Back to Journals » Clinical Ophthalmology » Volume 16
Topical Omega-3 Polyunsaturated Fatty Acids for the Treatment of Dry Eye – Results from a Pilot Randomized Controlled Masked-Observer Study
Authors Kaercher T , Messmer EM, Berninger T, Huber-van der Velden KK, Geiger R, Cipriano-Bonvin P, Jacobi C
Received 31 August 2022
Accepted for publication 17 November 2022
Published 8 December 2022 Volume 2022:16 Pages 4021—4031
DOI https://doi.org/10.2147/OPTH.S388294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Thomas Kaercher,1 Elisabeth M Messmer,2,3 Thomas Berninger,4 Klaudia K Huber-van der Velden,5 Raphaela Geiger,6 Pauline Cipriano-Bonvin,7 Christina Jacobi8,9
1Augenarztpraxis, Heidelberg, Germany; 2Department of Ophthalmology, Ludwig-Maximilian-University, Munich, Germany; 3Praxis Professor Kampik & Kollegen, Munich, Germany; 4Augenzentrum, Olching, Germany; 5Augenheilkunde Lindenthal, Köln-Lindenthal, Germany; 6TRB Chemedica AG, Feldkirchen, Germany; 7TRB Chemedica International SA, Geneva, Switzerland; 8Augen & Haut Zentrum-Praxis Dr. Jacobi, Nuremberg, Germany; 9Friedrich-Alexander University of Erlangen-Nuremberg, Erlangen, Germany
Correspondence: Thomas Kaercher, Augenarztpraxis, 48 Dossenheimer Landstr, Heidelberg, 69121, Germany, Tel +49 6221 400 888, Fax +49 6221 412 203, Email [email protected]
Purpose: To investigate the efficacy, safety and tolerability of topical omega-3 polyunsaturated fatty acids (PUFA) as an innovative treatment of dry eye disease (DED).
Patients and Methods: In a pilot, multicenter, masked-observer, randomized, active-controlled, non-inferiority study in Germany, patients self-treated their eyes with daily instillations of eye drops containing either omega-3 PUFA or povidone as major components for three months. At four and twelve weeks, efficacy was among others evaluated based on Ocular Surface Disease Index (OSDI), ocular surface symptoms intensity, general clinical impression, tear break-up time (TBUT), corneal fluorescein staining using the Oxford grading scale, tear volume, and matrix metalloproteinase-9 (MMP-9) concentration in the tear film. Safety evaluation included visual acuity, intraocular pressure, and the incidence of adverse events. Co-primary endpoints were the mean percent changes from baseline of TBUT and OSDI after four weeks.
Results: In total 80 patients were included, of whom 37 in the PUFA group and 39 in the povidone group were evaluable for the co-primary endpoints. Patients had a mean age of 52 years and > 80% were women. Both co-primary endpoints (TBUT and OSDI) significantly improved from baseline in both treatment groups, at Week 4 and Week 12 and the statistical analysis demonstrated topical omega-3 PUFA to be non-inferior to 2% povidone for these two parameters. Both treatments resulted in a significant improvement of most secondary efficacy endpoints as well, often with a slight difference in favor of PUFA, not reaching statistical significance though. One non-severe, treatment-related local AE was reported in each group.
Conclusion: Omega-3 PUFA-based eye drops proved to be non-inferior to povidone-containing eye drops in the treatment of signs and symptoms of dry eye. This treatment may thus be an additional tool for the management of DED.
Keywords: polyunsaturated fatty acids, povidone, dry eye disease, artificial tears, omega-3 fatty acid
Introduction
Dry eye disease (DED) is one of the most common ophthalmologic ailments, estimated to affect up to one-third of the population worldwide.1–5 The natural tear film is mainly formed from a triplet of aqueous, mucous and lipid layers, providing an equilibrium for the maintenance of a healthy ocular surface. Main functions of the tear film are to lubricate the ocular surface, transfer nutrients to the cornea, eliminate foreign matter and cellular debris, and act as first-line defense against infections.6 Consequently, eyes with abnormal tear film function were demonstrated to be more prone to optical aberrations.7,8 DED is today considered a multifactorial disease involving hyperosmolarity and instability of the tear film, neurosensory abnormalities, and inflammation of the ocular surface.9
Risk factors for developing DED are manifold and include advanced age, female sex, sexual hormone disturbances, smoking, extreme heat or cold weather conditions, low relative humidity, overuse of video screens, refractive surgery, contact lens wear, and certain medications,3 as well as an imbalance in the dietary intake of essential fatty acids.10 Various comorbidities may further contribute to dry eye-related symptoms.11–14
Due to its multifactorial nature, clinical and biological signs of DED can be inconsistent and sometimes discordant with symptomatology.15 Ocular manifestations include eye irritation, redness, a gritty or foreign body sensation, burning, tearing, photophobia, stinging, or intermittent sharp pain. Dry eye patients may have all, some, or none of these symptoms and due to its complex and varied presentations, DED may be misdiagnosed.
