Back to Journals » Veterinary Medicine: Research and Reports » Volume 11
Transsphenoidal Surgery in Canines: Safety, Efficacy and Patient Selection
Authors Hara Y
Received 25 November 2019
Accepted for publication 26 December 2019
Published 14 January 2020 Volume 2020:11 Pages 1—14
DOI https://doi.org/10.2147/VMRR.S175995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Yasushi Hara
Division of Veterinary Surgery, Nippon Veterinary and Life Science University, Tokyo, Japan
Correspondence: Yasushi Hara
Division of Veterinary Surgery, Nippon Veterinary and Life Science University, 1-7-1 Kyounan-cho, Musashino-shi, Tokyo 180-8602, Japan
Tel +81 422 31 4151
Fax +81 422 33 8836
Email [email protected]
Abstract: Pituitary-dependent hyperadrenocorticism (PDH) is the cause of approximately 80-85% of naturally occurring cases of hyperadrenocorticism(HAC) in canines and is triggered by an adrenocorticotropic hormone (ACTH)-producing pituitary adenoma or hyperplasia of the corticotroph in the pituitary anterior lobe or intermediate lobe. Transsphenoidal surgery(TSS) is an effective treatment that can directly remove pituitary tumors that cause PDH in canines under a single course of general anesthesia. However, careful evaluations of the definitive diagnosis, adenoma size and growth rate, relationship with surrounding tissue, general condition, and neurosurgical procedural skill involved in each case are important to determine TSS suitability. The basic principle is to confirm that the present HAC case is PDH, that is, an ACTH-producing adenoma or the hyperplasia of the corticotroph originating from either the pituitary anterior or intermediate lobe. Evaluations based on endocrinology, particularly plasma ACTH concentration, and imaging diagnosis, particularly MRI is essential for definitive diagnosis. Enlarged pituitary tumors can shorten the post-TSS survival time, increase the recurrence rate of clinical symptoms, and increase the risk for developing permanent central diabetes insipidus. Therefore, complete removal of adenomas of up to Grade IIIA according to the MRI-based classification system is relatively easy to achieve with TSS, and long-term remission and survival can be expected.
Keywords: canine, cushing’s syndrome, pituitary-dependent hyperadrenocorticism, transsphenoidal surgery
Introduction
Transsphenoidal surgery (TSS) typically refers to a craniotomy procedure where a pituitary tumor is surgically removed. In small animal practice, TSS is typically used for pituitary-dependent hyperadrenocorticism (PDH) and has recently been acknowledged as the most effective form of treatment for PDH accompanied by non-enlarged adrenocorticotropic hormone (ACTH)-producing adenomas.1 PDH is the cause of approximately 80–85% of naturally occurring cases of hyperadrenocorticism (HAC) in canines,2 and is triggered by ACTH-producing pituitary adenoma or hyperplasia of the corticotroph in the pituitary anterior lobe or intermediate lobe3–5 [Figure 1]. PDH is a life-threatening condition in canines as a result of two different mechanisms, paraneoplastic syndrome characterized by HAC (Cushing’s syndrome) and intracranial space-occupying lesions due to the growth of the adenoma itself6,7 [Figure 2]. Canine PDH is a clinically important disease, because the incidence is relatively high (1 or 2 case per 1000 dogs),8 but management methods for the treatment of PDH in small animal clinical practice often use cortisol biosynthesis inhibitors to dissolve the clinical signs caused by hypercortisolemia,9 which is not a curative treatment for pituitary adenoma. In PDH cases, if a bilateral adrenalectomy is performed or adrenocortical function is excessively suppressed owing to the administration of cortisol biosynthesis inhibitors, the resultant decreased cortisol production can weaken the negative feedbacks to the hypothalamus and pituitary gland, which in turn increases the risk of pituitary tumor growth.5,10−12 This phenomenon has been widely known as Nelson’s syndrome in the field of human medicine.13 In this context, selective pituitary adenomarectomy is considered the treatment of choice for patients with Cushing’s disease.14
Even in the field of small animal clinical practice, the widespread use of MRI and computed tomography (CT), and the subsequent improvements in diagnostic accuracy for pituitary adenomas, as well as the published data on the effectiveness of TSS for PDH by a research group from Utrecht University, have given renewed focus on the use of TSS for the treatment of PDH, with numerous new facilities being constructed globally. This review explains the effectiveness and safety of TSS for canine PDH and the patient selection for the treatment.
History and Efficacy of TSS for Canine PDH
With regard to canine hypophysectomy, experimental surgical reports in the medical field that aimed to study the physiological function of the pituitary gland were published in 1964.15 In the field of small animal veterinary practice, the pioneers of the use of TSS for canine PDH are undoubtedly the Utrecht University research group. The first reports on treatments using hypophysectomy in canines with Cushing’s syndrome were published by Rijnberk et al in 1968.16,17 Rijnberk et al performed TSS in four canines with PDH and showed a postoperative decrease in plasma 11β-hydroxycorticosteroid and urinary 17-hydroxycorticosteroid levels relative to the preoperative levels; postoperative improvements in clinical symptoms such as abdominal distension, polydipsia, and alopecia; and hair growth. Simultaneously, they reported for the first time that TSS can be a potentially effective treatment for canines with PDH as compared with bilateral adrenalectomy, of which the latter often resulted in death from circulatory disorders. However, given the anatomical differences between the skulls of canine breeds (e.g., brachycephalic vs dolichocephalic breeds) and the difficult nature of the surgical procedure, use of TSS never became widespread as a clinical treatment method for canines with PDH. In an era where CT and MRI were not widely available in small animal clinical practice, a larger focus was on methods that allowed for the accurate identification of the pituitary gland location during surgery, which was not directly visible in the operating field. To determine the location of the pituitary gland, Niebauer et al fixed three self-threading screws on the ventral side of the sphenoid bone during surgery and reported a strategy for locating the pituitary gland from this setup combined with venous sinus contrast imaging.18 TSS in that era was conducted while the canine was in a supine position, with the head and neck extended. A disadvantage of conducting the surgical operation in this position is that the cranium can become easily contaminated during surgery because the burr hole created in the sphenoid bone is in a lower