Back to Journals » Patient Related Outcome Measures » Volume 15
Translation and Cross-Cultural Adaptation into French of the Harris Hip Score and the Modified Harris Hip Score
Authors Bothorel H, Pernoud A , Christofilopoulos P
Received 11 September 2023
Accepted for publication 16 February 2024
Published 5 March 2024 Volume 2024:15 Pages 81—91
DOI https://doi.org/10.2147/PROM.S439707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Howland
Hugo Bothorel,1 Anthony Pernoud,1 Panayiotis Christofilopoulos2
1Research Department, la Tour Hospital, Geneva, Switzerland; 2Department of Orthopaedics and Trauma Surgery, La Tour Hospital, Geneva, Switzerland
Correspondence: Anthony Pernoud, Tel +41 22 719 78 74, Email [email protected]
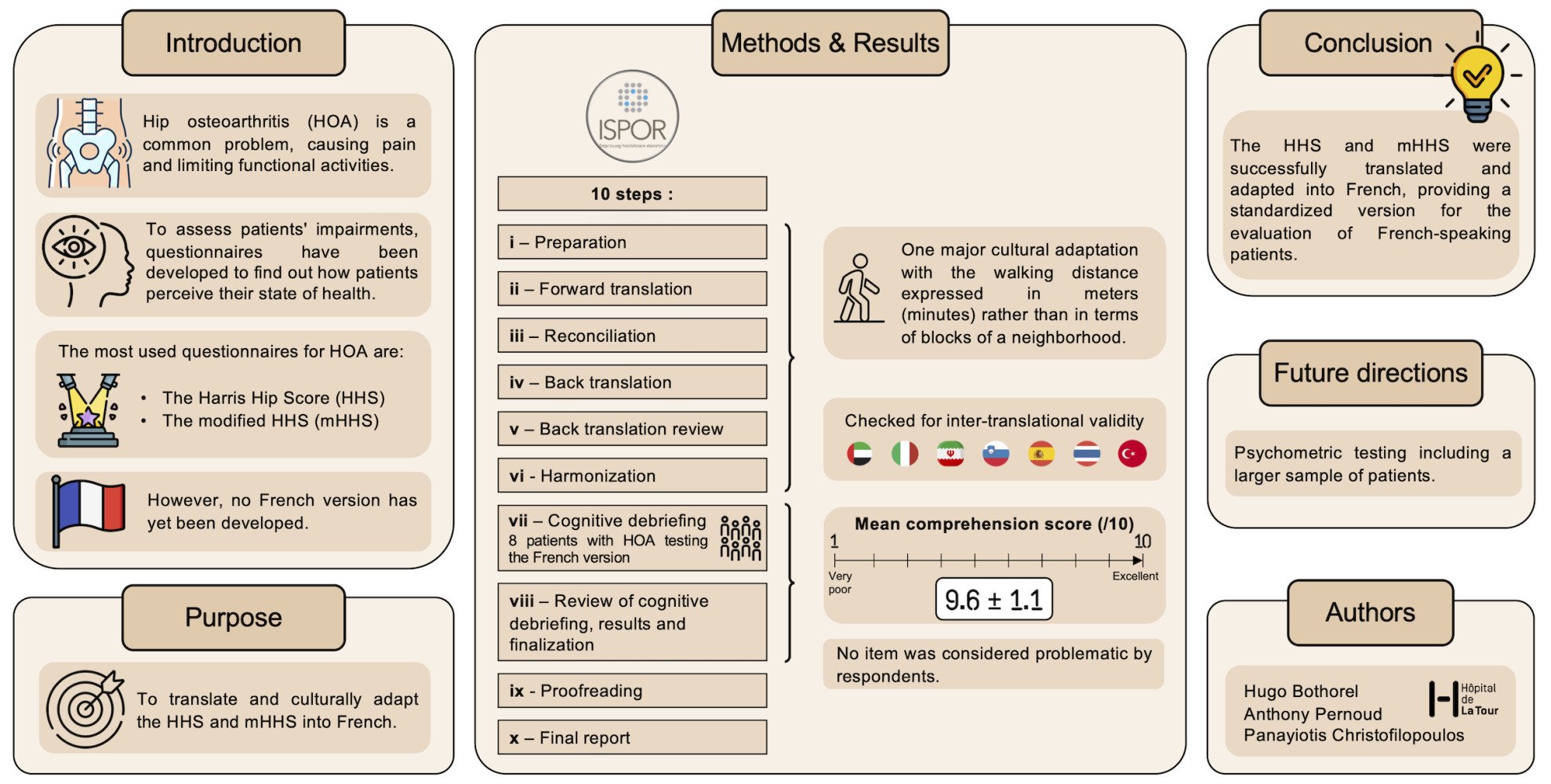
Purpose: The objective of this study was to develop a cross-cultural adaptation and translation into French of the original Harris Hip Score (HHS) and the modified Harris Hip Score (mHHS).