At present, there is no cure for DED and concerned patients depend on a life-long, regular treatment, consisting mostly of topical lubricants for tear replacement. Although at first sight the problem of DED does not seem to be serious, the considerable number of marketed eye drop products may indicate the difficulty to find a satisfactory solution for DED complaints. The goal of tear replacement is to increase humidity at the ocular surface and to enhance lubrication while decreasing evaporation. For this purpose, most commonly celluloses, carbomers, polyvinylalcohol, polyvinylpyrrolidones (povidone/polyvidone), or hyaluronic acid are used, with a preference for formulations without preservatives. If deemed appropriate, topical treatment with prescription drugs such as corticosteroids, antibiotics or cyclosporine can be used in second line.16
Recent research efforts have explored dietary modification, oral supplementation, and even topical application of polyunsaturated fatty acids (PUFA, eg omega-3) as an alternative strategy for the treatment of dry eye.17–20 Oral omega-3 PUFAs have for example been reported to improve DED symptoms and to reduce contact lens discomfort. PUFA were proposed to act as natural anti-inflammatory agents, improve the lipid layer composition of the tear film, decrease tear film osmolarity, and increase tear secretion from the lacrimal gland.21 This hypothesis has however been debated recently based on the results of the large Dry Eye Assessment and Management (DREAM) study, in which the daily intake of 300 mg omega-3 PUFA per os for twelve months did not result in additional benefits as compared to the use of placebo.22
Remogen® Omega was the first omega-3 PUFA-containing eye drop formulation approved for the topical management of dry eye. Its lipidic components (eicosapentaenoic acid, docosahexaenoic acid, vitamin E) supposedly counteract hyper-evaporation from the aqueous layer by replenishing and reconstituting the lipid layer of the natural tear film. Its aqueous components (carbomers, glycerol) help to restore the hydrating, mucomimetic, mucoadhesive and viscoelastic characteristics of the aqueous layer, leading to stabilization of the tear film and protection of the corneal and conjunctival epithelia. The hypoosmolarity of the eye drop solution may further compensate the hyperosmolarity caused by tear film instability and hyper-evaporation of water from the tear film.
In this study, the efficacy, safety and tolerability of topical omega-3 PUFA in the treatment of DED symptoms were compared to a 2% povidone formulation.
Materials and Methods
Study Design
This was a pilot, multicenter, single (=observer) masked, randomized, active-controlled, two-arm study with four visits scheduled over an observation period of 13 weeks. The screening visit, during which patients were checked for eligibility, was followed by a one-week run-in period. At the subsequent baseline visit, patients still fulfilling all eligibility criteria were randomized to treatment. At two further visits after four and twelve weeks of treatment, efficacy and safety/tolerability outcomes were measured. A treatment duration of four weeks was considered sufficient to evaluate efficacy, whereas twelve weeks were deemed required to evaluate any anti-inflammatory effects and treatment-associated risks.
The study was approved by local Ethics Committees (Ethikkommission der Landesärztekammer Baden-Württemberg, Ethik-Kommission der Ärztekammer Hamburg, Ethikkommission der Med. Fakultät der Ludwig-Maximilian-Universität), fully complied with the Declaration of Helsinki and all applicable local regulations, and was registered under clinicaltrials.gov (NCT02908282).
Patients
Eligible were men and women between 18 and 80 years of age with moderate to severe DED, defined by a mean tear break-up time (TBUT) ≤10 s, and an Ocular Surface Disease Index (OSDI) ≥20 for the same eye at both the screening and baseline visit. When both eyes met these criteria, the eye with the lower TBUT was studied as the index eye.
