Back to Journals » Journal of Inflammation Research » Volume 14
Transcriptome Sequencing Explores the Mechanism of Baicalin on Bone Cancer Pain
Authors Wang A, Guo D, Cheng H, Jiang H, Liu X, Yun Z
Received 25 August 2021
Accepted for publication 2 November 2021
Published 16 November 2021 Volume 2021:14 Pages 5999—6010
DOI https://doi.org/10.2147/JIR.S336028
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Aitao Wang,1 Dongmei Guo,1 Hongyu Cheng,2 Hui Jiang,3 Xiaojuan Liu,1 Zhizhong Yun4
1Department of Anesthesiology, Inner Mongolia People’s Hospital, Hohhot, Inner Mongolia, 010017, People’s Republic of China; 2Department of Anesthesiology, Inner Mongolia Medical University, Hohhot, Inner Mongolia, 010110, People’s Republic of China; 3Department of Anesthesiology, Baotou Medical College, Baotou, Inner Mongolia, 014040, People’s Republic of China; 4Department of Urinary Surgery, Inner Mongolia People’s Hospital, Hohhot, Inner Mongolia, 010017, People’s Republic of China
Correspondence: Zhizhong Yun
Department of Urinary Surgery, Inner Mongolia People’s Hospital, #20, Zhaowuda Road, Saihan District, Hohhot, Inner Mongolia, 010017, People’s Republic of China
Tel +86-18047191483
Email [email protected]
Introduction: Bone cancer pain is characterized by persistent pain, usually requiring drugs to relieve pain. Baicalin, a flavonoid compound extracted from Scutellaria baicalensis, which has antioxidant and analgesic effects. But, the effect of baicalin on bone cancer pain is unclear. Thus, this study aimed to explore the mechanism of baicalin on SD rats with bone cancer pain.
Materials and Methods: The MADB-106 breast cancer cells-induced bone pain model was constructed and carried out baicalin treatment. The therapeutic effect of baicalin on bone cancer pain model was observed by hematoxylin-eosin staining and immunofluorescence staining. We also performed transcriptome sequencing analysis of baicalin in the treatment of bone metastases. Also, RT-qPCR and ELISA were used to detect the expression levels of inflammation factors.
Results: After baicalin treatment, osteoclast activation was inhibited and the number of bone trabeculae was increased. Baicalin inhibited the protein expression level of inflammatory factors (IL-1β, IL-6, TNF-α and PGE2) in the bone metastases group. Based on the transcriptome sequencing of the bone metastases group and the baicalin treatment group, baicalin inhibited the expression of ALPP, DUSP1, CYR61, ALPPL2, SPP1 and TLR4. RT-qPCR was also used to validate the expression levels of these cytokine genes.
Conclusion: Baicalin had a certain inhibitory effect on the SD rat model of bone metastasis cancer. These insights can guide future research on the molecular mechanism of bone cancer pain and provide a theoretical basis for baicalin in the treatment of bone pain caused by breast cancer in the future.