position than the oral cavity and nasopharynx, into which secretions, body fluids, and lavage fluids in the surrounding area can easily accumulate. Thereafter, CT and MRI were eventually introduced in the field of small animal clinical practice in the 1990s. This allowed for visualizations of the skull and morphological evaluations of the brain and pituitary gland. The diagnostic accuracy for pituitary tumors was greatly improved with this development. CT and MRI scans obtained during surgery were useful for determining the positional relationships of the surrounding tissue (set as surgical landmarks) and the pituitary gland. In 1997, Meij et al from Utrecht University reported on a new TSS procedure that uses CT to determine the pituitary fossa location by using the distance from the hamular process, and elevating and restraining the canine’s head in a prone position19 [Figure 3]. Afterward, the same group reported postoperative evaluations of 52 cases of PDH in canines and 7 cases of PDH in cats.20,21 Thereafter, Hanson et al from the same group reported that TSS treatment for PDH in canines resulted in 127 cases of remission from among 150 cases (85%), with 124 canines showing remission of HAC symptoms within 8 weeks of operation.22 The 1-, 2-, 3-, and 4-year survival rates after the TSS procedure were 83.5%, 76.1%, 71.5%, and 67.8%, respectively. Meanwhile, the success rate of an o,p′-DDD-based treatment in 129 canines with PDH in the same facility was 61% (68/111 canines), and the 1-, 2-, and 3-year survival rates after the operation were 80%, 69%, and 61%, respectively. On the basis of the aforementioned findings, they reported that in long-term studies, TSS-based treatment was more effective than the o,p′-DDD-based treatment.22 In addition, Hanson et al reported on post-TSS factors that influenced the survival of canines with PDH and PDH recurrence in canines that had had a remission.23 In recent years, van Rijn et al from the same group conducted long-term performance evaluations of 306 canines with PDH that received TSS treatment. They showed a postoperative remission rate of 84%, postoperative mortality rate (i.e., death within 4 weeks following the operation) of 8.8%, median survival time of 781 days, median remission period of 951 days, and recurrence rate of 27% (median recurrence period: 555 days).24 These studies reported that in recent years, the number of PDH cases referred for surgery has increased after the introduction of trilostane-based treatment prior to TSS and among those with increasing pituitary-to-brain ratio (PBR) prior to operation.
 |
Figure 3 Patient posture for TSS. The dog is placed in sternal recumbency with its upper jaw fixed to a metal bar attached to the surgery table. The lower jaw was pulled downwards (A). Following midline incision of the soft palate and mucoperiosteum, a bur hole was made in the base of the pituitary fossa. The ventral surface of the pituitary tumor could be seen through the opening in the sphenoidal bone (B; arrow). During the operation, it is important to confirm that the opening in the sphenoidal bone is accurately located to the pituitary fossa by MRI or CT(C, D; arrow). (C) Gd-T1 weighted sagittal image. (D) Gd-T1 weighted transverse image. This was advocated by Dr. Meij BP in 1997.19 The advantage of conducting the surgery in this position is that lavage fluid flows downward with a low risk of contamination into the opening and intracranial cavity. |
The authors (Japanese research group) studied the fundamental points concerning the TSS procedure and postoperative management described by the formed mentioned group of Utrecht University, and applied TSS in 45 PDH cases. In the evaluation of postoperative performance in 25 cases in the early stages of a surgical series, 21 (84%) of 25 canines showed remission of clinical symptoms. The 1-, 2-, 3-, and 4-year postoperative survival rates were 92%, 81%, 81%, and 81%, respectively. The preoperative baseline and post-ACTH stimulation cortisol levels were rapidly reduced to a significant extent after the operation. The postoperative alkaline phosphatase and alanine aminotransferase levels were lower than the preoperative levels.25 In addition, clinically distinct alopecia and skin calcification symptoms showed improvements 3–6 months after the operation.26 Teshima et al reported that patients with PDH that have a large pituitary gland are at risk of developing central diabetes insipidus(CDI) even after the TSS procedure.27 Diabetes mellitus can sometimes develop after PDH, and insulin resistance is an accompanying symptom. For the cases that PDH that developed diabetes mellitus, the removal of ACTH-producing tumor via TSS might be effective to control the concurrent diabetes mellitus.21,28 With regard to TSS, the entire tumor can be difficult to sufficiently visualize during surgery when the pituitary tumor is large, which might affect the surgical performance. Thus, Sato reported on an MRI-based classification system using the pituitary tumor sizes in PDH cases.29 The system is used to classify cases to grades I-V based on the size of the pituitary tumor and positional relationships between the pituitary tumor and the cranial structure and to determine whether blood vessels are involved (A or B). Sato et al reported that the pituitary tumor was completely removed in 29 (93.5%) of 31 total PDH cases corresponding to grades I–IIIA. Recently, the TSS procedures for PDH cases have been the focus of research in the United States. Mamelak et al reported that TSS procedures can be safely performed in canines with PDH by using a high-resolution video telescope and ensuring an operating field for larger adenomas than previously reported in the literature.30 They reported the postoperative outcome results in 26 canines with PDH that received TSS. Although 5 of 26 canines died within 5 days after the procedure, remission that lasted >3 months was observed in 21 canines after the operation, with the 1-year postoperative survival and remission rates of 81% and 95%, respectively. In addition, Owen et al reported that an accurate TSS approach to the pituitary fossa was possible by using a neuronavigation system.31
Case Selection
Currently, no clear application standard of TSS for PDH cases is available. However, careful evaluations of the (1) definitive diagnosis, (2) adenoma size and growth rate, (3) relationship with surrounding tissue, (4) general condition, and (5) neurosurgical procedural skill involved in each case are important to determine TSS suitability.
Definitive Diagnosis
The basic principle is to confirm that the present HAC case is PDH, that is, an ACTH-producing adenoma or the hyperplasia of the corticotroph originating from either the pituitary anterior or intermediate lobe. In this case, evaluations based on endocrinology and imaging diagnosis is particularly essential.