Patients were excluded, if they presented: (a) a contraindication for any of the study treatments, (b) any disease or condition judged by the investigator to be incompatible with study assessments, or (c) if they had to take concomitant medications affecting the tear film, tear secretion or ocular surface integrity or that might have biased any treatment effect such as local/systemic corticosteroids or antibiotics, omega-3 oral supplements, topical cyclosporine A.
Wearing of contact lenses had to be discontinued and punctal plugs had neither to be removed nor replaced later than one month prior to inclusion until the end of the study. Any eye surgery had to date back ≥6 weeks. Patients with any concurrent systemic disease or with controlled glaucoma were eligible if on stable therapy. Participants of childbearing potential had to use adequate methods of birth control for the duration of the study; pregnant or lactating females were excluded. All eligible patients had to sign written informed consent prior to inclusion.
Treatments
After the run-in period with the use of 0.9% saline eye drops, patients were randomized to receive either omega-3 PUFA-containing lubricant eye drops (Remogen® Omega, TRB Chemedica, Feldkirchen, Germany) or 2% povidone eye drops (Wet-Comod®, Ursapharm, Saarbrücken, Germany). Patients had to instill the eye drops at least three times a day (as needed) for twelve weeks, in line with the approved label of both products (Figure 1).
 |
Figure 1 Study design. |
To ensure randomization, an experienced biostatistician created a computer-generated randomization list using blocks of two. The masked observer chronologically allocated eligible patients to the random treatment assignment code without knowing the underlying group allocation.
Outcome Measures
Efficacy
Efficacy was evaluated through specific symptom questionnaires (OSDI, Ocular Surface Symptoms Intensity), patient and investigator-rated clinical global impression (CGI), the inflammation marker matrix metalloproteinase-9 (MMP-9), stability of the tear film (TBUT), Oxford grading after conjunctival and corneal staining, and tear film volume (Schirmer I test). A strict order was followed by all investigators to perform the examinations.
The OSDI is a validated instrument for measuring DED severity and its impact on vision-related function, based on a scale of 0 to 100, with higher scores representing greater disability.23 The questionnaire covers: (a) ocular symptoms during the last seven days [five items], (b) environmental stimuli causing ocular discomfort [four items], and (c) limitations in daily activities [three items]. Each item was scored on a Likert-scale ranging from 0 (at no time) to 4 (at all times).
The Ocular Surface Symptoms Intensity (OSSI) was rated on the basis of a 100 mm visual analogue scale (VAS) for 14 symptoms, ie burning; stinging; foreign body sensation/grittiness; pain; redness; dryness sensation; pruritus; blurry vision; photophobia; eye fatigue; watery eyes; feeling of pressure; sticky eye lids; and their swelling. Single scores were summed up to a cumulative score ranging from 0 (no complaints) to 1400 mm (worst complaints).
The inflammation marker MMP-9 was measured on the index eye only, using the qualitative InflammaDry® test (Quidel Corporation, San Diego, United States of America) and following manufacturer’s instructions. This test returns a positive result at concentrations >40 ng/mL.
Conjunctival and corneal staining was performed to detect cell debris on the ocular surface and to delineate the area of epithelial loss using lissamine green and fluorescein-impregnated strips, respectively, followed by Oxford grading.24,25 Fluorescein staining was also used to determine TBUT as a measure of tear film stability. TBUT was defined as the time between the last eyelid flip after applying the dye and the appearance of the first dry spot on the surface of the cornea.
The tear volume was determined by the Schirmer I test, ie a strip of filter paper was applied with the folded end hooked onto the lateral third of the lower eyelid of the index eye, and the patient was instructed to blink normally. After five minutes, the strip was removed and the wet length on the strip [mm] was recorded to quantify the basal and reflex tear production rate.
Patients’ and investigators’ clinical global impression (CGI) of ocular complaints as compared to baseline was evaluated on a 7-point Likert scale from 1 (very much improved) to 7 (very much worse).
Mean percent change from baseline of OSDI score and TBUT at Week 4 were co-primary endpoints; all remaining efficacy measures were secondary parameters.
Safety and Tolerability
Safety parameters comprised all adverse events detected by slit lamp examination, general external ophthalmic examination, or directly reported by the patients. Moreover, at each visit, intraocular pressure and the best corrected distance visual acuity (BCVA) were measured for both eyes. Safety evaluation was based on the number, severity, and seriousness of adverse events (AE) possibly related to study treatment.