Keywords: breast cancer, bone cancer pain, baicalin, transcriptome sequencing
Introduction
With the continuous improvement of cancer treatment technology, the 3-year and 5-year survival rates of cancer patients have been greatly improved. However, cancer still cause pain to the patients, which seriously affects their quality of life. This is because in many common cancers, tumors can easily metastase to the spine, hips, ribs, femur and tibia,1 and then invade the surrounding soft tissue. Usually, tumor metastasis to bone will activate osteoclasts and lead to bone resorption, resulting in moderate to severe persistent pain.2,3 According to statistics, about 60–90% of patients with advanced cancer have suffered from varying degrees of pain, of which about 30% have suffered from continuous severe pain,4 which may be related to bone metastasis. As report went, up to 70% of breast and prostate cancer patients and up to 30% of thyroid, bladder and lung cancer patients had bone metastases.5
Currently, the treatment of pain in bone metastasis involved the use of a variety of adjuvant methods, including surgery, radiotherapy, chemotherapy, as well as drug treatment-bisphosphonates, calcitonin, analgesics. Nevertheless, the side effects of some drugs limit their clinical application, such as gastric ulcer and nephrotoxicity of non-steroidal anti-inflammatory analgesics.6 In recent years, Chinese herbal extracts have attracted the attention of many researchers. Baicalin (7-d-glucuronic acid, 5,6-dihydroxyflavone) is a flavonoid extracted from Scutellaria baicalensis and other Chinese herbal medicines. Baicalin has anti-tumor,7 anti-inflammatory,8 neuroprotective9 and anti-anxiety effects.10 In previous studies, baicalin can significantly inhibit the proliferation of human prostate cancer,11 and induce the apoptosis of human colorectal cancer cells SW620.12 Also, Wang et al revealed that baicalin suppressed the invasion and metastasis of human osteosarcoma cells by EMT induced by TGF-β1.13 However, the treatment mechanism of baicalin on bone metastases cancer has not been studied yet.
In the study, we explored the therapeutic effect of baicalin on bone metastasis by constructing SD rat model of bone metastasis cancer and carrying out baicalin treatment. And we used transcriptomics sequencing technology and RT-qPCR verification experiments to explore the treatment mechanism of baicalin on bone metastasis cancer. We hope to provide a theoretical basis for baicalin in the treatment of bone metastases=cancer.
Methods
Cell Culture
MADB-106 breast cancer cells were purchased from the Beijing Union Medical College Cell Center, and the cells were cultured into DMEM/F12 medium containing 10% FBS in a 37°C 5% CO2 incubator. When the cells reached the logarithmic phase (70–80%), trypsin was used to digest cells and collected cell suspension. After centrifugation, a cell suspension with a concentration of 1×105 cells/10 µL final dilution was prepared and put it on ice for standby.
Induction of Bone Cancer Pain and Treatment with Baicalin
SD rats were randomly divided into the NC group and the Bone metastases group after adaptive feeding for one week. SD rats were anesthetized with pentobarbital sodium (50 mg/kg). In a simple fixation device, the legs of SD rats were fixed with clips and the left leg was shaved. After disinfection with 70% v/v ethanol, the skin incision was parallel to the tibia. Drill a hole in the left tibial plateau with the disposable blood collection needle, and the breast cancer cell suspension (1 × 105 cells) diluted with 50 μL normal saline was slowly injected into the bone marrow cavity by a disposable sterile injection needle. The control group was injected with the same amount of normal saline into the bone marrow cavity.14,15 In order to prevent cells from leaking out of the bone, the intraosseous injection hole was closed with bone wax and the tumor cells left outside the bone marrow cavity were removed with 70% v/v ethanol. The animals were placed on a warm mat for recovery and then put in separate cages. During the whole operation, the aseptic operation was strictly followed. In addition, after injection, some SD rats with bone cancer pain were intraperitoneally injected with baicalin (30 mg/kg) every 2 days for 15 days.
Real-Time Fluorescence Quantitative PCR
Firstly, total RNA was extracted from the tumor tissues in bone marrow taken at 1 month by Tissues RNA Miniprep Kit (BW-R1-02, Biomiga) and was stored at −20°C. cDNA was synthesized by the PrimeScriptTM RT reagent Kit with gDNA Eraser (AJ51485A, Takara). The amplification reaction was completed by Hieff® qPCR SYBR® Green Master Mix (Low Rox) (H1911331, Shanghai Yisheng Biotechnology Co., Ltd) with the primers listed in Supplementary Table 1. The amplification procedure was performed by pre-denaturation at 50°C for 2 min, followed by 40 cycles of 95°C 10 sec and 60°C 30 sec. The final extension procedure was performed by 95°C 15 sec. Subsequently, the amplification curve was obtained at 60°C 1 min and 95°C 15 sec. Data were statistically analyzed with 2−ΔΔCt.
