Back to Journals » Clinical Optometry » Volume 15
Quality of Life in Digital Device Users Who are Treated with Systane Hydration PF
Authors Pucker AD , Lievens C , McGwin G Jr, Franklin QX, Logan A, Wolfe GS
Received 21 November 2022
Accepted for publication 1 March 2023
Published 7 March 2023 Volume 2023:15 Pages 45—54
DOI https://doi.org/10.2147/OPTO.S398496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Mr Simon Berry
Andrew D Pucker,1 Chris Lievens,2 Gerald McGwin Jr,1 Quentin X Franklin,1 Amy Logan,1 Gregory S Wolfe2,3
1University of Alabama at Birmingham, Birmingham, AL, USA; 2Southern College of Optometry, Memphis, TN, USA; 3Center for Eye and Health Outcomes, Memphis, TN, USA
Correspondence: Andrew D Pucker, University of Alabama at Birmingham, School of Optometry, 1716 University Blvd, Birmingham, AL, 35233, Tel +1 (920) 579-2900, Email [email protected]
Purpose: To understand the impact of Systane Hydration PF on dryness symptoms and quality of life in digital device users and to determine if participants prefer either the unit-dose or multi-dose dispensing system of Systane Hydration PF.
Materials and Methods: This 2-week, three visit study recruited regular digital device users. Participants were required to score ≤ 80 on the Impact of Dry Eye on Everyday Life (IDEEL) Quality of Life (QoL) Work domain and between 13 and 32 on the Ocular Surface Disease Index (OSDI) questionnaire. Participants were randomized to either Systane Hydration PF unit-dose or multi-dose for 1 week and switched to the alternative dosing system for the second week. Participations were evaluated by completing the full IDEEL-QoL module and OSDI questionnaire at each visit. Likert surveys were completed to probe dispensing system preferences.
Results: Thirty participants with a mean ± SD age of 28.6 ± 12.0 years (70% female) were recruited. Participants had significant improvements in all three IDEEL-QoL domains as well as in OSDI scores (all p < 0.0001). Participants had similar preferences for the two dispensing systems, though they were more likely to indicate that they thought that the multi-dose bottle was more environmentally friendly than the unit-dose vials.
Conclusion: Digital device users with dry eye symptoms had meaningful improvements in eye comfort and quality of life scores after being treated with Systane Hydration PF for 2 weeks. Participants did not have a clear dispensing system preference suggesting that the best dispensing system may depend on the patient.
Keywords: artificial tears, digital eye strain, dry eye disease, symptoms, Systane Hydration PF
Introduction
While high-powered computers and digital devices have greatly improved many aspects of modern life, such as improved social connections, providing lifesaving assistance, and making our transactions more efficient,1 the pervasive use of digital devices has caused some patients to develop a condition known as Digital Eye Strain (DES), which is also known as computer vision syndrome (CVS). DES is a condition where patients experience eye symptoms, which can be subcategorized as vision-related, oculomotor-related, ocular surface-related, environmentally related, or device-related.2 Seguí et al have specifically determined through an extensive literature search that CVS/DES symptoms include burning, foreign body sensation, tearing, excessive blinking, eye redness, eye pain, heavy eyelids, dryness, blurred vision, double vision, poor near focusing, photophobia, glare and halos, worsening vision, and headache.3
Extensive digital device use is no longer an employment-specific challenge, because digital devices are now common and socially expected during many home and leisure activities.4 Digital device use can produce a myriad of symptoms,2,4–6 with many of these symptoms also being indicative of dry eye disease (DED). Thus, it is not surprising that there is an established relationship between DED and DES.2,4–7 DES symptoms have been reported to be as high as 93% in digital device users depending upon how DES is defined.2,4 While the etiology of dryness symptoms in DES are probably multifactorial and likely include contact lens wear, incomplete blinks, tear film instability, and increased friction, much of the dryness symptoms in DES are likely due to tear film evaporation secondary to having a reduced number of blinks per minute while using digital devices.2,7 Since much of the ocular symptoms associated with DES likely stem from excessive tear evaporation, artificial tears have become an accepted treatment for dryness symptoms in DES patients.2,4
Systane Hydration PF (Alcon, Fort Worth, TX, USA) is a preservative-free, artificial tear that contains water-loving sodium hyaluronate and the viscosity enhancing agent hydroxypropyl guar. While Systane Hydration PF should in theory improve the dryness symptoms and subsequently the quality of life of patients who have DES, this clinical application has yet to be tested. This preservative-free drop furthermore is available in both unit-dose and multi-dose drop system options, but it is unclear if patients perceive a difference between these two dispensing systems. Thus, the purpose of this study was to recruit participants who have DES who also have mild-to-moderate dry eye symptoms and to treat them with Systane Hydration PF to determine how regular use of Systane Hydration PF over 2 weeks impacts a patient’s ocular symptoms and overall quality of life. This study secondarily compared the participant’s experience with the two Systane Hydration PF dispensing systems because this information could improve compliance and subsequently treatment effectiveness.
