Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Trajectories of Spirometric Patterns, Obstructive and PRISm, in a Population-Based Cohort in Latin America
Authors Perez-Padilla R, Montes de Oca M, Thirion-Romero I , Wehrmeister FC , Lopez MV, Valdivia G, Jardim JR , Muino A , B Menezes AM
Received 28 January 2023
Accepted for publication 30 May 2023
Published 21 June 2023 Volume 2023:18 Pages 1277—1285
DOI https://doi.org/10.2147/COPD.S406208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Rogelio Perez-Padilla,1 Maria Montes de Oca,2 Ireri Thirion-Romero,1 Fernando C Wehrmeister,3 Maria Victorina Lopez,4 Gonzalo Valdivia,5 Jose R Jardim,6 Adriana Muino,4 Ana Maria B Menezes3 On behalf of the PLATINO Group
1National Institute of Respiratory Diseases, Mexico City, Mexico; 2Pulmonary Division, Hospital Universitario de Caracas, Universidad Central de Venezuela, and Centro Medico de Caracas, Caracas, Venezuela; 3Post-Graduate Program in Epidemiology, Federal University of Pelotas, Pelotas, Brazil; 4Universidad de la Republica. Hospital Maciel, Montevideo, Uruguay; 5Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile; 6Paulista School of Medicine, Federal University of São Paulo, São Paulo, Brazil
Correspondence: Rogelio Perez-Padilla, Instituto Nacional de Enfermedades Respiratorias, Tlalpan 4502, Col. Sección XVI, 14080, CDMX, Mexico City, Mexico, Email [email protected]
Background: Preserved ratio impaired spirometry (PRISm) has been associated with adverse outcomes and increased transition to other spirometric categories over time. We aimed to examine its prevalence, trajectories over time, and outcomes in a population-based sample from Latin America.
Methods: Data were obtained from two population-based surveys of adults from three cities in Latin America (PLATINO study), conducted on the same individuals 5– 9 years after their baseline examination. We estimated the frequency of PRISm defined by FEV1/FVC≥ 0.70 with FEV1 < 80%, describing their clinical characteristics, longitudinal transition trajectories over time, factors associated with the transition.
Results: At baseline, 2942 participants completed post-bronchodilator spirometry, and 2026 at both evaluations. The prevalence of normal spirometry was 78%, GOLD-stage 1 10.6%, GOLD 2– 4 6.5%, and PRISm was: 5.0% (95% CI 4.2– 5.8). PRISm was associated with less schooling, more reports of physician-diagnosis of COPD, wheezing, dyspnea, missing days at work, having ≥ 2 exacerbations in the previous year but without accelerated lung function decline. Mortality risk was significantly higher in PRISm (HR 1.97, 95% CI 1.2– 3.3) and COPD GOLD 1– 4 categories (HR 1.79, 95% CI 1.3– 2.4) compared with normal spirometry. PRISm at baseline most frequently transitioned to another category at follow-up (46.5%); 26.7% to normal spirometry and 19.8% to COPD. The best predictors of transition to COPD were closeness of FEV1/FVC to 0.70, older age, current smoking, and a longer FET in the second assessment.
Conclusion: PRISm, is a heterogeneous and unstable condition prone to adverse outcomes that require adequate follow-up.
Keywords: preserved ratio impaired spirometry PRISm, airflow obstruction, COPD, lung function decline
Introduction
A reduced spirometric function, whether with a restrictive or obstructive pattern is associated with adverse outcomes including shorter survival.1,2 There is a need to identify people with reduced lung function or at risk of progressing to airflow obstruction (COPD or other conditions), or other abnormal spirometric patterns over time for closer monitoring and risk management.
The spirometric restrictive pattern defined as Preserved Ratio Impaired Spirometry (PRISm) has been reported in 6–17% of different surveys3–6 and is a heterogeneous and unstable population in terms of its longitudinal transition to different lung function categories (reverting to normal spirometry, developing COPD, or remaining as persistent PRISm), and associated with cardiovascular disease (CVD) burden, or early mortality.3–6 Limited information exists in the field, in particular in a population-based study that also included never-smokers.