Enzyme-Linked Immunosorbent Assay
The blood sample of SD rats in the control group, the Bone metastases group and Bone metastases + Baicalin group were taken from the eyes of anesthetized mice. Enzyme linked immunosorbent assay (ELISA) was used to measure the protein level of tumor-derived cytokines (PGE2/IL-1β/IL-6/TNF-α) in mouse serum samples. Mouse derived PGE2 ELISA Kit was purchased from Shanghai renjie biotechnology Co., Ltd. Also, mouse derived ELISA Kit (IL-1β/IL-6/TNF-α) was purchased from Shanghai Biyuntian Biotechnology Co., Ltd. The specific steps were as follows: (1) The whole blood collected from the anesthetized mouse was put into the prepared 96-well ELISA plate, wrapped in an aluminum foil bag and placed at room temperature for 20 min. (2) We set up the standard hole and the sample hole. Add 50 μL of different concentrations of the standard, 50 μL of sample to be tested into the standard hole and the sample hole, respectively. (3) Then add 100 μL of antibody labeled with peroxidase (HRP) into the sample hole and standard hole, seal the orifice plate, and incubate in an incubator for 60 min. (4) Discard the liquid and add the washing solution for multiple washing. (5) Add 50 μL of substrate A and B, and incubate at 37°C for 15 min. (6) The OD value of each hole at the wavelength of 450nm was detected by Thermo Scientific microplate reader.
Hematoxylin-Eosin Staining
The tibia of the inoculated side was taken about 22 days after inoculation, and the surrounding muscle soft tissue was stripped and put it into 15% hydrochloric acid formaldehyde decalcification solution for 3 days. After softening, the tissue was fixed in 70% ethanol, and then dehydrated, embedded in paraffin, sectioned and stained with hematoxylin-eosin. Tumor growth and bone structure damage were observed under microscope.
Immunofluorescence Staining
Firstly, the tissue sections were fixed with 4% paraformaldehyde for 15 min and permeated with 0.1% Triton-X 100 for 10 min. The section was incubated with anti-iNOS primary antibody (Santa Cruz Biotechnology, SC-651) overnight. The primary antibody was washed without hybridization by PBS, and then incubated with the fluorescent secondary antibody. Subsequently, the sections were immersed in tartrate resistant acid phosphatase (Trap, Servicebio, G1050) solution and counterstained with hematoxylin dye solution (Servicebio, G1004) to display the nuclei. After multiple washing and drying, anhydrous ethanol and xylene were dehydrated and sealed. Under the optical microscope (Nikon, ECLIPSE E100) and imaging system (Nikon, DS-U3), the tissue sections were examined and analyzed by Imaris 8 image analysis software.
Functional Analyses
Based on the results of the transcriptome sequencing, Geno ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) were used to observe the function of differentially expressed genes (DEGs) presented by Fisher’s exact test using the clusterProfiler 2.2.1 version. Biological process (BP), cell composition (CC) and molecular function (MF) were included in GO annotation analysis. KEGG enrichment analysis mainly focused on the mRNAs-related signaling pathways.
Statistical Analysis
SPSS 20.0 software was used for data management and statistical analysis. The Student’s t-tests was used for comparison among three groups. p-values obtained from all figures were two-way analysis of variance. All the data were repeated for 3 times. p-value <0.05 was considered to have significant differences.