Patients and Methods: The translation and cultural-adaptation of the questionnaire were performed following a 10-step process as recommended by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). During the cognitive debriefing, each item of the questionnaire has been rated between 1 and 10 according to the comprehension level of 8 patients who underwent total hip arthroplasty.
Results: The cross-cultural adaptation process required the modification of the walking distance item by converting the number of blocks into meters but also in walking time (minutes). All the different steps have been performed without any other important changes on the translated questionnaire which has been found highly understandable by interviewed patients (9.6 ± 1.1).
Conclusion: This study successfully reports the French version development of the original HHS (HHS-Fr) and its modified patient-reported version (mHHS-Fr), thereby providing to clinicians a standardised version for the evaluation of French-speaking patients suffering from hip osteoarthritis.
Keywords: hip, PROM, patient-reported outcome measure, osteoarthritis, French
Graphical Abstract:
Introduction
Osteoarthritis (OA) is a highly prevalent condition, especially on the hip joint which symptomatically affects 25% of the people who live to age 85.1,2 Beyond its high prevalence among the elderly population, it is worth noting that the symptoms triggered by hip OA represent a leading cause of disability and pain,3 thereby considerably reducing patient quality of life, work productivity and physical activities.4 Conservative treatment, involving weight management and exercise therapy, is an effective first-line treatment to alleviate such symptoms in mild to moderate OA.5 When patients are unresponsive, however, surgical intervention is required.6 Often defined as “the operation of the century”,7 total hip arthroplasty (THA) remains the gold standard operation to successfully treat this pathology and currently represents one of the most common surgery performed in healthcare institutions with an increasing demand.8
Evaluating healthcare treatments is essential to better appreciate patients’ clinical evolution after surgery. Several instruments have been created for THA evaluation,9–11 including the Harris Hip Score (HHS) which remains one of the most commonly used questionnaire.12,13 Aligned with the emerging concept of value-based healthcare (VBHC), evaluating the success of surgery from the patients’ perspective becomes to date of great interest to ensure that a real clinical benefit is given in what matters the most to them.14,15 Therefore, patient-reported outcome measures (PROMs) have been increasingly used during the last decades to have a relevant and unbiased assessment of the patient health status before treatment and throughout recovery.16–18 The HHS includes both patient-reported and clinician-reported items. The patient-reported items assess pain and physical functioning in daily activities. The clinician-reported items investigate the range of motion and physical deformities. PROMs are easy to use as they do not require the presence of the clinician, thereby limiting any influence. Therefore, a modified version of the HHS (mHHS) has been developed. In the mHHS, the clinician-reported items from the original HHS (eg range of motion, limb length discrepancy) have been removed, providing a valid19 and fully patient-reported version of the questionnaire.20
PROMs development follows multiple validation steps to ensure their validity and correct patient interpretation, hence, their translation towards a different language which preserves the aforementioned characteristics while integrating the new cultural specificities needs to be thoroughly performed. The HHS and/or its modified version (mHHS) have been translated from English into different languages, such as Arabic,21 Greek,22 Italian,23 Portuguese,24 Slovenian,25 Spanish26 and Turkish,27 though no official French version has been proposed yet despite its wide use.12 Therefore, the purpose of this study was to develop a French translation and cross-cultural adaptation of the HHS (HHS-Fr) and mHHS (mHHS-Fr) for French-speaking patients.
