plan.
Funding
This work was kindly supported by the National Nature Science Foundation of China under Grant (81760382), the Science and Technology Plan of Jiangxi Provincial Health and Family Planning Commission (20181073), the Youth Science Foundation of Jiangxi Science and Technology Department (20202BABL216027) and the project from Education Department of Jiangxi Province (GJJ190123).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1). doi:10.5334/aogh.2419
2. Tsoukalas N, Aravantinou-Fatorou E, Baxevanos P, et al. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann Transl Med. 2018;6(8):145. doi:10.21037/atm.2018.03.31
3. Agorreta J, Hu J, Liu D, et al. TRAP1 regulates proliferation, mitochondrial function, and has prognostic significance in NSCLC. Mol Cancer Res. 2014;12(5):660–669. doi:10.1158/1541-7786.MCR-13-0481
4. Restall IJ, Lorimer IAJ. Induction of premature senescence by Hsp90 inhibition in small cell lung cancer. PLoS One. 2010;5(6):e11076. doi:10.1371/journal.pone.0011076
5. Lee JH, Kang KW, Kim JE, et al. Differential expression of heat shock protein 90 isoforms in small cell lung cancer. Int J Clin Exp Pathol. 2015;8:9487.
6. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
7. Caballero Vázquez A, Garcia Flores P, Romero Ortiz A, García Del Moral R, Alcázar Navarrete B. Small cell lung cancer: recent changes in clinical presentation and prognosis. Clin Respir J. 2020;14(3):222–227. doi:10.1111/crj.13119
8. Hoter A, El-Sabban M, Naim H. The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci. 2018;19(9):2560. doi:10.3390/ijms19092560
9. Basit F, van Oppen LM, Schöckel L, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8(3):e2716–e2716. doi:10.1038/cddis.2017.133
10. Faienza F, Rizza S, Giglio P, Filomeni G. TRAP1: a metabolic hub linking aging pathophysiology to mitochondrial S-Nitrosylation. Front Physiol. 2020;11. doi:10.3389/fphys.2020.00340
11. Rasola A, Neckers L, Picard D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014;24(8):455–463. doi:10.1016/j.tcb.2014.03.005
12. Matassa D, Agliarulo I, Avolio R, Landriscina M, Esposito F. TRAP1 regulation of cancer metabolism: dual role as oncogene or tumor suppressor. Genes-Basel. 2018;9(4):195. doi:10.3390/genes9040195
13. Kuchitsu Y, Nagashio R, Igawa S, et al. TRAP1 is a predictive biomarker of platinum-based adjuvant chemotherapy benefits in patients with resected lung adenocarcinoma. Biomed Res. 2020;41(1):53–65. doi:10.2220/biomedres.41.53
14. Tan L, Zhang Y, Jiang Y, et al. The clinical significance of anti-mitotic spindle apparatus antibody (MSA) and anti-centromere antibody (ACA) detected in patients with small cell lung cancer (SCLC). Am J Clin Exp Immunol. 2017;6(2):21–26.
15. Wu Y, Tan L, Chen J, et al. MAD2 combined with Mitotic Spindle Apparatus (MSA) and Anticentromere Antibody (ACA) for diagnosis of Small Cell Lung Cancer (SCLC). Med Sci Monit. 2018;24:7541–7547. doi:10.12659/MSM.909772
16. Pinsky P, Gierada DS. Long-term cancer risk associated with lung nodules observed on low-dose screening CT scans. Lung Cancer. 2020;139:179–184. doi:10.1016/j.lungcan.2019.11.017
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