Di He et al4 using data from the English Longitudinal Study of Aging (ELSA) cohort, reported that patients defined as having severe PRISm (both FEV1 and FVC<80% predicted values, FEV1/FVC≥0.70) had increased respiratory symptoms, greater risk of mortality and greater likelihood of progressing to COPD (GOLD 2–4) than those defined as mild PRISm (either of FEV1 and FVC<80% predicted, FEV1/FVC≥0.70). Similar results have been reported in other studies related to the increased risk of mortality in PRISm subjects and their transition to other spirometric categories over time.3,4,7
The Proyecto Latinoamericano de Investigación en Obstrucción Pulmonary (PLATINO study) described cross-sectionally8 and longitudinally9,10 the main characteristics of COPD in a population-based sample of Latin America (LA) Utilizing the data from the PLATINO follow-up study, the present study aimed to identify the prevalence of the PRISm group (FEV1/FVC≥0.70 based on low FEV1 <80% predicted), assess their stability (longitudinal transition trajectories) over time, their main characteristics, and the impact on survival, exacerbations and lung function decline in comparison with normal spirometry group at baseline. In addition, we examined the factors associated with the transitions to different lung function categories over time.
Methods
Study Design and Population
The detailed methods of the PLATINO baseline and follow-up studies have been described elsewhere.8,9 The PLATINO study was a population-based study carried out in five centers in Latin America (Montevideo, Santiago de Chile, São Paulo, Mexico City, and Caracas) from 2002 to 2004, among adults aged ≥40 years.8 The study was conducted in two phases: 1) the baseline survey that occurred in all the five centers8 and 2) the follow-up visit that occurred in three of the five centers; Montevideo, Santiago, and São Paulo 5, 6, and 9 years after the baseline surveys, respectively.9
The baseline study sample (with post-bronchodilator spirometry testing) consisted of 5183 subjects (Montevideo: 884; Santiago: 1140; São Paulo: 918; Mexico City: 964, and Caracas: 1277), and the prevalence of post-bronchodilator (post-BD) FEV1/FVC ratio <0.70 ranged from 7.8% to 19.7% across the five centers. At follow-up, subjects in Montevideo, Santiago de Chile, and São Paulo from the original baseline sample were located and re-interviewed and performed post-BD spirometry. In addition, mortality data were prospectively collected from the time of the baseline visit to the follow-up visit. Individuals were visited at their homes based on the contact information provided by them during the baseline exam.
Ethical approval was obtained from the Institutional Review Boards at all five sites (Instituto Nacional de Enfermedades Respiratorias, Universidad Central de Venezuela, Pontificia Universidad Católica de Chile, Federal University of Pelotas, the Federal University of São Paulo) for the baseline exam and the three sites participating in the follow-up exams. All participants signed a written informed consent approved by the Boards. The study was done in accordance with Good Clinical Practice including the Declaration of Helsinki.
Assessments and Measurements
The PLATINO questionnaire is available at the website: http://www.platino-alat.org. The same core questionnaire was used at the PLATINO baseline and follow-up visits. Spirometry was undertaken on individuals who did not present any exclusion criteria (99% of the sample) using an ultrasonic spirometer (EasyOne; ndd Medical Technologies, Zurich, Switzerland). Spirometry was performed before (pre-BD) and 15 minutes after the administration of 200 μg of Salbutamol (post-BD) according to the American Thoracic Society (ATS) criteria of acceptability and reproducibility.11
Lung function categories were defined using post-BD spirometry and based on the FEV1/FVC ratio, and the FEV1 expressed as a percentage of predicted (FEV1%) by the PLATINO reference values12 as follows:
- PRISm group: FEV1/FVC≥0.70 post-BD and FEV1 <80% predicted.