Subjective tolerability and acceptability were separately rated using a tripartite questionnaire with independent cumulative scores. Tolerability was assessed using four positive (sense of eye wellbeing, alleviation of ocular DED symptoms, moistening, refreshment feeling) and four negative items (foreign body and burning sensations, sticky eyelids, long-lasting blurred vision), each of them to be rated for intensity on a 5-point Likert scale (none, slight, moderate, severe, extreme). Accordingly, the cumulative score ranged from 0 (worst) to 32 (best tolerability). Acceptability was also rated on a 5-point Likert scale regarding four categories (comfortability, alleviation of ocular DED complaints, long-term alleviation, and normal eye feeling). Cumulative acceptability scores thus ranged from 4 (best) to 20 (worst).
Statistical Analysis
As this is a pilot study, no formal sample size calculation was performed. A sample of 100 subjects (50 per group) was deemed sufficient to detect efficacy outcomes.
Three data sets were defined prior to the statistical analysis. The safety data set comprises all randomized patients who received the study treatment at least once, irrespective of deviations recorded. The Intention-To-Treat (ITT) data set comprises randomized patients who received at least one dose of the study treatment, had at least one follow-up visit for a primary efficacy criterion and had no severe protocol deviation. Finally, the Per-Protocol (PP) population was a subgroup of the ITT consisting of all randomized patients who met all inclusion and exclusion criteria and who completed the study without major and/or severe protocol deviations.
The one-sided (alpha = 0.025) multivariate Wilcoxon-Mann–Whitney (MW)-U test was used to evaluate differences between both groups in the percent change from baseline of the co-primary endpoints (OSDI and TBUT) at Week 4 for non-inferiority (with H0 being a MW-measure ≤ 0.36) and superiority (with H0 being a MW-measure ≤ 0.5), using an a priori ordered hypotheses scheme. For missing values, the last observation was carried forward. Secondary endpoints were analyzed descriptively also using non-parametric tests if appropriate.
In an additional exploratory post-hoc analysis, intra-group comparisons (Week 4 vs baseline, and Week 12 vs baseline) were performed for the OSDI, TBUT, Schirmer I test, and Oxford grading in each group. Continuous variables were evaluated using a two-sided Student’s t-test and ordinally scaled variables with a Wilcoxon signed-rank test, both with alpha = 0.05 in the ITT population.
Inter-group comparisons (PUFA versus povidone) were performed in the PP and ITT populations for OSDI, TBUT, and Schirmer I test using a one-sided (alpha = 0.025) non-inferiority Student’s t test at Week 4 and Week 12 on the absolute change from baseline with non-inferiority margins equal to 7.3, −2.5 s, and −4 mm, respectively. The inter-group comparisons of OSSI at Week 4 and Week 12 have been performed using a two-sided Student’s t-test (alpha = 0.05) in the ITT population.
For all parametric tests, the normality assumption was checked using Shapiro test (alpha = 0.01) and graphical visualization. The homogeneity of variances assumption was checked using a Fisher’s test for variance equality (alpha = 0.05). When the normality assumption was met, but the test for variance homogeneity was found significant (p < 0.05), a Welch’s t-test was used instead. As these analyses were post-hoc, no correction for multiple testing was applied.
Results
Eighty patients were randomized in five ophthalmologic outpatient clinics in Germany from October 24, 2016 until March 31, 2018. Of these, 76 had at least one post-dose measurement of a primary endpoint, no severe protocol deviation and were included in the analysis (Figure 2). Patients had a mean age of 52.5 ± 15.3 years and the majority were women (Table 1). Patients in both groups were suffering from DED on average for 4.2 (1.1–7.8) years (median and interquartile range).
 |
Table 1 Patients and Disease Characteristics at Baseline |
 |
Figure 2 CONSORT chart. |
Efficacy
Both treatments showed a significant improvement versus baseline in the two co-primary endpoints (OSDI and TBUT) (Figure 3). At Week 4, the absolute mean change from baseline of the OSDI score in the ITT set was significant both in the PUFA group (−9.1 ± 15.17; p<0.001) and in the povidone group (−5.1 ± 12.02; p=0.012). The non-inferiority of PUFAs versus povidone was demonstrated by a Wilcoxon-MW-U test on the percent change from baseline and these results were confirmed by the non-inferiority of the absolute change from baseline (Figure 4A). Similar results were observed at Week 12 (Table 2) and in the PP population (data not shown).