Materials and Methods
Participants
This 2-week, three-visit study was conducted at the University of Alabama at Birmingham (Birmingham, AL, USA). A total of 30 participants were recruited. Thirty participants were deemed appropriate since 29 participants yielded sufficient data to understand patient reported outcomes in a recent study completed by the lead investigator.8,9 Adult (>18 years) participants who answered yes to the following two questions and used digital devices (eg, computers, tablets, or smartphones) ≥8 hours per day were recruited:
Are your eyes dry, irritated, or itchy or do they burn while using a digital screen, like a computer or smartphone? Yes or No
Eye fatigue is the physical discomfort of your eyes after spending periods of time throughout the day in front of a digital screen, like a computer or smartphone.10 Do you have eye fatigue based upon this definition? Yes or No
Participants were also required to score ≤80 on the Impact of Dry Eye on Everyday Life (IDEEL) Quality of Life (QoL) Work domain to ensure that they had clinically meaningful symptoms,11 and they were required to have an Ocular Surface Disease Index (OSDI) score between 13 and 32 (inclusive) to ensure that they had mild-to-moderate dry eye symptoms, which is the typical indication for artificial tear monotherapy.12,13 Each subset of the IDEEL-QoL questionnaire has a 0 to 100 range with higher scores being better. The OSDI questionnaire likewise has a 0 to 100 range; however, lower scores are better. Participants were excluded if they had worn contact lenses in the past week, had a known systemic health conditions that is thought to alter tear film physiology, had a history of ocular surgery within the past 12 months, had a history of ocular trauma, active ocular infection or inflammation, were currently using isotretinoin-derivatives or ocular medications, were currently using a dry eye treatment other than artificial tears, were currently using more than 4 drops of artificial tears per day in each eye, or if they were pregnant or breastfeeding.14 If a participant was using artificial tears or rewetting drops, they were required to refrain from using them for at least 24 hours before their baseline visit, and they were only allowed to use Systane Hydration PF during the course of the study. No contact lenses wear was allowed during the study.
Surveys and Clinical Tests
This study was registered with Clinicaltrials.gov (NCT04837807). It was approved by the University of Alabama at Birmingham’s Institutional Review Board (IRB-300007063), and it followed the Declaration of Helsinki. Participants were asked to bring their spectacles to the study visit and to adhere to the contact lens exclusion factors described above. The first visit commenced with reviewing the IRB approved screening form to ensure that they still qualified for the study. The OSDI questionnaire was completed, and it was confirmed that they had a score between 13 and 32 (inclusive). The participants then completed the IDEEL-QoL Work questionnaire, and it was confirmed that they had a score ≤80. Non-eligible participants were dismissed. Participants who meet all study criteria were enrolled following informed consent.
Participant demographics were collected (age, sex, race, ethnicity). The remaining two subsections of the IDEEL-QoL questionnaire were then completed (Daily Activities and Feelings).15 Visual acuity was measured with a Bailey-Lovie high-contrast Logarithm of the Minimum Angle of Resolution (logMAR) chart with their presenting correction if applicable. A slit-lamp biomicroscope was used to monitor ocular safety. After completing entrance testing, participants were randomized with Research Electronic Data Capture (REDCap) to determine if they would start with Systane Hydration PF multi-dose or Systane Hydration PF unit-dose.16,17 Participants were educated that they should use Systane Hydration PF at least four times per day in each eye. During this education session, the participants were also given a daily log to record the number of drops used each day, their eye comfort throughout the day, and the duration digital device use by type across the study. When comfort was being evaluated, it was done so with a 0 to 100 visual analog scale (VAS). Higher scores were more comfortable.