ACTH
Although several effective endocrinological analyses can be used for the definitive diagnosis of PDH, the following are primarily used: resting-state plasma ACTH concentration, corticotrophin-releasing hormone (CRH) test, ACTH stimulating test, high-dose dexamethasone suppression test, and urinary cortisol/creatinine ratio (UCCR).1,2,32 Excess ACTH produced from the ACTH-producing adenoma itself is the primary cause of PDH cases. Thus, plasma ACTH concentration can be used as a tumor marker in canine PDH. Numerous reports have shown the correlation between pituitary gland size and plasma ACTH concentration in canine PDH cases.6,33,34 Furthermore, plasma ACTH concentration and UCCR are useful not only in clinical diagnosis but also for post-TSS recurrence and post-treatment monitoring.22,29,35
MRI
Cases of suspected PDH require cerebral MRI and/or CT analysis to evaluate the pituitary gland size and morphology. In the field of human medicine, MRI is generally known to be superior to CT in terms of usefulness for the evaluation of pituitary glands with Cushing’s syndrome.36 In the field of small animal clinical practice, MRI is generally chosen for pituitary gland evaluation owing to its high image resolution.29,37,38 However, small pituitary gland adenomas can be detected using dynamic CT with contrast agent administration.39 In PDH cases, the enlargement of the pituitary gland is detected prior to the onset of clinical neurological symptoms.40 T1-weighted, T2-weighted, and gadolinium-enhanced T1-weighted images should be taken in the MRI analyses of cases of suspected PDH. These images are used for evaluation focused on pituitary gland size, condition of the posterior lobe, and relationships with the surrounding tissue.
Evaluation of the pituitary gland size from a cross-sectional image must be standardized across various dog breed sizes. Considering this point, calculation methods that focus on pituitary lesion size (height) relative to the canine brain cross-sectional area (i.e., PBR; the ratio of pituitary gland height [mm] to the total brain cross-sectional area [mm2]) are generally used.41 The pituitary gland is considered enlarged when the PBR is >0.31 and non-enlarged when the PBR is <0.31 [Figure 4].
 |
Figure 4 Measurements of pituitary brain ratio (PBR). PBR can be calculated by both CT and MRI. Among Gd-enhanced T1 weighted transverse images, the image that contained the largest cross section of the pituitary was selected. For the brain area, the edge of the brain were traced on the display (shown in blue line), and the enclosed area was calculated. On the same image, the height of the pituitary gland was measured (shown in yellow arrow). A vertical bar in both figures represent 10 mm. The PBR was calculated according to the following formula: PBR = (height of the pituitary gland)/(cross sectional area of the brain) x 102. This was advocated by Dr. Kooistra HS in1997.41 (A) T1-weighted transverse image of the healthy dog. The brain area, pituitary height, and PBR were 1608mm2, 4.7mm, and 0.292, respectively. (B) T1-weighted transverse image of the dog with pituitary tumor. The brain area, pituitary height, and PBR were 1650mm2, 11.4mm, and 0.691, respectively. |
A correlation was observed between the signal intensity of the pituitary posterior lobe on T1-weighted images and the arginine vasopressin (AVP) content in humans, and a high signal intensity is related to vasopressin-neurophysin II complexes within neurosecretory granules.42,43 In healthy canines, the posterior lobe is located in the central area of the pituitary gland in T1-weighted, cross-sectional, and medial sagittal images. This contributes to the high signal intensity, which indicates its relationship to AVP, as is the case with humans.44 A phenomenon referred to as displacement of the posterior lobe is observed in canine PDH where an adenoma that had formed in either the pituitary anterior or intermediate lobe, compressing the posterior lobe, increased in size and moved to the edge from its central position.45 The displacement of posterior lobe is caused by pituitary tumor growth and, as such, can be determined prior to the stage at which the pituitary tumor is designated as enlarged on the basis of a PBR analysis; therefore, it can be useful as an early PDH diagnostic tool [Figure 5].
 |
Figure 5 Displacement of the posterior lobe of the pituitary gland. (A) T1-weighted sagittal image and its magnified image (B) of the healthy dog. (C) T1-weighted transverse image and its magnified image (D) of the healthy dog. (E) T1-weighted sagittal image and its magnified image (F) of the dog with pituitary microadenoma. (G) T1-weighted transverse image and its magnified image (H) of the dog with pituitary microadenoma. A vertical bar in the figure (A), (C), (E) and (G) represent 10 mm, respectively. In the healthy dog, the pituitary posterior lobe is located at the center of the gland in both the sagittal and transverse planes. The signal intensity of the posterior lobe on MR T1-weighted image is higher compared to the surrounding anterior and intermediate lobes. In contrast, in the PDH case with microadenoma in the anterior or intermediate lobe of the pituitary, the posterior lobe is compressed and displaced peripherally due to the enlargement of the microadenoma (F, H; arrow).45 |
Adenoma Size and Growth Rate
The pituitary tumor size suitable for operation has currently not been determined but is an important factor when considering the use of TSS. As TSS approaches the tumor via a small burr hole created in the sphenoid bone, the entire adenoma that has grown on the dorsal side from the operating field could not be confirmed. At the same time, direct observation of the adhesion status between the adenoma and the surrounding tissue, as well as of blood vessel intrusion, is not possible. Thus, when a massive pituitary tumor is treated with TSS, the possibility of incomplete excision and the risk of blood vessel injuries during excision or iatrogenic injuries to the surrounding tissue structure increase. In addition, reports have shown the correlation of pituitary tumor size with surgical removal success rate, post-TSS recurrence rate, and survival.22,24 The PBR of surgical cases in reports that evaluated TSS outcome was as follows: the Netherlands group (n=306), 0.13–1.40 (median, 0.39);24 US group (n=26), 0.35–1.54 (median, 0.73),30 and Japan group (n=33), 0.23–0.83.29 However, successful TSS has been reported in the extraction of massive pituitary tumors measuring 15.0 × 21.4 × 18.3 mm (height × width ×length).46 Even if complete removal of the massive pituitary tumor was not possible with TSS, the procedure can still be effective as a volume-reduction surgery for intracranial space-occupying lesions. Thus, pituitary adenoma size may not be necessarily the deciding factor for determining the suitability of surgery.
When MRI is used to confirm the enlargement of the pituitary gland in PDH cases, the clinical focus is on the adenoma growth rate. Reports have indicated that plasma ACTH concentration and resistance to dexamethasone were indicators that correlated to pituitary tumor size.33,41 Regarding the relationship between pituitary tumor origin and size, some reports have indicated that adenomas originating from the intermediate lobe were larger than those originating from the anterior pituitary;23 conversely, other reports have shown that adenomas originating from the anterior pituitary grow larger than those originating from the intermediate lobe.3,47 However, currently, no indicators have been identified for predicting the adenoma growth rate after the diagnosis of pituitary tumors in canines. Reports have analyzed the Ki-67 and minichromosome maintenance-7 (MCM7) expression levels in sampled pituitary tumors, and suggested that in canine pituitary adenomas, MCM7 level may be a more effective growth marker than Ki-67 level.47,48 However, a clinical indicator that can predict adenoma growth rate prior to surgery is still needed.