 |
Table 2 Study Parameters After 4 and 12 Weeks of Treatment |
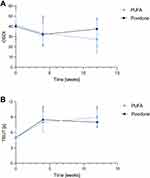 |
Figure 3 Change in the primary endpoints during the course of the study. (A) OSDI total score (B) TBUT. Values are presented as median ± quartiles. |
TBUT increased significantly between baseline and Week 4 both in the PUFA (3.2 ± 3.41 s; p<0.001) and in the povidone groups (3.5 ± 3.26 s; p<0.001). The significant improvement was maintained until Week 12 (4.5 ± 3.39 s; p<0.001 in the PUFA group, and 3.5 ± 3.52 s; p<0.001, in the povidone group). The non-inferiority of the TBUT percent change from baseline of PUFA compared to povidone was demonstrated at Week 4 and Week 12 using a one-side, non-inferiority Wilcoxon-MW-U analysis test. These results were confirmed by the non-inferiority of the absolute mean change from baseline of the TBUT (Figure 4B).
Improvements versus baseline were demonstrated in both groups for the majority of the secondary efficacy parameters as well (Table 2). The OSSI score showed a numerically greater mean relative decrease from baseline with PUFA compared to povidone (Week 4: −15% vs −10%; Week 12: −17% vs −3%, respectively). However, the inter-group difference was not statistically significant.
During the treatment with topical omega-3 PUFA, the proportion of patients tested positive for MMP-9 decreased from baseline (21.6%) to Week 4 (13.5%) and even further to Week 12 (5.6%), whereas in the povidone group the decrease was smaller and not persistent (23.1%, 18.0% and 19.4%, respectively); however, the difference between the two groups did not reach significance (p= 0.599 at week 4 and 0.086 at week 12).
Significant improvements versus baseline were demonstrated in both groups for the Oxford grading at Week 4 and Week 12 (Supplementary Tables S1–S4). By contrast, no significant change versus baseline was observed in either group for the tear volume of the index eye measured using a Schirmer I test (Table 2).
Improvement from baseline in the CGI was generally speaking more common after four than after twelve weeks (Table 2). Investigators tended to report improvements more frequently than patients did. At Week 12, 59.5% of patients in the PUFA group reported an improvement of their condition as compared to 48.7% in the povidone group.
Patients were instructed to instill the eye drops at least three times per day, but were allowed to increase the number of instillations if deemed necessary. The actual number of instillations per day was estimated by the patients at each visit. It was similar in both treatment groups over the whole study period (data not shown).
Safety and Tolerability
In total, three AEs were reported by three patients (3.8%), two in the PUFA group and one in the povidone group. All AEs were of moderate severity and resolved without sequelae; none was serious. One AE in each group was considered at least possibly related to treatment, ie instillation site pain (povidone group) and instillation site erythema (PUFA group), with the former leading to permanent treatment discontinuation. Additionally, one patient in the PUFA group reported a keratoconjunctivitis epidemica, which was evaluated as not being related to study treatment.
There was no significant change in BCVA, nor in intraocular pressure while slit lamp examination; external ocular examination indicated good tolerability of both treatments. Acceptability and tolerability scores of both treatments were similar, particularly at the end of treatment (Table 2).
Discussion
We report here the results of a controlled clinical trial investigating the efficacy and tolerability of a topical treatment of DED with an omega-3 PUFA formulation. It confirmed the results of preclinical studies demonstrating the efficacy of topical omega-3 PUFA in alleviating symptoms in DED models in mouse26,27 and rabbit.28
Our study proved the topical omega-3 PUFA formulation to be non-inferior to a marketed 2% povidone-based formulation. In fact, numerically greater improvements of OSDI, TBUT, OSSI, MMP-9, Oxford grading, and CGI evaluation were observed with topical omega-3 PUFA, even if for none of these parameters superiority versus povidone could actually be demonstrated. Still, beyond statistical significance, only the improvements observed with topical omega-3 PUFA exceeded the threshold of minimal clinically important differences (MCID). For the OSDI score, the MCID was reported to range from 7.0 to 9.9.29 Such a change from baseline was achieved with topical omega-3 PUFA after four weeks and maintained until twelve weeks of treatment (−9.1 ± 15.2 and −10.1 ± 19.5), whereas with povidone, it remained below this threshold at Week 4 and even showed a relapse after 12 weeks (−5.1 ± 12.0 and 5.3 ± 14.1, respectively; Figure 2). Both formulations were well tolerated, not signaling any potential safety issue.