Results
Baicalin Recovered the Bone Resorption in Bone Cancer Pain Rats
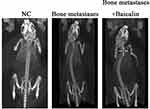
After breast cancer cell injection about 3 weeks, CT was used to examine the degree of tibial destruction in the NC group, the Bone metastases group and the Bone metastases + Baicalin group, including bone volume fraction (BV/TV), trabecular thickness (Tb.Th), the number of trabeculae (Tb.N) and the mean spacing of trabecular bone (Tb.Sp). Compared with the control group, BV/TV, Tb.Th and Tb.N were downregulated in the Bone metastases group. Although the trend of Tb.Sp was opposite, BV/TV, Tb.Th and Tb.N were significantly increased after baicalin treatment (Supplementary Figure 1). These results showed that the bone mineral density (BMD) was reduced and the bone trabeculae was sparse in the Bone metastases group, which proved the success of establishment of cancer pain model from the perspective of radiology. The above results showed that the bone resorption in the Bone metastases group was significantly reduced compared to the NC group. The bone resorption was recovered after Baicalin treatment (Figure 1).
Baicalin Inhibited Osteoclast Activation and Promoted the Increase of Bone Trabeculae in Bone Cancer Pain Rats
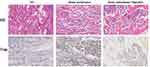
Besides, the number of trabeculae and osteoclasts in SD rats were also detected in the NC group, the Bone metastases group and the Bone metastases + Baicalin group detected by HE staining and Trap staining, respectively. Therefore, Trap is a typical marker of osteoclasts, and the results of Trap staining was that the cytoplasm of osteoclasts was wine red and the nucleus was light blue, illustrating that osteoclasts count was significantly increased in the Bone metastases group and was decreased after Baicalin treatment. HE staining also showed that the number of trabeculae decreased in the Bone metastases group and increased in the Bone metastases + Baicalin group (Figure 2).
Baicalin Inhibited the Expression of iNOS in Bone Cancer Pain Rats
NO has been reported to promote tumor metastasis, and iNOS is one of the four subtypes of NOS, which is the only rate limiting enzyme for NO synthesis. Thus, immunofluorescence detection was utilized to detect the expression of iNOS in the groups. The results revealed that the expression of iNOS was significantly increased in the Bone metastases group and decreased after Baicalin treatment (Figure 3).
Baicalin Inhibited the Expression of Inflammatory Factors in Bone Cancer Pain Rats
Subsequently, the protein level of inflammatory factors (IL-1β, IL-6, TNF-α and PGE2) were examined in the three groups shown by ELISA. In Figure 4, the IL-1β, IL-6, TNF-α and PGE2 protein level in the Bone metastases group was significantly higher than that of in the NC group. After Baicalin treatment, IL-1β, IL-6, TNF-α and PGE2 expression levels were significantly inhibited.
Transcriptome Sequencing Was Used to Detect the Therapeutic Effect of Baicalin on Bone Cancer Pain
In order to further study the therapeutic effect of Baicalin on bone cancer pain, we took the spinal cord tissues of SD rats in the NC group, the Bone metastases group and the Bone metastases + Baicalin group for transcriptome sequencing. The GO and KEGG enrichment analysis of DEGs were utilized to study the transcriptome differences in the Bone metastases group vs the NC group (Tables 1 and 2) and the Bone metastases + Baicalin group vs the NC group (Tables 3 and 4). In Tables 1 and 2, the results of top 10 enriched GO pathways of upregulated DEGs showed that the BP changes were in regulation of cellular process, regulation of nitrogen compound metabolic process, ribosome biogenesis, cell cycle, ncRNA processing, response to stimulus, rRNA metabolic process, rRNA processing, organelle organization and positive regulation of cellular metabolic process. Also, the CC changes of DEGs were obviously abundant in nucleus, nucleoplasm, nuclear lumen, intracellular membrane-bounded organelle, cytoplasm, cytosol, mitochondrion and mitochondrial membrane. Changes in MF were mainly enriched in protein binding, protein binding, enzyme binding, metal ion binding, cation binding, catalytic activity, nucleoside-triphosphatase activity and pyrophosphatase activity. The KEGG pathway analysis revealed that the upregulated DEGs were mainly enriched in ribosome biogenesis in eukaryotes, cell cycle, DNA replication, colorectal cancer, pyrimidine metabolism, mismatch repair, biosynthesis of amino acids, estrogen signaling pathway, alanine aspartate and glutamate metabolism, purine metabolism.