Materials and Methods
HHS Score
The HHS (Table 1) is composed of two parts. The first one (91 points), patient-reported, contains 8 questions assessing equally pain (44 points) and function (47 points), with the latter divided into daily activities (14 points) and gait (33 points). The second part of the HHS (9 points), clinician-reported, tackles absence of deformity (4 points) and range of motion (5 points).
 |
Table 1 Original Harris Hip Score Questionnaire |
mHHS Score
The mHHS encompasses only the patient-reported part of the HHS. Accordingly, the mHHS evaluates pain (44 points) and function (47 points), with function covering daily activities (14 points) and gait (33 points). As a result, the overall score for the mHHS ranges from 0 (indicating the worst condition) to 91 (indicating the best condition). To convert the mHHS score into a range from 0 (indicating the worst condition) to 100 (indicating the best condition), the overall mHHS score is multiplied by 1.1 and rounded to the nearest integer.
Cultural Adaptation and Translation Process
The cross-cultural adaptation and translation process was carried out according to the recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), also known as the Professional Society for Health Economics and Outcomes Research,28 comprising 10 steps (Figure 1): preparation, forward translation, reconciliation, back translation, back translation review, harmonization, cognitive debriefing, cognitive debriefing review, proofreading and final report. All patients who participated in this study provided a written informed consent for the use of their answers in research projects.This study was conducted in accordance with the Declaration of Helsinki., but since this research was qualitative and only relied on non-medical data, an a priori approval from the local ethical committee was, however, not required.
 |
Figure 1 Process for the cross-cultural adaptation and translation into French of the HHS and mHHS. |
Translators being professional native French speakers and fluent in English, living in Switzerland and having experience in PROMs translation were contacted.
Two independent translations from English to French were developed (V1 and V2) by two translators (T1 and T2) contacted in step i.
A selection of the most appropriate translations of V1 and V2 was made between the translators and the project manager (PM), experienced in PROMs use for research and clinical practice, in order to preserve the conceptual equivalence. This step resulted in the development of a third version (V3).
Two new independent translators (T3 and T4) performed a back translation of V3, producing two versions (V4 and V5). Those translators were professional English native speakers and fluent in French. They had no prior knowledge of the HHS and had not seen the source language or any other versions before or during the back translation.
The translators and PM reviewed the two back translations (V4 and V5) against the original to avoid mistranslation or omission, and to make sure conceptual equivalence was preserved. Any discrepancies identified between the back translations and the original questionnaire will lead to a reviewing of the reconciled version (V3) and potential revisions.
In this step, the PM compares V3 with other existing translations to ensure inter-translation validity.
This stage included a small, relevant sample of patients to test the French version. The purpose was to assess the comprehensibility, interpretation and cultural relevance of V3, as well as to explore for alternative wordings. The clinician-reported part of the HHS was not subjected to a cognitive debriefing as there is no need to culturally adapt the items that only concern measurements (eg range of motion or limb length discrepancy). The objective of this step is to test the proposed translation on the targeted patients. Therefore, the patient-reported part of the HHS (mHHS) was distributed to patients with either hip OA or hip prosthesis. Respondents had a 10-point Likert scale to assess the level of comprehension for each question ranging from 1 (not understood at all) to 10 (fully understood). Respondents who rated their comprehension level as ≤6 for a specific item were asked for a suggestion to improve comprehension. A translation was validated if the median score of the item was ≥7.