A significant relief of dry eye symptoms by oral supplementation of omega-3 PUFA is generally considered established, as demonstrated in various clinical trials of dry eye patients of different origins.10,17–19,30,31 Large clinical trials and meta-analyses have however failed to demonstrate consistent improvements in dry eye patients.21,22 The baseline consumption of fatty acids, the daily PUFA dosage, the EPA/DHA ratio or the presence of additional fatty acids besides EPA and DHA have been proposed to impact clinical improvements and might therefore explain inconsistent results observed in clinical studies.32 In this context, the topical application of omega-3 PUFA might be an interesting alternative as it allows to circumvolve the issues associated with individual variations of lipid metabolism.
MMP-9 is a gelatinase with key functions in the physiologic desquamation of corneal epithelium and higher levels of this metalloproteinase have been reported in DED patients.33–35 MMP-9 is now proposed to promote inflammatory pathways and disruption of the corneal barrier in DED.35 In our patient population, the application of topical PUFA directly on the surface of the eye appears to reduce the prevalence of elevated MMP-9 concentration (>40 ng/mL). Although only preliminary, these data might indicate that topical omega-3 PUFA inhibit inflammatory processes occurring at the ocular surface. The putative anti-inflammatory role of these lipids needs to be further investigated in larger studies in order to confirm the trend observed here.
Interestingly, exploratory analyses reported a trend towards a decrease of ocular inflammation following oral supplementation with PUFAs (containing omega-3 fatty acids, omega-6 fatty acids or a mix of both). The tear concentration of the interleukins IL-1β, IL-6 or IL-17A and the percentage of human leukocyte antigen (HLA)-DR positive cells decreased for example significantly after three months of oral supplementation.20,36,37 Tear osmolarity, a surrogate marker of eye inflammation, also evolved positively following PUFA oral supplementation.31,38 This reduction of inflammation was however only poorly associated with clinical improvements. The use of topical PUFAs could be an alternative option to relieve ocular inflammation and to consequently improve the signs and symptoms of DED.
Our promising efficacy results are in line with other recent studies on the topical use of omega-3 PUFA for DED. A study with a similar design compared the efficacy and safety of an artificial tear formulation containing omega 3-PUFA (flaxseed oil) and trehalose with a marketed formulation lacking these two components in 242 patients with DED. As in our study, both treatments significantly improved OSDI, ocular staining, and TBUT over 90 days; however, between-group differences in combined ocular, corneal, and conjunctival staining were in favor of the omega 3-PUFA formulation, which also resulted in fewer treatment-related AEs (6.7 vs 9.8%) and in particular in no blurred vision (omega 3-PUFA 0% vs 4.1%).39 In a crossover study, an omega-3 PUFA containing eye drop formulation showed the increase in the lipid layer thickness of the tear film and dryness symptom relief to be superior to non-emollient aqueous formulation.40
Moreover, topical omega 3-PUFA proved to be beneficial in related ophthalmic indications, eg a faster regeneration of corneal nerve fibers was shown in patients with keratoconus after epi-off cross-linking41 and reduced tear concentrations of pro-inflammatory cytokines in patients suffering from contact lens discomfort.20 However, in this study, there was no effect on tear osmolarity and a greater symptomatic relief compared with placebo turned out not to be significant.
Lessons learned during the clinical evaluation of systemic omega-3 PUFA could help us to further characterize the efficacy and safety of topical PUFA. The main evidence for the efficacy of oral supplementation of omega-3 PUFA in alleviating DED has been provided by the well-controlled studies of Bhargava et al, which differed in three major aspects from ours, ie they were larger in size – having enrolled between 100 and 500 patients – lasted longer for up to six months and used placebo as comparator.17–19 This clearly points at the limitations of our study. As we initially had no precise estimation possible of the effect size, we did not implement any power considerations. Retrospectively, with <100 patients enrolled and the effect size being diminished by the choice of an active comparator, our study was certainly underpowered to demonstrate superiority.