- GOLD COPD criteria: FEV1/FVC<0.70 post-BD (GOLD-1 FEV1≥80%; GOLD-2 FEV1% ≥50 and <80%; GOLD-3 FEV1 ≥30% and <50%; GOLD-4 FEV1<30%).
Exacerbation was self-reported and defined as a deterioration of breathing symptoms that affected usual daily activities or caused missed work, asking also the number of such episodes, the episodes that needed to see a doctor or required hospitalization within the 12 months preceding the study.
Statistical Analyses
The main comparison was among the PRISm group and participants with airflow obstruction according to GOLD criteria (COPD), having as control those with normal spirometry. The main groups (normal, PRISm, and COPD) were compared by analysis of variance (ANOVA) with Bonferroni adjustment for multiple comparison tests for quantitative variables or by Fisher’s exact test for nominal variables. The groups were also compared on their survival using Cox proportional hazard models, lung function decline using multiple regression models, and 2 or more exacerbations in the last year using logistic regression models. The results of the models were reported crude and adjusted by covariables, such as age, gender, current smoking (expressed as yes or no and by the number of cigarettes smoked per day), cumulative smoking in pack-years, body mass index (BMI>30 kg/m2), years of schooling, and previous medical diagnosis of respiratory diseases or other comorbidities obtained through a questionnaire.
Concordance of lung function categories at baseline (normal, PRISm, and COPD) and follow-up was evaluated in the same individuals using the Kappa statistic. Logistic regression models were fitted to explore baseline characteristics associated with a transition to a different category at follow-up, including age, sex, BMI, and self-reported physician-diagnosed comorbidities (asthma, chronic bronchitis, COPD, heart disease, and diabetes mellitus).
The impact of the participants’ FEV1% and the FEV1/FVC proximity to the category’s thresholds (FEV1% of 80% and FEV1/FVC of 70%), utilizing the difference between the measured FEV1/FVC or FEV1% from the thresholds, were evaluated in logistic regression models as independent variables, as well as the impact of the spirometry test quality and particularly the forced expiratory time (FET), the duration of the forced expiration during spirometric testing at baseline and follow-up. In repeated tests, transitions to a different category are more likely if baseline results are borderline13 and also if the expiratory duration (FET) was different, since, with longer expiration, FEV1/FVC tends to drop, rising the probability of diagnosing airflow obstruction.14 Finally, we analyzed the groups with consistent PRISm in both assessments versus those that migrated to COPD or normal spirometry in the second evaluation, to determine the predictors of the group change.
Results
Baseline Characteristics
From the 3151 study participants in the three cities, 2942 completed post-BD spirometry at baseline (Table 1), 2815 were evaluated at follow-up, 2136 performed spirometry at follow-up (858 from Santiago, 683 from Montevideo, and 595 from Sao Paulo) and 301 deaths were documented (population considered for survival analysis). Follow-up rates for each independent variable category were around 80%.15
 |
Table 1 Baseline Characteristics of the Study Population by Different Lung Function Categories Groups |
The baseline prevalence of normal spirometry was 78.0% (2294 individuals, 95% CI 76.4–79.5), 10.6% for GOLD stage 1 (311 subjects, 95% CI 9.5–11.7), 6.5% for GOLD 2–4 (191 subjects. 95% CI 5.6–7.4), and for PRISm 5.0% (146 subjects, 95% CI 4.2–5.8) (Table 1).
The baseline clinical characteristics and lung function of the study population by the different lung function categories are shown in Table 1 and Table 2. Subjects in the PRISm group in comparison with those in the GOLD 1 and GOLD 2–4 were more often female, younger, with higher BMI and obesity, and less frequently current smokers (Table 1). PRISm group compared with GOLD 1 subjects, had more exacerbations, physician consultations in the last year, and worse health perception, whereas compared with GOLD 2–4 category, PRISm group reported fewer physician diagnoses of asthma, tuberculosis, and chronic bronchitis, exacerbations, physician consults, missing days at work in the last year, use of respiratory medication, and reports of feeling depressed or with little energy (Table 1). The perception of good or excellent health was similar among the PRISm and GOLD 2–4 subjects. The groups had a similar proportion of good-quality pre-BD and post-BD spirometry tests (Table 2).