Translators (T1 and T2) and PM evaluated patients’ comments on the comprehensibility, interpretation and cultural relevance of V3, and suggested alternative formulations. If any items were found to be problematic, T1, T2 and PM addressed them by making the necessary changes to V3, incorporating respondents’ suggestions. This resulted in a final version (VF).
The PM proofread the final version to check for grammatical and spelling errors.
A report on the development of the HHS-Fr was written to detail the concept and wording choices to harmonized subsequent translations.
Results
Forward Translation
One item was found problematic in the function domain (walking distance) due to conceptual differences and was therefore adapted by favoring the cultural adaptation over the literary translation (see Discussion).
Backward Translation
The PM deemed that V3 was an adequate French translation with a valid cross-cultural adaptation as both V4 and V5 captured the full meaning of the original mHHS.
Cognitive Debriefing and Final Version
Eight native French speakers with either hip OA or hip prosthesis were included in the cognitive debriefing. Following their review of the French translation (V3), the mean (± standard deviation) level of comprehension for the whole mHHS was 9.6 ± 1.1. No comments were proposed from patients for all the items. No major revisions were carried out as the median understanding for all the items was 10. The level of comprehension of the different items can be found in Table 2.
 |
Table 2 Level of Comprehension for the 8 Items |
Final Version
The PM reviewed the final version (VF) and checked for spelling and/or grammar errors. The final version of the original HHS (HHS-Fr) comprising the final version of the mHHS (mHHS-Fr) can be found in Table 3.
 |
Table 3 Final Version of the HHS-Fr and mHHS-Fr |
Discussion
Assessing the patient health status from their own perspective enables an unbiased evaluation of the clinical benefits delivered by a treatment. The modified version (patient-reported) of the original HHS is one of the most relevant and commonly used PROM for patients undergoing THA; however, this questionnaire has never been translated and cross-culturally adapted into French. In this study, the authors therefore developed a French version of the original and modified HHS following the ISPOR recommendations thereby allowing their use in the French speaking population.
For standardization purposes and to avoid errors, the ISPOR recommendations were used as a framework to cross-culturally adapt and translate the mHHS.28–30 These recommendations included 10 steps among which several forward translations and several backward translations were required. All the different steps were respected so that the final French versions of the mHHS and HHS preserve the original meaning of the standard English questionnaire. Indeed, all patients interviewed in this study rated the different questions as highly understandable with no particular remark.
During the whole process, conceptual equivalence was preferred over literary translation,31 which is particularly important in medical practice, as patients may not understand medical terms. Different translators and the PM experienced in PROMs instruments analyzed the translations in order to ensure meaning and conceptual equivalence with the standard English questionnaire. This method essentially highlighted one problematic item about the walking distance which was originally expressed in number of blocks. However, in Switzerland, and more generally in Europe, the walking distance is expressed in meters or minutes. We therefore converted blocks into miles, assuming that a block equals 1/9 mile,32 then into meters. To improve the representation of the distance, we also expressed the meters in minutes, based on the walking speed reported for postoperative THA patients.33,34 Thus, the conceptual equivalence was favored on this item for a better understanding of patients, as proposed in the translation into Slovenian.25
Although the authors thoroughly translated into French and cross-culturally adapted the HHS and the mHHS to the French speaking patients, this study did not assess their psychometric characteristics. It is worth noting that reevaluating psychometric properties for a questionnaire that has already been validated on this aspect in a different language22 remains controversial.29 As a matter of fact, an appropriate translation and cross-cultural adaptation following the ISPOR guidelines should not alter the original characteristics of the standard questionnaire.28,35
Future Directions, Clinical Implications and Lessons Learned
The HHS-Fr and mHHS-Fr will be useful to clinicians who evaluate in daily practice French-speaking patients suffering from hip OA. Future work will include a psychometric testing of the HHS-Fr and the mHHS-Fr to ensure that properties from the source questionnaire were maintained. Beyond the robust methodology followed in this study, it is important to note that different contributors (translators, patients, clinician and research experts) were needed in the translation and adaptation of these questionnaires owing to their valuable and complementary feedback.