When participants returned for their one-week visit, they repeated the OSDI questionnaire, all three subsets of the IDEEL-QoL questionnaire, visual acuity, and an evaluation with the slit-lamp biomicroscope as described above. The participants then completed an investigator-designed Likert questionnaire based upon the work of Denis et al that probed their experience with the drops.18 This questionnaire also queried if participants had any mobility issues since the Likert questionnaire was aimed at understanding how the participants perceived applying the drops. The compliance log was collected at this visit, and they were given a new compliance log to be completed over the following week. Participants were then given the alternative drop delivery system, and they were released until their follow-up visit. No washout period was deemed necessary since participants were using the same drops.
When participants returned for their two-week visit, the same testing was completed as at the one-week visit; however, the investigator designed questionnaire included a question that specifically asked the participants to select which dispensing method they preferred. After the completion of this testing, participants were released from the study.
Statistical Analysis
All data were analyzed with SAS Version 9.4 (SAS; Cary, NC, USA) or Excel (Microsoft Corporation, Redmond, WA, USA). Means and standard deviations (SD) were presented to understand data trends. ANOVA and chi-square tests were used to compare continuous and categorical variables, respectively. The patient reported outcomes questionnaire data were analyzed to determine the frequency of each response, and these data are presented as percentages. When analyzing the treatment effect of Systane Hydration, the data were analyzed chronologically, but when dispensing methods were being analyzed, data were sorted by dispensing method.
Results
This study recruited 30 participants with 29 of them completing all three visits (Figure 1). The participant who discontinued the study did so because they lost interest in the study. The mean age ± SD of the participants was 28.6 ± 12.0 years, and 70% of the participants were female. Thirteen percent of the participants identified as Hispanic with 3%, 37%, 0%, 3%, 50%, 3%, and 3% of the participants identifying as American Indian or Alaskan Native, Asian, Native Hawaiian or Pacific Islander, Black or African American, White, more than one race, or unknown or not reported, respectively. Thirteen percent of the participants reported using artificial tears or rewetting drops more than once per week before starting the study. None of the participants had been diagnosed with a mobility-related disease. No adverse events related to the study were noted by the investigators or reported by the participants.
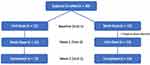 |
Figure 1 Participant Flow Diagram. |
When asked about drop usage, the participants indicated that on average that they applied their drops 4.4 ± 0.8, 5.0 ± 1.5, and 5.1 ± 1.4 times per day on the first day, at 1 week, and at 2 weeks, respectively. Only two participants at each of these timepoints used their drops fewer than four times per day. The participants used their digital devices an overall average of 12.4 ± 3.9, 12.9 ± 5.0, and 10.7 ± 4.2 hours per day on the first day, at 1 week, and at 2 weeks (p = 0.08), respectively. A breakdown of hours by device type can be found in Table 1.
 |
Table 1 Digital Device Use in Participants Treated with Systane Hydration PF |
The primary goal of this study was to understand how Systane Hydration PF affects dryness symptoms and quality of life in participants who are symptomatic digital device users. The participants indicated that they had significant improvements in their VAS eye comfort upon waking, eye comfort at the beginning of digital device use, eye comfort at midday of digital device use, eye comfort at the end of the digital device use day, and end of day eye comfort (all p < 0.003; Table 2). This study found that there was a significant improvement in Daily Activity, Feelings, and Work IDEEL scores after being treated with Systane Hydration PF for 2 weeks (all p < 0.0001; Table 3). A full breakdown of IDEEL responses by question can be found in Supplemental Table 1. OSDI scores also significantly improved with treatment (p < 0.0001; Table 3). Although this study found a significant improvement in dry eye symptoms leading to improved quality of life, it did not find any clinically meaningful differences in right eye (baseline 0.05 ± 0.14; 1-week 0.03 ± 0.17; 2-week 0.02 ± 0.15 [p=0.05]) or left eye (baseline 0.03 ± 0.18; 1-week 0.01 ± 0.15; 2-week 0.02 ± 0.18 [p=0.21]) logMAR visual acuities.
 |
Table 2 Eye Comfort Visual Analog Scale (VAS) Scores in Digital Eye Strain Participants with Dry Eye Treated with Systane Hydration PF |
 |
Table 3 Standardized Symptoms Questionnaire Scores by Study Visit |
The secondary goal of this study was to understand if the participants preferred the unit-dose or multi-dose Systane Hydration PF dispensing systems. This study found that 55% of the participants preferred the unit-dose Systane Hydration PF dispensing system while 45% of the participants preferred multi-dose Systane Hydration PF dispensing system. Furthermore, when asked, participants indicated that they were overall “very satisfied” or “satisfied” with the unit-dose (76%) and multi-dose (66%) Systane Hydration PF dispensing systems (Table 4). Participants indicated that they would or probably would continue using the unit-dose (55%) and multi-dose (80%) Systane Hydration PF dispensing systems after the study. The participants found the unit-dose and multi-dose Systane Hydration PF dispensing systems “very easy” or “easy” to open (100% vs 100%), handle (100% vs 90%), target their eye (97% vs 76%), remove the residual drops (89% vs 89%), squeeze to obtain a drop (97% vs 76%), and instill the drop (100% vs 89%) (Table 4). Participants finally were more likely to indicate that the multi-dose (86%) system of Systane Hydration PF was environmentally friendly or probably environmentally friendly than the unit-dose (45%) system of Systane Hydration PF (Table 4).