Relationship Between the Pituitary Tumor and the Surrounding Tissue
To determine the indication of TSS, the relationship between the pituitary adenoma and its surrounding tissue must also be determined, especially whether blood vessels are involved. Surgery for complete removal will become exceedingly difficult if the pituitary tumor has undergone invasive growth into the nearby blood vessels or tissues such as the arterial circle of Willis or cavernous sinus, or if it had progressed to the adjacent tissue. Sato reported on an MRI-based classification system that uses MRI to classify pituitary tumor size and its influence on the surrounding tissue into five grades and two subtypes29 [Figure 6]. The image types used in this study were sagittal and cross-sectional gadolinium-enhanced T1-weighted MRI, in which the pituitary tumor size is maximized. The five-grade classification uses anatomical structures around the pituitary gland (i.e., sella sellae, third ventricle, optic chiasm, mammillary body, and interthalamic adhesion) as indicators. Pituitary tumors were classified in to 5 types according to extent: Grade 1, no tumor extension beyond the dorsum sellae; Grade 2, tumor extension beyond the dorsum sellae up to the third ventricle but no contact with the optic chiasm and mammillary body; Grade 3, tumor extension beyond the dorsum sellae up to the third ventricle and contact with the optic chiasm and/or mammillary body but not the interthalamic adhesion; Grade 4, tumor extension beyond the dorsum sellae and contact with the optic chiasm, mammillary body and interthalamic adhesion; and Grade 5, tumor occupation of the third ventricle. In addition, each grade is classified into two subtypes, with type A referring to cases without involvement of the cavernous sinus and/or arterial circle of Willis, and type B referring to cases with involvement of either or both. In cases where complete tumor removal is the objective, TSS is most suited for canine PDH of Grades I–IIIA according to the MRI-based classification system. In these cases, complete cure of PDH can be expected if TSS is performed at this stage. Meanwhile, complete tumor removal using TSS is difficult in many grade >3 and type B cases. Furthermore, postoperative recurrence is more likely to occur. Thus, TSS should be considered a tumor volume-reduction surgery for cases of Grade ≥3B.29
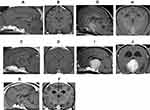 |
Figure 6 MRI grading system for the pituitary tumor The MRI-based classification system categorizes pituitary tumor into five grades and two subtypes, based on the tumor size and its influence on the surrounding tissue.29 Gd-enhanced sagittal and transverse T1 weighted images are used for evaluation. (A and B) Grade 1: No tumor extension beyond the dorsum sellae (red line). (C and D) Grade 2: Tumor extension beyond the dorsum sellae (red line) up to the third ventricle but no contact with the optic chiasm and mammillary body. (E and F) Grade 3: Tumor extension beyond the dorsum sellae up to the third ventricle and contact with the optic chiasm and/or mammillary body but without interthalamic adhesion. (G and H) Grade 4: Tumor extension beyond the dorsum sellae and contact with the optic chiasm, mammillary body and interthalamic adhesion. (I and J) Grade 5: Tumor occupation of the third ventricle. In addition, each grade is classified into two subtypes, with type A referring to cases without involvement of the cavernous sinus and/or arterial circle of Willis, and type B referring to cases with involvement of either or both. |
Additional care must be given to cases where meningiomas have formed around the pituitary gland.49 In such cases in canines, Cushing’s syndrome is clinically triggered in the early stages. However, this confers the risk of subsequent pituitary dysfunction, as the tumor continues to grow. Complete removal of meningiomas that had formed in these areas is difficult in many cases if they had grown while invading other important structures (e.g., arterial circle of Willis, optic nerve) [Figure 7].
 |
Figure 8 Treatment of the pituitary tumor via TSS using a high definition arthroscope. As Mamelak AN reported in 2014,30 magnifying and illuminating the operative field helps surgeons perform TSS. A high definition arthroscope with a xenon fiber optic light is also useful for TSS. An eleven-year-old, castrated Toy Poodle was referred with complaints of polyuria, polydipsia, and alopecia (A). Preoperative MR T2-weighted images revealed an enlarged pituitary tumor (B; sagittal, C; transverse). PBR was 0.73, and MRI-grading was III-A. TSS was performed using a high definition arthroscope. Series of figures (D) to (G) show the process of TSS. (D) The opening in the sphenoid bone. The ventral surface of the pituitary mass was confirmed. (E): Incision of the dura in a cruciate pattern was made. (F): Tumors were removed using a grasper and suction through the opening of the sphenoidal bone. (G): After the tumor was almost completely removed, the arthroscope was introduced into the third ventricle, which was enlarged by the growth of the tumor. The ventral side of the interthalamic adhesion was seen (*). Immediate postoperative MR T2-weighted images revealed an enlarged and air-filled space in the third ventricle, due to the removal of the tumor (h; sagittal, i; transverse). Figure (J) represents the gross appearance of this case at 6 months after TSS. The systemic alopecia observed prior TSS gradually remitted. |
General Condition of the Patients
In patients with PDH, the metabolic disorder progresses at a cellular/tissue level throughout the body if tissues throughout the body have been exposed to HAC. The extent varies with the degree of hypercortisolemia and exposure duration. Of these cases, some make the induction of the general anesthesia difficult owing to their general conditions. Although a HAC stage classification based on the effects of HAC on tissues throughout the body and a strategy for objectively determining the range of surgical indication have been suggested, currently, no such classification system exists. Thus, surgical candidate must be determined on a case-to-case basis. Complications associated with HAC include soft palate elongation, tracheal collapse, thrombosis or a thrombosis-inducing hypercoagulable state, infection (e.g., cystitis), hypertension, mitral valve regurgitation, excessive intraperitoneal adipose tissue, and diabetes.1,50–53
Furthermore, the cranial structure of each case (e.g., cranial morphology [brachycephalic vs dolichocephalic breeds], sphenoid bone thickness, and canine size [large vs toy breeds]) can affect TSS performance as well. Burring of the sphenoid bone is essential when undertaking a surgical approach from the nasopharynx to the pituitary fossa. Here, the thicker the sphenoid bone, the more limited the operating field will be when the burr hole reaches the pituitary fossa. This can also result in the risk of incomplete removal of the pituitary adenoma.23 In addition, care must be given to canine size. Ishino et al reported a successful TSS procedure on a 3-kg chihuahua, but generally surgical procedures can be difficult in toy breeds, whose body sizes are extremely small.54
Neurosurgical Procedural Skill