 |
Table 1 Top 10 Enriched GO Pathways of DEGs Between the Bone Metastases Group and the NC Group |
 |
Table 2 Top 10 Enriched KEGG Pathways of DEGs Between the Bone Metastases Group and the NC Group |
 |
Table 3 Top 10 Enriched GO Pathways of DEGs Between the Baicalin Treatment Group and the NC Group |
 |
Table 4 Top 10 Enriched KEGG Pathways of DEGs Between the Baicalin Treatment Group and the NC Group |
In the Tables 3 and 4, the results of top 10 enriched GO pathways of downregulated DEGs displayed that the BP changes were in regulation of cellular process, regulation of nitrogen compound metabolic process, cell cycle, mitotic cell cycle, regulation of cell cycle, positive regulation of cellular metabolic process, regulation of RNA metabolic process, regulation of nucleic acid-templated transcription, regulation of transcription, regulation of RNA biosynthetic process. CC changes were enriched in cytoplasm, cytosol, nucleoplasm, membrane, membrane-bounded organelle, endomembrane system, cytoskeleton and organelle membrane. The MF changes were mainly enriched in protein binding, metal ion binding, cation binding, alpha-catenin binding, cytoskeletal protein binding, DNA binding, cyclin binding, catalytic activity, transcription regulator activity and enzyme regulator activity. As for the top 10 KEGG KEGG pathway enrichment, the mRNAs were mainly enriched in AGE-RAGE signaling pathway in diabetic complications, herpes simplex virus 1 infection, salmonella infection, prion disease, malaria, endocytosis, cellular senescence, transcriptional misregulation in cancer, NF-kappa B signaling pathway, TNF signaling pathway.
RT-qPCR Was Used to Verify the Sequencing Results
According to the transcriptomic sequencing results, the mRNA expression levels of ALPP, DUSP1, CYR61, ALPPL2, SPP1 and TLR4 were significantly up-regulated in the Bone metastases group compared with the NC group. By comparing the Bone metastases + Baicalin group and the NC group, the protein expression level of these genes was significantly down-regulated. The RT-qPCR results of these genes were consistent with the results obtained by transcriptomic sequencing (Figure 5).
Discussion
It has been reported that the development of bone cancer pain was accompanied by the expression of many genes in peripheral and central nervous system were changed.16–19 Some researchers also found the changes of bone cancer pain gene expression profile by transcriptome sequencing. Some genes for the progression of pain hypersensitivity have also been discovered. However, the specific mechanism is still not very clear. In the study, we constructed the SD rat model of bone metastasis induced by MADB-106 cells and treated with baicalin. Based on the transcriptome sequencing results and RT-qPCR validation experiments, we found that baicalin had a certain inhibitory effect on the SD rat model of bone metastasis cancer.
Natural products of plants were said to treat chronic diseases, such as pain.20 Baicalin is a flavonoid extracted from Huang Qin, which has a variety of physiological functions. It has antioxidant properties,21 and has analgesic effect on migraine induced by nitroglycerin in rats,22 neuropathic pain in rats with spinal nerve ligation.23,24 Also, it was reported that it possessed anti-tumor effect in osteophilic breast cancer through inducing cell apoptosis.25 However, the presumptive effect of baicalin on bone cancer pain and its underlying mechanism were not yet clear. In the latest research, Li et al26 found that baicalin can improve mechanical hyperalgesia and thermal hyperalgesia in SD rats with bone cancer pain. In chicken liver inflammation induced by lipopolysaccharide, Cheng et al27 reported that baicalin can inhibit the expression of iNOS by TLR4-Mediated NF-κB Pathway. Min et al28 revealed that baicalin suppressed the higher expression level of iNOS in the process of UVB-induced inflammatory injury by TLR4 pathway. The above results showed that baicalin may regress the expression of iNOS in the process of inflammatory response by TLR4 pathway or TLR4-mediated pathway. In this study, baicalin can inhibit the expression of iNOS in the SD rat model of bone metastasis cancer. In the follow-up, we still need to further explore what pathway baicalin plays a role. Additionally, the results showed that baicalin recovered the bone mineral density and the number of bone trabeculae in the SD rat with bone cancer pain, and inhibited the activation of osteoclasts. On the basis of the transcriptome sequencing results, the expression levels of some inflammatory cytokines were significantly decreased after baicalin treatment of bone cancer pain model. The results illustrated that baicalin had a certain inhibitory effect on the rat model of bone metastasis cancer. These insights can guide future research on the molecular mechanism of bone cancer pain, lay a theoretical foundation for new molecular markers related to pain response, and provide a theoretical basis for baicalin in the treatment of bone pain caused by breast cancer in the future.