 |
Table 2 Baseline Lung Function of the Study Population by Different Lung Function Categories Groups |
In a multinomial logistic regression, the PRISm group was associated with less schooling, report of wheezing, dyspnea, missing days of work, and previous COPD diagnosis, whereas COPD was associated with older age, male gender, lower BMI, current tobacco smoking, cumulative smoking as pack-years, previous COPD diagnosis, chronic bronchitis, emphysema, asthma, tuberculosis, cough or phlegm, wheezing, use of respiratory medicine and missing days of work (Table S1).
Survival, Exacerbations and Lung Function Decline
Table 3 describes the association of the PRISm group at baseline with survival, exacerbations in the past year, and lung function decline in the follow-up. The reference group was participants with normal spirometry. The PRISm group was not associated with accelerated lung function decline and had on average an increase in FEV1 post-BD during follow-up compared with the normal spirometry group (coefficients on FEV1 decline were positive). The impact of PRISm on the time to death and exacerbations was reduced after adjustment by FEV1 (adjusted 2).
 |
Table 3 Adjusted Association (HR 95% CI) Between PRISm Group at Baseline with Survival, Lung-Function Decline, and Exacerbations in the Follow-Up |
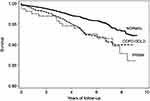
Figure 1 shows the survival curves of subjects with normal spirometry, COPD (GOLD 1–4) and PRIMs adjusted by age, female gender, education, pack-years of smoking, and comorbidities. Mortality risk was significantly higher in PRISm and COPD GOLD 1–4 categories compared with normal spirometry.
Longitudinal Transitions into the Different Lung Function Categories
Table 4 shows the longitudinal transition trajectories by baseline lung function categories. PRISm subjects at baseline more often changed towards a different lung function category (46.5%) than subjects with COPD (30.2%) or normal spirometry (12.4%). In the PRISm group, 26.7% turned to normal spirometry, and 19.8% towards COPD. The great majority of normal subjects remained normal in the second evaluation (87.6%), 7.9% moved towards COPD, and 4.5% to PRISm. Among subjects with COPD (GOLD 1–4), the majority remained in the same category (69.8%), 6.7% transited toward PRISm, and 23.5% to normal spirometry.
 |
Table 4 Longitudinal Transition Trajectories Among Groups with Normal Spirometry, Airflow Obstruction (GOLD 1–4), and PRISm (FEV1<80%) |
Agreement between lung function categories at baseline and follow-up was moderate. The overall agreement in the PRISm group, was 83.5% (Kappa statistic=0.54, 95% CI 0.51–0.58). Individuals persisting in the PRISm group were younger, more often female, and had a shorter forced expiratory time (FET) at the second assessment. Those who switched from PRISm to COPD were older, had lower baseline FEV1%, and FEV1/FVC were more often female, had a longer FET at the second assessment (2.1 seconds on average), and more frequently reported diagnosis of asthma and smoking. Those who switched to normal spirometry were more often men, with diabetes or heart disease were less often current smokers, and had less often an asthma diagnosis.
Individuals who switched from COPD to PRISm had lower FEV1%, shorter FET at the second assessment, higher BMI, and more frequent heart disease. On the other hand, individuals who switched from normal spirometry to PRISm had lower baseline FEV1 and FVC%, shorter FET at the second assessment (2 s on average), and more frequently reported heart disease.
The most important predictors of transition from normal spirometry or PRISm to COPD were closeness of FEV1/FVC to 0.70 (OR 18, 95% CI 9.7–36), age (OR 1.05, 95% CI 1.03–1.08), current smoking (OR 2.0, 95% CI 1.2–3.2), self-assessment of good health (OR 0.52, 95% CI 0.33–0.85) and a longer FET in the second assessment (OR 0.69, 95% CI 0.65–0.73) (Table S2).