Even among neurosurgical procedures, TSS with an approach from the base of the skull is a difficult surgical procedure. Particularly in TSS, ensuring a large operating field by removing large parts of the skull is impossible, given the vascular structure around the pituitary gland. Tumor resection is performed through a small burr hole with a diameter of approximately 5–10 mm created on the base of the sphenoid bone. The left and right sides of the pituitary gland are in contact with the cavernous sinus, the arterial circle of Willis is located in the outer dorsal side of the pituitary gland, and there is the optic chiasma in front of the pituitary gland. The diencephalon and third ventricle are located in the dorsal side through the pituitary stalk. When the tumor size continues to increase, the tumor will grow in the dorsal direction and extend into the third ventricle, significantly reducing the field of view during surgery. Thus, to perform the TSS operation, neurosurgeons must have extensive knowledge on the paths and positional relationships of the blood vessel and cerebral nerves in the skull, and the regional anatomy of the nasopharynx. Mamelak et al advocated the use of a high definition video telescope to more safely conduct TSS operations on larger pituitary tumors. They additionally introduced the pilot hole technique, which is aimed at identifying the pituitary gland location during surgery, and the two-hand two-suction technique for the extraction of large pituitary tumors. In the two-hand two-suction technique, the pituitary tumor is guided into the sphenoid bone burr hole, with the suction maintained in one hand of the operating surgeon while the tissue is debulked using another suction maintained in the other hand.30 Furthermore, recent reports have indicated an accurate surgical approach that uses a neuronavigation system in TSS operations for canines as well. Even in cases where complete removal of the tumor was usually difficult, increases in operation safety and complete removal rates can be expected31 [Figure 8].
In small animal clinical practice, all operations have a learning curve and surgeons improve their operation performance with experiences. Similarly, on the basis of reports on clinical outcome after TSS, the postoperative death rates in later series were lower than those in earlier series; thus, we can infer that the progress in the learning curve was related to the increased number of cases with a surgical operation.20,24,30 Therefore, it will be essential for an inexperienced surgeon to receive the systemic education on TSS before treating the actual clinical case.
Complications
All surgical treatments confer the risk of postoperative complications. Similarly, several known complications are associated with TSS, including hemorrhage, CDI, respiratory distress, recurrence of HAC, bacterial meningitis, keratoconjunctivitis sicca, and transitory visual impairment.20,26 Furthermore, the mortality rate within 4 weeks of TSS operation was reported to range from 8.8% to 19%.24,30 The reported causes of death post-TSS include severe bronchopneumonia, intraoperative hemorrhaging and the associated hematomas in the hypothalamus and meninges, hypernatremia, thrombotic endocarditis in the right atrium, and glucocorticoid-associated myotonia.20,22,24,30
Hemorrhage
Hemorrhage from cancellous bone or the cavernous sinus surrounding the pituitary gland can occur during burring of the sphenoid bone, but this can be relatively easy to control using hemostatic materials such as Gelform. However, because injuries to the artery forming the arterial circle of Willis can be fatal, careful MRI evaluation of the positional relationships between the pituitary tumor and blood vessels prior to surgery is exceedingly important. The risk of adhesion between the pituitary tumor and blood vessels can be high in animals with advanced age.55 However, whether the pituitary gland is adhering to the blood vessels is difficult to confirm with MRI. Suction and graspers are used during removal of the pituitary tumor, and nerve hooks are used when confirming the presence of residual tissue after tumor removal, but care must be given during surgery to ensure that these surgical tools do not excessively pull on any tissues in the area.20
CDI
With regard to postoperative management, all cases must be carefully monitored for CDI with acute hypernatremia, which can occur immediately after surgery. Unlike TSS in humans, TSS in canines can completely remove pituitary tumors including normal tissues. This is associated with cases in which the adenoma in the pituitary gland has grown, with its volume becoming several times larger than the normal tissue volume during diagnosis and surgery. With the loss of the posterior lobe (which functions as an AVP secretion area) after surgery, AVP hyposecretion and the resultant CDI subsequently develop.56 Acute hypernatremia (Na+ > 150 mmol/L) caused by the large amount of low specific-gravity urine discharge can occur 4–16 hrs (median, 12 hrs) after TSS in healthy canines. Plasma Na+ concentration levels can reach 160–180 mmol/L in these cases and, if left untreated, can subsequently lead to hemorrhage in the brain stem (pons and medulla oblongata) and trigger fatal encephalopathy. Therefore, after TSS, a urethral catheter is left in the treated animal for 3–5 days after awakening from anesthesia, with frequent monitoring of urine volume and specific gravity every 1–5 hrs. Antidiuretic hormone treatment and transfusion therapy can be used to treat postoperative CDI. Antidiuretic hormone treatment is highly compatible with V2 receptors, and management based on desmopressin acetate (DDAVP; 4 μg conjunctival administration, twice daily, or 0.1–0.2 μg/kg subcutaneous injection, once to twice daily), which has a short reaction time, is considered effective.56 However, care must be taken since excessive DDAVP administration can trigger water intoxication with the hyponatremia. At the maintenance stage (ie, 1 week after operation), hormone replacement therapy must be regulated on a case-to-case basis. Generally, if preoperative MRI has confirmed that the pituitary tumor has not expanded into the third ventricle, postoperative CDI will be transitory and returns to normal approximately 2 weeks after operation. A possible explanation of this phenomenon is that a pseudo-posterior lobe is formed in the pituitary stalk after hypophysectomy, which restarts AVP secretion.57 However, Taoda et al reported that the AVP secretion ability did not sufficiently recover in healthy dogs even at 3 months after the TSS procedure.58 An explanation for this may be the possible involvement of compensatory mechanisms such as V2 receptor upregulation in the kidney. In addition to DDAVP, hormone replacement therapy with two drugs (glucocorticoids and thyroid hormone) is necessary after TSS.19,20 In cases with small pituitary tumor, postoperative CDI is transitory and DDAVP administration becomes unnecessary 1–2 weeks after surgery. However, if preoperative MRI has confirmed that the pituitary tumor has expanded into the third ventricle, postoperative CDI will become permanent.22,27 In these cases, postoperative hormone replacement therapy requires the administration of three drugs (antidiuretic, glucocorticoids, and thyroid hormones). This situation may indicate that the supraoptic and/or paraventricular nucleus of the hypothalamus facing the third ventricle is being damaged by the pituitary tumor, and AVP production is disrupted.