Conclusion
This study reports the development of the original HHS (HHS-Fr) and its modified patient-reported version (mHHS-Fr) that followed the ISPOR recommendations for both translation and cultural adaptation of the English standard questionnaire into French. This work will provide clinicians a standardised version for the evaluation of French-speaking patients suffering from hip OA.
Abbreviations
HHS, Harris Hip Score; HHS-Fr, French version of the Harris Hip Score; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; mHHS, Modified Harris Hip Score; mHHS-Fr, French version of the Modified Harris Hip Score; OA, Osteoarthritis; PM, Project Manager; PROM, Patient-reported Outcome Measure; THA, Total Hip Arthroplasty; T1 and T2, Translators performing V1 and V2; T3 and T4, Translators performing V3 and V4; VBHC, Value-based Healthcare; VF, Final French version; V1 and V2, Adaptation from English to French; V3, French resulting version; V3 and V4, Adaptation from French resulting to English.
Data Sharing Statement
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study does not rely on patient health data. However, interviewed patients provided their written informed consent for the use of their answers for research purposes.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murphy LB, Helmick CG, Schwartz TA, et al. One in four people may develop symptomatic Hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18(11):1372–1379.
2. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138.
3. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi:10.1038/nrrheum.2014.44
4. Cross M, Smith E, Hoy D, et al. The global burden of Hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi:10.1136/annrheumdis-2013-204763
5. Sinatti P, Sanchez Romero EA, Martinez-Pozas O, Villafane JH. Effects of patient education on pain and function and its impact on conservative treatment in elderly patients with pain related to Hip and Knee Osteoarthritis: a systematic review. Int J Environ Res Public Health. 2022;19(10):6194. doi:10.3390/ijerph19106194
6. Gunther KP, Deckert S, Lutzner C, et al. Total Hip replacement for osteoarthritis-evidence-based and patient-oriented indications. Dtsch Arztebl Int. 2021;118(43):730–736. doi:10.3238/arztebl.m2021.0323
7. Learmonth ID, Young C, Rorabeck C. The operation of the century: total Hip replacement. Lancet. 2007;370(9597):1508–1519.
8. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision Hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi:10.2106/00004623-200704000-00012
9. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the Hip or knee. J Rheumatol. 1988;15(12):1833–1840.
10. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford Hip and knee scores. J Bone Joint Surg Br. 2007;89-B(8):1010–1014. doi:10.1302/0301-620X.89B8.19424
11. Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total Hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi:10.1186/1471-2474-4-10
12. Longo UG, Ciuffreda M, Candela V, Berton A, Maffulli N, Denaro V. Hip scores: a current concept review. Br Med Bull. 2019;131(1):81–96. doi:10.1093/bmb/ldz026
13. Riddle DL, Stratford PW, Bowman DH. Findings of extensive variation in the types of outcome measures used in Hip and knee replacement clinical trials: a systematic review. Arthritis Rheum. 2008;59(6):876–883. doi:10.1002/art.23706
14. Briffa N. The employment of patient-reported outcome measures to communicate the likely benefits of surgery. Patient Relat Outcome Meas. 2018;9:263–266.