In a logistic regression model having as the dependent variable a transition to any other spirometric category, having an FEV1% close to 80% and an FEV1/FVC close to 0.70 (borderline results) were predictors of a group change at follow-up. The risk of a transition increased if the FET in the follow-up was longer than at baseline (OR 1.09, 95% CI 1.04–1.12), and if the follow-up post-BD spirometry had lower quality than the first spirometry (OR 1.7 95% CI 1.2–2.4), supporting the contribution of technical issues to category transitions. If FET at follow-up was longer than at baseline, there was a tendency to transit to COPD14 and if the second was shorter the change was more likely towards PRISm or normal pattern.
Current smoking increased the risk to change to COPD (OR 2.0, 95% CI 1.2–3.2), and reduced the chance to move to normal spirometry (OR 0.47, 95% CI 0.26–0.87). Advanced age increases the risk of transitioning to COPD (OR 1.05, 95% CI 1.03–1.08), and a self-assessment of “good health” reduces the risk of changing to COPD (OR 0.50, 95% CI 0.30 to 0.80) or normal (OR 0.53, 95% CI 0.30–0.95).
Discussion
The main findings of this study on prevalence, and trajectories of spirometric categories in a population-based cohort from Latin America were: first, the PRISm prevalence ranged from 5% if defined by an FEV1 <80%, it was associated with less schooling, more reports of COPD physician-diagnosis, wheezing, dyspnea, absences from work, increased risk of death, and exacerbations, but without accelerated lung function decline; second, PRISm group compared to COPD were more often female, younger, with higher BMI and fewer current smokers; third, PRISm subjects at baseline switched most frequently than COPD or individuals with normal spirometry switched to a different lung function category (46.5%), and the best predictors of transition to COPD were closeness of FEV1/FVC to 0.70, older age, current smoking and a longer expiration (FET) in the second assessment.
Few population-based studies have evaluated the prevalence of PRISm subjects and their transition to other spirometric categories over time.3,7,16 Mild PRISm in the ELSA study tended to transition toward normal spirometry (40.2%), 7.6% changed to GOLD 1, and 10% to GOLD 2–4, while severe PRISm tended to maintain the status (42.4%) or transition toward GOLD 2–4 (28.3%).4 PRISm groups had higher BMI, the highest inactive physical activity (severe PRISm group), more cardiovascular disease (CVD) and cancer.4
In the Rotterdam population-based cohort, with the crude prevalence of PRISm at baseline was 7.1% and 7.6% if age-adjusted, more often transitioned towards a different lung function category (43.0%), 32.6% transitioned to COPD, and 10.4% to normal spirometry.7 Overall, PRISm subjects had the highest BMI at baseline, were more often female, and smoked less.7 Another longitudinal study (COPDGene) in current and former smokers showed that the prevalence of PRISm remained consistent (10.4%–11.3%) between the study phases and nearly one-half of them transitioned into or out of PRISm at each visit.5
The results of the present study, are consistent with the previous findings: PRISm category with a prevalence of 5%, changed more often (almost half) over time.4,5,7 They also coincide in terms of the clinical characteristic of PRIMs subjects (more often female, younger, with higher BMI, and fewer smokers). In addition, our results showed that the best predictors of transition from PRISm to COPD were closeness of FEV1/FVC to 0.70, older age, current smoking, and a longer FET in the second assessment.