Ethics Approval and Consent to Participate
All experimental procedures have been approved by the Ethics Committee of People’s Hospital of Inner Mongolia (202021009L). Animal treatment was performed according to the guidelines of the International Association for pain research.29
Acknowledgments
We thank all participants in the study.
Funding
This work was supported by the mechanism of Baicalin inhibiting TLR-4 mediated central immune response to alleviate bone cancer pain (2019GG124).
Disclosure
The authors state that they have no conflicts of interest.
References
1. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
2. Jung Koo H, Sohn EH, Kim YJ, Jang SA, Namkoong S, Chan Kang S. Effect of the combinatory mixture of Rubus coreanus miquel and astragalus membranaceus bunge extracts on ovariectomy-induced osteoporosis in mice and anti-RANK signaling effect. J Ethnopharmacol. 2014;151:951–959. doi:10.1016/j.jep.2013.12.008
3. Zhu XC, Zhang JL, Ge CT, et al. Advances in cancer pain from bone metastasis. Drug Des Devel Ther. 2015;9:4239–4245.
4. Turabi A, Plunkett AR. The application of genomic and molecular data in the treatment of chronic cancer pain. J Surg Oncol. 2012;105:494–501. doi:10.1002/jso.21707
5. Guerra Liberal FDC, Tavares AAS, Tavares J. Palliative treatment of metastatic bone pain with radiopharmaceuticals: a perspective beyond Strontium-89 and Samarium-153. Appl Radiat Isot. 2016;110:87–99. doi:10.1016/j.apradiso.2016.01.003
6. Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med. 2008;58:220–233.
7. Zhou QM, Wang S, Zhang H, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30:1648–1658. doi:10.1038/aps.2009.166
8. Jung MA, Jang SE, Hong SW, Hana MJ, Kim DH. The role of intestinal microflora in anti-inflammatory effect of baicalin in mice. Biomol Ther. 2012;20:36–42. doi:10.4062/biomolther.2012.20.1.036
9. Liu YF, Gao F, Li XW, et al. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res. 2012;37:1670–1680. doi:10.1007/s11064-012-0771-8
10. Huynh DL, Ngau TH, Nguyen NH, Tran GB, Nguyen CT. Potential therapeutic and pharmacological effects of wogonin: an updated review. Mol Biol Rep. 2020;47:9779–9789. doi:10.1007/s11033-020-05972-9
11. Yu Z, Zhan C, Du H, Zhang L, Liang C, Zhang L. Baicalin suppresses the cell cycle progression and proliferation of prostate cancer cells through the CDK6/FOXM1 axis. Mol Cell Biochem. 2020;469(1–2):169–178. doi:10.1007/s11010-020-03739-1
12. Chen WC, Kuo TH, Tzeng YS, Tsai YC. Baicalin induces apoptosis in SW620 human colorectal carcinoma cells in vitro and suppresses tumor growth in vivo. Molecules. 2012;17:3844–3857. doi:10.3390/molecules17043844
13. Wang Y, Wang H, Zhou R, et al. Baicalin inhibits human osteosarcoma cells invasion, metastasis, and anoikis resistance by suppressing the transforming growth factor-β1-induced epithelial-to-mesenchymal transition. Anticancer Drugs. 2017;28:581–587. doi:10.1097/CAD.0000000000000495
14. Zhang J, Wang LS, Ye SL, Luo P, Wang BL. Blockage of tropomyosin receptor kinase a (TrkA) enhances chemo-sensitivity in breast cancer cells and inhibits metastasis in vivo. Int J Clin Exp Med. 2015;8:634–641.