15. Field J, Holmes MM, Newell D. PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas. 2019;10:233–241. doi:10.2147/PROM.S156291
16. Churruca K, Pomare C, Ellis LA, et al. Patient-reported outcome measures (PROMs): a review of generic and condition-specific measures and a discussion of trends and issues. Health Expect. 2021;24(4):1015–1024. doi:10.1111/hex.13254
17. Zini MLL, Banfi G. A narrative literature review of bias in collecting patient reported outcomes measures (PROMs). Int J Environ Res Public Health. 2021;18(23):12445. doi:10.3390/ijerph182312445
18. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi:10.2147/PROM.S156279
19. Edwards PK, Queen RM, Butler RJ, Bolognesi MP, Lowry Barnes C. Are range of motion measurements needed when calculating the Harris Hip Score? J Arthroplasty. 2016;31(4):815–819. doi:10.1016/j.arth.2015.10.016
20. Byrd JW, Jones KS. Prospective analysis of Hip arthroscopy with 2-year follow-up. Arthroscopy. 2000;16(6):578–587. doi:10.1053/jars.2000.7683
21. Al-Qahtani AN, Alsumari OA, Al Angari HS, Alqahtani YN, Almogbel RA, AlTurki AA. Cultural adaptation and validation of an Arabic version of the Modified Harris Hip Score. Cureus. 2021;13(4):e14478. doi:10.7759/cureus.14478
22. Stasi S, Papathanasiou G, Diochnou A, Polikreti B, Chalimourdas A, Macheras GA. Modified Harris Hip score as patient-reported outcome measure in osteoarthritic patients: psychometric properties of the Greek version. Hip Int. 2021;31(4):516–525. doi:10.1177/1120700020901682
23. Dettoni F, Pellegrino P, La Russa MR, et al. Validation and cross cultural adaptation of the Italian version of the Harris Hip Score. Hip Int. 2015;25(1):91–97. doi:10.5301/hipint.5000184
24. Guimarães RP, Alves DPL, Azuaga TL, et al. Translation and transcultural adaptation of the modified Harris Hip score. Acta Ortop Bras. 2010;18(6):339–342. doi:10.1590/S1413-78522010000600007
25. Josipovic P, Moharic M, Salamon D. Translation, cross-cultural adaptation and validation of the Slovenian version of Harris Hip score. Health Qual Life Outcomes. 2020;18(1):335.
26. Lara-Taranchenko Y, Soza D, Pujol O, Gonzalez-Morgado D, Hernandez A, Barro V. Cross-cultural adaptation for the Spanish population of the modified Harris score for functional and symptomatic Hip joint assessment. Rev Esp Cir Ortop Traumatol. 2022;66(2):128–134. doi:10.1016/j.recot.2021.08.002
27. Celik D, Can C, Aslan Y, Ceylan HH, Bilsel K, Ozdincler AR. Translation, cross-cultural adaptation, and validation of the Turkish version of the Harris Hip score. Hip Int. 2014;24(5):473–479. doi:10.5301/hipint.5000146
28. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. doi:10.1111/j.1524-4733.2005.04054.x
29. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–1432.
30. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25(24):3186–3191. doi:10.1097/00007632-200012150-00014
31. Brammen D, Greiner F, Dormann H, et al. Lessons learned in applying the international society for pharmacoeconomics and outcomes research methodology to translating Canadian emergency department information system presenting complaints list into German. Eur J Emerg Med. 2018;25(4):295–299. doi:10.1097/MEJ.0000000000000450
32. Yang Y, Diez-Roux AV. Walking distance by trip purpose and population subgroups. Am J Prev Med. 2012;43(1):11–19. doi:10.1016/j.amepre.2012.03.015
33. Queen RM, Butler RJ, Watters TS, Kelley SS, Attarian DE, Bolognesi MP. The effect of total Hip arthroplasty surgical approach on postoperative gait mechanics. J Arthroplasty. 2011;26(6 Suppl).66-–71
34. Gerhardt D, Mors TGT, Hannink G, Van Susante JLC. Resurfacing Hip arthroplasty better preserves a normal gait pattern at increasing walking speeds compared to total Hip arthroplasty. Acta Orthop. 2019;90(3):231–236. doi:10.1080/17453674.2019.1594096
35. Tuthill EL, Butler LM, McGrath JM, et al. Cross-cultural adaptation of instruments assessing breastfeeding determinants: a multi-step approach. Int Breastfeed J. 2014;9:16. doi:10.1186/1746-4358-9-16
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