Standard spirometric diagnostics have included the restrictive disease pattern, and several conditions may explain its presence in addition to the presence of true restrictive disease with reduced Total Lung Capacity (TLC) due to a stiff lung or chest wall or weak inspiratory muscles. The nonspecific spirometric pattern, characterized by low FVC with normal TLC, may be due to early obstructive or restrictive disease, small airway disease, or emphysema with air-trapping.17,18 Incomplete inspiration or expiration due to poor effort can also lead to low FVC and FEV1.19 A poor effort may lead to pattern change in follow-up studies due to changes in spirometry quality or FET. Known longitudinal variations in lung function can modify functional category, especially if results at baseline are close to spirometric thresholds.13 Furthermore, repeated tests, acceptable and classified as good quality tests, can lead to a modified FEV1/FVC and thus the diagnosis of airflow obstruction if FETs are variable, a situation improved by utilizing FEV1/FEV6 with clearly defined measuring time (6 seconds) compared with FVC than can be measured at different times.14 In our study, a transition to a restrictive pattern occurred more frequently if the second study FET was shorter than the first, whereas a change to an obstructive pattern occurred if the second FET was longer. Transitions to a different group were strongly favored by borderline tests, understandable even with “normal” variations in longitudinal lung function, but also if FET changes in consecutive tests. In addition to technical variations, there were other relevant risk factors for changing groups at follow-up. Transiting to airflow obstruction at the follow-up was more likely in older men, those with poor health perception, with a previous diagnosis of asthma, and current smokers. Change to PRISm was more likely if BMI was higher, with a previous diagnosis of heart disease.
In comparison with reference groups, PRISm and COPD GOLD 2–4 were predictors of all-cause mortality in adjusted survival analyses and also predictors of CVD mortality.7 Data from the studies NHLBI and ELSA reported that PRISm compared with normal spirometry significantly increased the risks of all-cause, respiratory, and CVD mortality.1,3,4 Our results confirm the previous findings and show a mortality risk significantly higher in PRISm and COPD GOLD 1–4 categories compared with normal spirometry and similar between PRISm and COPD GOLD 1–4 categories. Comorbid diseases that contribute to CVD mortality, may affect lung function due to cardiomegaly, parenchymal or pleural fluid accumulation, or airway edema. However, the prevalence of CVD and diabetes in our study was similar among the different lung function categories, and also the survival analysis if adjusted for comorbidities. Therefore, this makes it unlikely that comorbidities explain the difference in survival.
This study has some limitations. Being a population-based study, few cases of severe airflow obstruction were observed. Comorbidities and exacerbations were self-reported and may be underestimated compared to what can be expected from a comprehensive clinical search. Only two spirometric evaluations were performed in three cities from the five analyzed at baseline, which may offer less accurate estimates of lung function decline, but measurements were done with the same equipment and well-trained technicians and were separated by 6–9 years with high rates of follow-up. In contrast to large cohorts of smokers, or groups of patients with COPD, having a proper control group in a non-obstructed population, exposed and non-exposed to tobacco and other known risks are key for relevant comparisons.
In conclusion, PRISm is an unstable and heterogeneous population that, due to its association with exacerbations and reduced survival, requires additional evaluations and follow-up. In this category, severe conditions, such as those associated with a true restriction may be present or develop (for example, interstitial lung diseases, muscle weakness, among others), as well as, early changes in restrictive or obstructive disease. However, we must not forget the basic instability of borderline cases, considering repeating the tests during the follow-up, and complementing the evaluation with other lung function tests or imaging, depending on the cases.