Conclusion
Topical omega-3 PUFA proved its efficacy and tolerability as topical treatment of DED and demonstrated non-inferiority versus 2% povidone. The improvements observed with topical omega-3 PUFA were carried over 12 weeks.
Abbreviations
AE, adverse events; BCVA, best corrected distance visual acuity; CGI, clinical global impression; DED, dry eye disease; ITT, intention-to-treat; MCID, minimal clinically important difference; MMP-9, matrix metalloproteinase-9; MW, Mann–Whitney; OSDI, Ocular Surface Disease Index; OSSI, Ocular Surface Symptoms Intensity; PP, per protocol; PUFA, polyunsaturated fatty acids; TBUT, tear break-up time; VAS, visual analogue scale.
Acknowledgments
We thank Uwe Totzke for medical writing support funded by TRB Chemedica and Dr. Sabine Collaud-Basset for her valuable scientific input.
Disclosure
The study was sponsored by TRB Chemedica and the sponsor was involved in all stages of the study, including study design, statistical analyses and preparation of the manuscript. RG and PCB are employees of TRB Chemedica. Professor Elisabeth M Messmer reports personal fees from TRB Chemedica, during the conduct of the study; personal fees from several companies, outside the submitted work. Dr Christina Jacobi reports personal fees from Théa, personal fees from Santen, outside the submitted work. The authors report no other conflict of interest in this work.
References
1. Peral A, Dominguez-Godinez CO, Carracedo G, Pintor J. Therapeutic targets in dry eye syndrome. Drug News Perspect. 2008;21(3):166–176.
2. Quinto GG, Camacho W, Behrens A. Postrefractive surgery dry eye. Curr Opin Ophthalmol. 2008;19(4):335–341. doi:10.1097/ICU.0b013e3283009ef8
3. Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi:10.2147/OPTH.S5555
4. Tavares Fde P, Fernandes RS, Bernardes TF, Bonfioli AA, Soares EJ. Dry eye disease. Semin Ophthalmol. 2010;25(3):84–93. doi:10.3109/08820538.2010.488568
5. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocular Surface. 2017;15:334–365. doi:10.1016/j.jtos.2017.05.003
6. Dilly PN. Structure and function of the tear film. Adv Exp Med Biol. 1994;350:239–247.
7. Albarran C, Pons AM, Lorente A, Montés-Mico R, Artigas JM. Influence of the tear film on optical quality of the eye. Cont Lens Anterior Eye. 1997;20:129–135. doi:10.1016/S1367-0484(97)80011-2
8. Montes-Mico R. Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg. 2007;33(9):1631–1635. doi:10.1016/j.jcrs.2007.06.019
9. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocular Surface. 2017;15:438–510. doi:10.1016/j.jtos.2017.05.011
10. Barabino S, Horwath-Winter J, Messmer EM, Rolando M, Aragona P, Kinoshita S. The role of systemic and topical fatty acids for dry eye treatment. Prog Retin Eye Res. 2017;61:22–34. doi:10.1016/j.preteyeres.2017.05.003
11. Palmowski AM, Ruprecht KW. The cornea and systemic diseases. Curr Opin Ophthalmol. 1995;6(4):17–20. doi:10.1097/00055735-199508000-00004
12. Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home Healthc Now. 2018;36(2):74–83; E71–E72. doi:10.1097/NHH.0000000000000652
13. Vehof J, Snieder H, Jansonius N, Hammond CJ. Prevalence and risk factors of dry eye in 79,866 participants of the population-based Lifelines cohort study in the Netherlands. Ocular Surface. 2021;19:83–93. doi:10.1016/j.jtos.2020.04.005
14. Shah R, Amador C, Tormanen K, et al. Systemic diseases and the cornea. Exp Eye Res. 2021;204:108455. doi:10.1016/j.exer.2021.108455
15. Baudouin C, Aragona P, van Setten G, et al. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98(9):1168–1176. doi:10.1136/bjophthalmol-2013-304619
16. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocular Surface. 2017;15:575–628. doi:10.1016/j.jtos.2017.05.006
17. Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6(6):811–816. doi:10.3980/j.issn.2222-3959.2013.06.13
18. Bhargava R, Kumar P. Oral omega-3 Fatty Acid treatment for dry eye in contact lens wearers. Cornea. 2015;34(4):413–420. doi:10.1097/ICO.0000000000000386
19. Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Cont Lens Anterior Eye. 2015;38(3):206–210. doi:10.1016/j.clae.2015.01.007
20. Downie LE, Gad A, Wong CY, et al. Modulating contact lens discomfort with anti-inflammatory approaches: a randomized controlled trial. Invest Ophthalmol Vis Sci. 2018;59(8):3755–3766. doi:10.1167/iovs.18-24758
21. Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. 2019;12(12):CD011016. doi:10.1002/14651858.CD011016.pub2
22. Asbell PA, Maguire MG, Pistilli M, et al. N-3 fatty acid supplementation for the treatment of dry eye disease. New Engl J Med. 2018;378(18):1681–1690. doi:10.1056/NEJMoa1709691
23. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
24. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi:10.1097/00003226-200310000-00008
25. Begley C, Caffery B, Chalmers R, Situ P, Simpson T, Nelson JD. Review and analysis of grading scales for ocular surface staining. Ocular Surface. 2019;17(2):208–220. doi:10.1016/j.jtos.2019.01.004
26. Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi:10.1001/archophthalmol.2007.61
27. Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr Eye Res. 2014;39(9):871–878. doi:10.3109/02713683.2014.884595
28. Verbey NL, van Haeringen NJ, de Jong PT. Modulation of immunogenic keratitis in rabbits by topical administration of poly-unsaturated fatty acids. Curr Eye Res. 1988;7(6):549–556. doi:10.3109/02713688809031810
29. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi:10.1001/archophthalmol.2009.356
30. Wang L, Chen X, Hao J, Yang L. Proper balance of omega-3 and omega-6 fatty acid supplements with topical cyclosporine attenuated contact lens-related dry eye syndrome. Inflammopharmacology. 2016;24(6):389–396. doi:10.1007/s10787-016-0291-2
31. Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124(1):43–52. doi:10.1016/j.ophtha.2016.09.023
32. Mendoza RL. Clinical trials with multiple endpoints can establish a correlation, but not (yet) causality, between dietary supplementation with omega-3 fatty acids and keratoconjunctivitis sicca. J Med Econ. 2018;21(8):733–744. doi:10.1080/13696998.2018.1478838
33. Sambursky R, Davitt WF, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–28. doi:10.1001/jamaophthalmol.2013.561
34. Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–2308. doi:10.1016/j.ophtha.2016.07.028
35. Shoari A, Kanavi MR, Rasaee MI, Afsar Aski S, Tooyserkani R. Inhibition of matrix metalloproteinase-9 for the treatment of dry eye syndrome; a review study. Exp Eye Res. 2021;46(1):7–13. doi:10.1080/02713683.2020.1782943
36. Pinazo-Duran MD, Galbis-Estrada C, Pons-Vazquez S, Cantu-Dibildox J, Marco-Ramírez C, Benítez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interv Aging. 2013;8:139–148. doi:10.2147/CIA.S40640
37. Brignole-Baudouin F, Baudouin C, Aragona P, et al. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89(7):e591–e597. doi:10.1111/j.1755-3768.2011.02196.x
38. Oydanich M, Maguire MG, Pistilli M, et al. Effects of omega-3 supplementation on exploratory outcomes in the dry eye assessment and management study. Ophthalmology. 2020;127(1):136–138. doi:10.1016/j.ophtha.2019.07.009
39. Downie LE, Hom MM, Berdy GJ, et al. An artificial tear containing flaxseed oil for treating dry eye disease: a randomized controlled trial. Ocular Surface. 2020;18(1):148–157. doi:10.1016/j.jtos.2019.11.004
40. Fogt JS, Fogt N, King-Smith PE, Liu H, Barr JT. Changes in tear lipid layer thickness and symptoms following the use of artificial tears with and without omega-3 fatty acids: a randomized, double-masked, crossover study. Clin Ophthalmol. 2019;13:2553–2561. doi:10.2147/OPTH.S228261
41. Cagini C, Messina M, Torroni G, Riccitelli F, Mariniello M, Dua HS. Efficacy of topical microemulsion of fatty acids of the omega-3 series on the sub-epithelial corneal nerves regeneration after epithelium-off corneal collagen cross-linking for keratoconus. Int Ophthalmol. 2020;40(1):205–212. doi:10.1007/s10792-019-01170-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