Respiratory Distress After Surgery
Not many reports have described cases of respiratory distress as a complication of surgery,20 but at our facility, this is sometimes observed immediately after TSS in small canines with either soft palate hypertrophy or an elongated soft palate. In animals with an elongated soft palate, nasopharyngeal stenosis can occur as a result of the inflammatory swelling of the soft palate itself due to incisions and suturing during surgery, which can induce difficulty breathing (e.g., upper respiratory tract obstruction). These complications typically occur within a few days after the TSS procedure and is characterized by progressive labored breathing. In these cases, an oxygen-supplying catheter is transdermally inserted in the cervical trachea, and continuous oxygen supply is provided for a few days (typically 2–4 days). The nasopharyngeal stenosis is then relieved once the soft palate inflammation has subsided, which in turn relieves the canine of respiratory distress. The oxygen-supplying catheter is removed once return of canine respiration to normal is confirmed, and the oxygen supply from the catheter is cut.
Recurrence
The recurrence rate of canine PDH after TSS has been reported to be 13.8–27%.24,29 The recurrence mechanisms include the incomplete removal of the ACTH-producing adenoma or adenomatosis of a residual ACTH-producing tissue after surgery.57 Studies that used experimental canines showed that suction-based TSS procedures are possibly incomplete because residual pituitary cells may be present in the fibrous tissue of the sella turcica after surgery.55,59 Meij et al also conducted histological analyses of the diencephalon region in experimental canines treated with TSS and euthanized 10 weeks after surgery. Although they did not confirm the persistence of pituitary gland tissue in the ventral side of the hypothalamus, they detected pituitary gland tissue embedded within the fibrous tissue of the sella turcica.19
Researchers have focused on tumor recurrence related to TSS treatment of PDH and analyzed its predictive factors. Significantly higher PBR and UCCR values have been observed in recurring cases relative to non-recurring cases.22 Hanson et al reported that the influencing factors of post-TSS recurrence in canines with PDH include sphenoid bone thickness, pituitary gland size, plasma α melanocyte-stimulating hormone(αMSH), and UCCR.23 In addition, van Rijn et al from the same group indicated that high ACTH concentration levels at 5 hrs after TSS, high cortisol concentration levels at 1 and 4 hrs after TSS, high standardized ACTH concentration levels at 3 hrs after TSS, high standardized cortisol concentration levels at 4 hrs after TSS, and irregular shifts in cortisol concentration were factors related to the reduction of remission duration after TSS. However, evaluations using other indicators such as PBR are still necessary for evaluating the risk of HAC after TSS.35 The recurrence of clinical symptoms in canine PDH cases treated with TSS is usually defined as an endocrinal recurrence. However, this definition changes when the pituitary tumor is considered from another perspective as an intracranial space-occupying lesion (i.e., a brain tumor). In our experience, in cases where most of the tumor was visually removed in the first round of TSS and the tumor was not identified on MRI immediately after surgery, the tumor is often undetectable even with repeated MRI after the recurrence of clinical symptoms. In other words, TSS may prevent pituitary tumor mass effects related to neurological disorders that are triggered in PDH patients.
Prognostic Fators After TSS
Hanson et al reported on the prognostic factors of PDH in canines after TSS.22 They reported that cases with an enlarged pituitary tumor had statistically significantly shorter survival times and remission durations than those with a non-enlarged pituitary tumor. In other words, preoperative pituitary tumor size can affect prognosis. Factors other than pituitary size that affect the post-TSS survival of canines with PDH include resting-state plasma ACTH concentration and age at the time of operation. Regarding the growth markers of the pituitary adenoma itself, the survival time is short when the Ki-67 index is >3%.47 In addition, Vastenhout et al reported recently that the pituitary tumor-transforming gene 1 (PTTG1) mRNA expression level was three times higher than normal in an ACTH-producing pituitary tumor and that tumors with higher PTTG expression levels had shorter remission durations after TSS.60
Conclusion
The most typical cause of Cushing’s syndrome in canines is ACTH-producing pituitary adenoma. Clinically, Cushing’s syndrome is often the treatment focus in canines with PDH, however ACTH-producing pituitary adenoma also manifest as intracranial tumors. In cases with a definitive diagnosis of PDH, a cerebral MRI is essential in the early stages, prior to starting treatment. If the intracranial tumor is left untreated, the tumor growth over time will trigger neurological symptoms in the patient. Furthermore, the tumor growth can trigger intracranial hypertension, which can have fatal results such as cerebral herniation. TSS is an effective treatment that can directly remove pituitary tumors that cause PDH in canines under a single course of general anesthesia. However, its success is largely dependent on the surgeon’s technical expertise and postoperative management. Neurosurgeons, endocrinologists, and diagnostic imaging specialists must work together as a team to achieve treatment success. Enlarged pituitary tumors can shorten the post-TSS survival time, increase the recurrence rate of clinical symptoms, and increase the risk for developing permanent CDI. Therefore, complete removal of adenomas of up to Grade IIIA according to the MRI-based classification system is relatively easy to achieve with TSS, and long-term remission and survival can be expected.
Consent for Publication
For the owners of cases that I showed in this article, I have gotten the permission of using them for this article.
Data Sharing Statement
All data which I used for this text depend on article quotation.
Acknowledgment
I would like to thank Editage for English language editing.
Funding
The author received no financial support nor other conflicts related to this article.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Behrend EN. Canine hyperadrenocorticism. In: Feldman EC, Nelson RW, Reusch CE, Scott-Moncrieff JCR, Behrend EN, editors. Canine & Feline Endocrinology.
2. Feldman EC, Nelson RW. Canine hypersdrenocorticism(Cushing’s syndrome). In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. Philadelphia: WB Saunders; 2004:252–357.