The PLATINO group for this project also comprised Francisco Franco-Marina MD1, Dolores Moreno MD2, Carmen Lisboa5, Julio Pertuze MD5, Oliver A. Nascimento MD6, Mariana R. Gazzotti PhD6, Graciane Laender PhD6, and Beatriz Manzano PhD.6
Abbreviations
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; LLN, lower limit of normality (5th percentile); GOLD, Global Obstructive Lung Disease organization; FEV6, forced expiratory volume in 6 seconds; LA, Latin America; ATS, American Thoracic Society; BD, bronchodilator; BMI, body mass index; 95% CI, 95% confidence interval; ALAT, Asociación Latinoamericana de Tórax, Latin American Thoracic Association; PRISm, Preserved ratio impaired spirometry.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; all took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The PLATINO study has been sponsored by The Asociación Latinoamericana de Tórax (ALAT), Boehringer Ingelheim GmbH, GlaxoSmithKline, and Novartis for the collection of the data during the fieldwork.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Trajectory of preserved ratio impaired spirometry: natural history and long-term prognosis. Am J Respir Crit Care Med. 2021;204(8):910–920. doi:10.1164/rccm.202102-0517OC
2. Washio Y, Sakata S, Fukuyama S, et al. Risks of Mortality and Airflow Limitation in Japanese Individuals with Preserved Ratio Impaired Spirometry. Am J Respir Crit Care Med. 2022;206(5):563–572. doi:10.1164/rccm.202110-2302OC
3. Wan ES, Balte P, Schwartz JE, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA. 2021;326(22):2287. doi:10.1001/jama.2021.20939
4. He D, Sun Y, Gao M, et al. Different Risks of Mortality and Longitudinal Transition Trajectories in New Potential Subtypes of the Preserved Ratio Impaired Spirometry: evidence From the English Longitudinal Study of Aging. Front Med. 2021;8:755855. doi:10.3389/fmed.2021.755855
5. Wan ES, Hokanson JE, Regan EA, et al. Significant Spirometric Transitions and Preserved Ratio Impaired Spirometry Among Ever Smokers. Chest. 2022;161(3):651–661. doi:10.1016/j.chest.2021.09.021
6. Wijnant SRA, Lahousse L, Brusselle GG. The global significance of PRISm: how data from low- and middle-income countries link physiology to inflammation. Eur Respir J. 2020;9(55).
7. Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55(1):1901217. doi:10.1183/13993003.01217-2019
8. Menezes AMB, Perez-Padilla R, Jardim JRB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet Lond Engl. 2005;366(9500):1875–1881. doi:10.1016/S0140-6736(05)67632-5
9. Menezes AMB, Pérez-Padilla R, Wehrmeister FC, et al. FEV1 Is a Better Predictor of Mortality than FVC: the PLATINO Cohort Study. PLoS One. 2014;9(10):e109732. doi:10.1371/journal.pone.0109732
10. Perez-Padilla R, Wehrmeister FC, Montes de Oca M, et al. Outcomes for symptomatic non-obstructed individuals and individuals with mild (GOLD stage 1) COPD in a population based cohort. Int J Chron Obstruct Pulmon Dis. 2018;13:3549–3561. doi:10.2147/COPD.S175527
11. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
12. Pérez-Padilla R, Valdivia G, Muiño A, et al. Valores de referencia espirométrica en 5 grandes ciudades de Latinoamérica para sujetos de 40 o más años de edad. Arch Bronconeumol. 2006;42(7):317–325. doi:10.1157/13090581
13. Perez-Padilla R, Wehrmeister FC, Montes de Oca M, et al. Instability in the COPD diagnosis upon repeat testing vary with the definition of COPD. PLoS One. 2015;10(3):e0121832. doi:10.1371/journal.pone.0121832
14. Perez-Padilla R, Wehrmeister FC, Celli BR, et al. Reliability of FEV1/FEV6 to Diagnose Airflow Obstruction Compared with FEV1/FVC: the PLATINO Longitudinal Study. PLoS One. 2013;8(8):e67960. doi:10.1371/journal.pone.0067960
15. Menezes AMB, Muiño A, López-Varela MV, et al. Estudio de cohorte de base poblacional sobre la enfermedad pulmonar obstructiva crónica en Latinoamérica: métodos y resultados preliminares. Fase II del estudio PLATINO. Arch Bronconeumol. 2014;50(1):10–17. doi:10.1016/j.arbres.2013.07.014
16. Jankowich M, Elston B, Liu Q, et al. Restrictive Spirometry Pattern, Cardiac Structure and Function, and Incident Heart Failure in African Americans. The Jackson Heart Study. Ann Am Thorac Soc. 2018;15(10):1186–1196. doi:10.1513/AnnalsATS.201803-184OC
17. Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions Associated With an Abnormal Nonspecific Pattern of Pulmonary Function Tests. Chest. 2009;135(2):419–424. doi:10.1378/chest.08-1235
18. Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test. Chest. 2011;139(4):878–886. doi:10.1378/chest.10-0804
19. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi:10.1183/13993003.01499-2021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.