15. Linher-Melville K, Sharma M, Nakhla P, et al. Inhibiting STAT3 in a murine model of human breast cancer-induced bone pain delays the onset of nociception. Mol Pain. 2019;15:1744806918823477. doi:10.1177/1744806918823477
16. Hu XF, He XT, Zhou KX, et al. The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells. J Neuroinflammation. 2017;14:213. doi:10.1186/s12974-017-0988-1
17. Chiou CS, Chen CC, Tsai TC, Huang CC, Chou D, Hsu KS. Alleviating bone cancer-induced mechanical hypersensitivity by inhibiting neuronal activity in the anterior cingulate cortex. Anesthesiology. 2016;125:779–792. doi:10.1097/ALN.0000000000001237
18. Hua B, Gao Y, Kong X, Yang L, Hou W, Bao Y. New insights of nociceptor sensitization in bone cancer pain. Expert Opin Ther Targets. 2015;19:227–243. doi:10.1517/14728222.2014.980815
19. Zhai M, Yang S, Lin S, et al. Distinct gene expression patterns of ion channels and cytokines in rat primary sensory neurons during development of bone cancer and cancer pain. Front Mol Neurosci. 2021;14:665085. doi:10.3389/fnmol.2021.665085
20. Yuan QL, Guo TM, Liu L, Sun F, Zhang YG. Traditional Chinese medicine for neck pain and low back pain: a systematic review and meta-analysis. PLoS One. 2015;10:e0117146. doi:10.1371/journal.pone.0117146
21. Chou TC, Chang LP, Li CY, Wong CS, Yang SP. The antiinflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesth Analg. 2003;97:1724–1729. doi:10.1213/01.ANE.0000087066.71572.3F
22. Sun YY, Zhang WJ, Dong CL, et al. Baicalin alleviates nitroglycerin-induced migraine in rats via the trigeminovascular system. Phytother Res. 2017;31:899–905. doi:10.1002/ptr.5811
23. Cherng CH, Lee KC, Chien CC, et al. Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. J Formos Med Assoc. 2014;113:513–520. doi:10.1016/j.jfma.2013.04.007
24. Li P, Xiong DL, Sun WP, Xu SY. Effects of baicalin on diabetic neuropathic pain involving transient receptor potential vanilloid 1 in the dorsal root ganglia of rats. Neuroreport. 2018;29:1492–1498. doi:10.1097/WNR.0000000000001138
25. Wang B, Huang T, Fang Q, et al. Bone-protective and anti-tumor effect of baicalin in osteotropic breast cancer via induction of apoptosis. Breast Cancer Res Treat. 2020;184:711–721. doi:10.1007/s10549-020-05904-y
26. Li P, Bi Y, Deng Y, Xiong D, Li A. Baicalin ameliorates bone cancer pain by suppressing TRPV1 in rat dorsal root ganglia. Nat Prod Commun. 2020;15:1934578X19899562. doi:10.1177/1934578X19899562
27. Cheng P, Wang T, Li W, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. 2017;8:547. doi:10.3389/fphar.2017.00547
28. Min W, Ahmad I, Chang ME, Burns EM, Qian Q, Yusuf N. Baicalin protects keratinocytes from toll-like receptor-4 mediated DNA damage and inflammation following ultraviolet irradiation. Photochem Photobiol. 2015;91(6):1435–1443. doi:10.1111/php.12505
29. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi:10.1016/0304-3959(83)90201-4
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.