3. Capen CC, Martin SL, Koestner A. Neoplasms in the adenohypophysis of dogs. Path Vet. 1967;4:301–325.
4. Halmi NS, Peterson ME, Colurso GJ, et al. Pituitary intermediate lobe in dogs: two types and high bioactive adrenocorticotrophin content. Science. 1981;211:72–74.
5. Peterson ME, Krieger DT, Drucker WD, et al. Immunocytochemical study of the hypophysis in 25 dogs with pituitary-dependent hyperadrenocorticism. Acta Endocrinol. 1982;101:15–24.
6. Kipperman BS, Feldman EC, Dybdal NO, et al. Pituitary tumor size, neurologic signs, and relation to endocrine test results in dogs with pituitary-dependent hyperadrenocorticism: 43 cases(1980–1990). J Am Vet Med Assoc. 1992;201(5):762–767.
7. Sarfaty D, Carrillo JM, Peterson ME. Neurologic, endocrinologic, and pathologic findings associated with large pituitary tumors in dogs: eight cases(1976–1984). J Am Vet Med Assoc. 1988;193(7):854–856.
8. Willeberg P, Priester W. Epidemiological aspects of clinical hyperadrenocorticism in dogs(canine Cushing’s syndrome). J Am Anim Hosp Assoc. 1982;18:717–724.
9. Fracassi F, Corradini S, Floriano D, et al. Prognostic factors for survival in dogs with pituitary-dependent hypercortisolism treated with trilostane. Vet Rec. 2015. doi:10.1136/vr.102546
10. Nelson RW, Ihle SL, Feldman EC. Pituitary macroadenomas and macroadenocarcinomas in dogs treated with mitotane for pituitaery-dependent hyperadrenocorticism: 13 cases(1981–1986). J Am Vet Med Assoc. 1989;194(11):1612–1617.
11. Taoda T, Hara Y, Takekoshi S, et al. Effect of mitotane on pituitary corticotrophs in clinically normal dogs. Am J Vet Res. 2006;67(8):1385–1394. doi:10.2460/ajvr.67.8.1385
12. Teshima T, Hara Y, Takekoshi S, et al. Trilostane-induced inhibition of cortisol secretion results in reduced negative feedback at the hypothalamic-pituitary axis. Domest Anim Endocrinol. 2009;36:32–44. doi:10.1016/j.domaniend.2008.10.002
13. Nelson D, Meakin J, Thorn G. ACTH-producing pituitary tumors following adrenalectomy for cushing’s syndrome. Ann Intern Med. 1960;52:560–569.
14. Barker FG
15. Markowitz J, Archibald J, Downie HG. Hypophysectomy in dogs. In: Markowitz J, editor. Exp Surg Bartimore. The William & Wilkins Co; 1964:630–643.
16. Rijnberk A, der Kinderen PJ, Thijssen JHH. Spontaneous hyperadrenocorticism in the dog. J Endocrinol. 1968;41:397–406. doi:10.1677/joe.0.0410397
17. Rijnberk A, der Kinderen PJ, Thijssen JHH. Canine cushing’s syndrome. Zentralbatt Fur Veterunarmedizin. 1969;16:13–28. doi:10.1111/j.1439-0442.1969.tb01035.x
18. Niebauer GW, Evans SM. Transsphenoidal hypophysectomy in the dog: a new technique. Vet Surg. 1988;17(6):296–303. doi:10.1111/j.1532-950X.1988.tb01021.x
19. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Transsphenoidal hypophysectomy in beagle dogs: evaluation of a microsurgical technique. Vet.Surg. 1997;26:295–309. doi:10.1111/j.1532-950X.1997.tb01502.x
20. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Results of transsphenoidal hypophysectomy in 52 dogs with pituitary-dependent hyperadrenocorticism. Vet Surg. 1998;27:246–261. doi:10.1111/j.1532-950X.1998.tb00123.x
21. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Transsphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in 7 cats. Vet.Surg. 2001;30:72–86.
22. Hanson JM, Van’t Hoofd MM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med. 2005;19:687–694.
23. Hanson JM, Teske E, Voorhout G, et al. Prognostic factors for outcome after transsphenoidal hypophysectomy in dogs with pituitary-dependent hyperadrenocorticism. J Neurosurg. 2007;107:830–840.
24. van Rijn SJ, Galac S, Tryfonidou MA, et al. The influence of pituitary size on outcome after transsphenoidal hypophysectomy in a large cohort of dogs with pituitary-dependent hypercortisolism. J Vet Intern Med. 2016;30:989–995. doi:10.1111/jvim.14367
25. Hara Y, Teshima T, Taoda T, et al. Efficacy of transsphenoidal surgery on endocrinological status and serum chemistry parameters in dogs with Cushing’s disease. J Vet Med Sci. 2010;72(4):397–404.
26. Hara Y, Tagawa M, Masuda H, et al. Transsphenoidal hypophysectomy for four dogs with pituitary ACTH-producing adenoma. J Vet Med Sci. 2003;65(7):801–804.
27. Teshima T, Hara Y, Taoda T, et al. Central diabetes insipidus after transsphenoidal surgery in dogs with Cushing’s disease. J Vet Med Sci. 2011;72(1):33–39.
28. Ishino H, Hara Y, Teshima T, et al. Hypophysectomy for a dog with coexisting Cushing’s disease and diabetes mellitus. J Vet Med Sci. 2010;72(3):343–348.
29. Sato A, Teshima T, Ishino H, et al. A magnetic resonance imaging-based classification system for indication of trans-sphenoidal hypophysectomy in canine pituitary-dependent hypercortisolism. J Small Anim Pract. 2016;57:240–246. doi:10.1111/jsap.12474
30. Mamelak AN, Owen TJ, Bruyette D. Transsphenoidal surgery using a high definition video telescope for pituitary adenomas in dogs with pituitary dependent hypercortisolism: methods and results. Vet Surg. 2014;9999:1–11. doi:10.1111/j.1532-950X.2014.12146.x
31. Owen TJ, Chen AV, Frey S, et al. Transsphenoidal surgery: accuracy of an image-guided neuronavigation system to approach the pituitary fossa(sella turcica). Vet Surg. 2018;47:664–671. doi:10.1111/vsu.12906
32. Feldman EC, Nelson RW, Feldman MS. Use of low- and high-dose dexamethasone tests for distinguishing pituitary-dependent from adrenal tumor hyperadrenocorticism in dogs. J Am Vet Med Assoc. 1996;209(4):772–775.
33. Bosje JT, Rijnberk A, Mol JA, et al. Plasma concentrations of ACTH precursors correlate with pituitary size and resistance to dexamethasone in dogs with pituitary-dependent hyperadrenocorticism. Domest Anim Endocrinol. 2002;22:201–210.
34. Gallelli MF, Cabrera Blatter MF, Castillo V. A comparative study by age and gender of the pituitary adenoma and ACTH and α-MSH secretion in dogs with pituitary-dependent hyperadrenocorticism. Res Vet Sci. 2010;88:33–40. doi:10.1016/j.rvsc.2009.06.011
35. van Rijn SJ, Hanson JM, Zierikzee D, et al. The prognostic value of perioperative profiles of ACTH and cortisol for recurrence after transsphenoidal hypophysectomy in dogs with corticotroph adenomas. J Vet Intern Med. 2015;29:869–876. doi:10.1111/jvim.12601
36. Escourolle H, Abecassis JP, Bertagna X, et al. Comparison of computerized tomography and magnetic resonance imaging for the examination of the pituitary gland in patients with Cushing’s disease. Clini Endocrinol(Oxf). 1993;39:307–313.
37. Bertoy EH, Feldman EC, Nelson RW, et al. One-year follow-up evaluation of magnetic resonance imaging of the brain in dogs with pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1996;208(8):1268–1273.
38. Duesberg CA, Feldman EC, Nelson RW, et al. Magnetic resonance imaging for diagnosis of pituitary macrotumors in dogs. J Am Vet Med Assoc. 1995;206(5):657–662.
39. van der Vlugt-meijer RH, Meij BP, van den Ingh TSGAM, et al. Dynamic computed tomography of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med. 2003;17:773–780.
40. Bertoy EH, Feldman EC, Nelson RW, et al. Magnetic resonance imaging of the brain in dogs with recently diagnosed but untreated pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1995;206(5):651–656.
41. Kooistra HS, Voorhout G, Mol JA, et al. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J Endocrinol. 1997;152:387–394.
42. Graham JP, Roberts GD, Newell SM. Dynamic magnetic resonance imaging of the normal canine pituitary gland. Vet Radiol Ultrasoud. 2000;41(1):35–40.
43. Kurokawa H, Fujisawa I, Nakano Y, et al. Posterior lobe of the pituitary gland: correlation between signal intensity on T1-weighted MR images and vasopressin concentration. Radiology. 1998;207:79–83.
44. Teshima T, Hara Y, Masuda H, et al. Relationship between arginine vasopressin and high signal intensity in the pituitary posterior lobe on T1-weighted MR images in dogs. J Vet Med Sci. 2008;70(7):693–699.
45. Taoda T, Hara Y, Masuda H, et al. Magnetic resonance imaging assessment of pituitary posterior lobe displacement in dogs with pituitary-dependent hyperadrenocorticism. J Vet Med Sci. 2011;73(6):725–731.
46. Fracassi F, Mandrioli L, Shehdula D, et al. Complete surgical removal of a very enlarged pituitary corticotroph adenoma in a dog. J Am Anim Hosp Associ. 2014;50:192–197. doi:10.5326/JAAHA-MS-5987
47. Miller MA, Owen TJ, Bruyette DS, et al. Immunohistochemical evaluation of canine pituitary adenomas obtained by transsphenoidal hypophysectomy. Vet Pathol. 2018;55(6):889–895. doi:10.1177/0300985818784160
48. Ishino H, Hara Y, Takekoshi S, et al. Ki-67 and minichromosome maintenance-7(MCM7) expression in canine pituitary corticotroph adenomas. Domest Anim Endocrinol. 2011;41:207–213. doi:10.1016/j.domaniend.2011.07.002
49. Miller Jr WH Parapituitary meningioma in a dog with pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1991;198(3):444–446.
50. Burns MG, Kelly AB, Hornof WJ, et al. Pulmonary artery thrombosis in three dogs with hyperadrenocorticism. J Am Vet Med Assoc. 1981;178(4):388–393.
51. Chen HY, Lien YH, Huang HP. Assessment of left ventricular function by two-dimensional speckle-tracking echocardiography in small breed dogs with hyperadrenocorticism. Acta Vet Scand. 2014;56:88. doi:10.1186/s13028-014-0088-5
52. Park FM, Blois SL, Abrams-Ogg ACG, et al. Hypercoagulability and ACTH-dependent hyperadrenocorticism in dogs. J Vet Intern Med. 2013;27:1136–1142. doi:10.1111/jvim.12162
53. Teshima T, Hara Y, Taoda T, et al. Cushing’s disease complicated with thrombosis in a dog. J Vet Med Sci. 2008;70(5):487–491.
54. Ishino H, Takekoshi S, Teshima T, et al. Hyperadrenocorticism caused by a pituitary ganglioglioma in a dog. Vet Pathol. 2018. doi:10.1177/0300985819829530
55. Niebauer GW, Eigenmann JE, van Winkle TJ. Study of long-term survival after transsphenoidal hypophysectomy in clinically normal dogs. Am J Vet Res. 1990;51(4):677–681.
56. Hara Y, Masuda H, Taoda T, et al. Prophylactic efficacy of desmopressin acetate for diabetes insipidus after hypophysectomy in the dog. J Vet Med Sci. 2003;65(1):17–22.
57. Meij BP, Mol JA, van den Ingh TSGAM, et al. Assessment of pituitary function after transsphenoidal hypophysectomy in beagle dogs. Domest Anim Endocrinol. 1997;14(2):81–97.
58. Taoda T, Hara Y, Masuda H, et al. Functional and morphological changes in the hypothalamus-pituitary posterior lobe system after hypophysectomy in the dog. J Vet Med Sci. 2006;68(1):1–7.
59. Lantz GC, Ihle SL, Nelson RW, et al. Transsphenoidal hypophysectomy in the clinically normal dog. Am J Vet Res. 1988;49(7):1134–1142.
60. Vastenhout N, van Rijn SJ, Riemers FM, et al. The mRNA expression of PTTG1 is a strong prognostic indicator for recurrence after hypopfysectomy in dogs with corticotroph pituitary adenomas. Vet J. 2018;240:19–21. doi:10.1016/j.tvjl.2018.08.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.