 |
Table 4 Likert Questions That Probe Participant Drop Preferences18 |
Discussion
The primary goal of this study was to understand how Systane Hydration PF affects dryness symptoms and quality of life in participants who are symptomatic digital device users. This goal was partially accomplished by evaluating participant reported outcomes with two different validated questionnaires.19,20 The first device was the OSDI questionnaire, which is a widely used dry eye symptoms questionnaire that evaluates three different domains related to dry eye (symptoms, tasks, environment) while also grading the severity of the condition.12,13 The second device was the IDEEL-QoL module (Daily Activities, Feelings, Work), which specifically evaluates how dry eye effects a patient’s quality of life.21 The current study found significant improvements in OSDI scores and all three domains of the IDEEL-QoL module after 2 weeks of treatment with Systane Hydration PF compared to the baseline visit. These data indicate that treatment with Systane Hydration PF improves a participant’s ability to engage in visual related activities while improving their quality of life and dryness symptoms in participants who are regular digital device users. The work domain furthermore showed the greatest magnitude of improvement with participants feeling less distracted, having improved concentration, needing fewer breaks from work, and not needing to change the way that they work or the environment where they reside. These validated survey results are corroborated by the VAS data, which found significant improvements in comfort at every timepoint measured compared to baseline. These results indicate that Systane Hydration PF can have a significant impact on the quality of life and comfort of those who suffer from DES.
Guillon et al, Reddy et al, Acosta et al, and Skilling et al have previously explored the effects of artificial tears on DES. Guillon et al (n = 21; 35.6 ± 11.6 years) evaluated three different dosing schedules in contact lens wearers who completed 4 hours’ worth of computer use in office; dose schedules were trialed on different study days.22 The authors found that each artificial tear regiment significantly reduced ocular symptoms compared to no treatment during computer use equally. Reddy et al found in a survey of 795 students (age range = 18 to 25 years) that respondents significantly indicated that artificial tears helped reduce the symptoms associated with DES.23 While studying blink rates, Acosta et al (n = 20; age = 21.1 ± 0.5 years) found that artificial tears but not saline improved ocular symptoms during short computer sessions (10 to 30 minutes) with wind stress compared to no treatment.24 Skilling et al lastly performed a 5-day, randomized trial (n = 50) with participants who used digital devices 4 or more hours per day. Participants were required to apply 1 to 2 drops of artificial tears (age = 30.6 ± 12.7 years) or Visine Original (age = 25.7 ± 11.0 years; contains tetrahydrozoline), and the author’s goal was to understand their effect on ocular comfort. While the authors found both drops to significantly improve ocular comfort, they failed to find any between-drop comfort differences.25 While all the above studies found artificial tears to benefit participants with DES, much like this study, the current study is one of the first to evaluate digital device users for more than 1 week while being treated with a modern artificial tear formulation dosed 4 or more times per day.
This study’s secondary outcome was to compare participant preferences for drop dispensing systems (unit-dose vs multi-dose dispensing system). This study specifically found that a similar proportion of participants overall preferred the multi-dose (55%) and unit-dose (45%) dispensing system. While there has been limited data related to formally comparing multi-dose and unit-dose dispensing systems in the dry eye arena, this question has been asked by others while evaluating glaucoma patients.18,26,27 Denis et al specifically conducted a retrospective survey of 788 glaucoma patients (68.1 ± 12.1 years) who were prescribed a new multi-dose dispensing system, and the authors found overall that 81.6% of the participants who had past experience with unit-dose drops and 74.4% of the participants with past experience with multi-dose drops preferred the novel multi-dose dispensing system with regards to handling. The authors likewise evaluated how satisfaction with the multi-dose delivery system was correlated with the level of shoulder, arm, and hand disability as determined with the QuickDASH® questionnaire, which is a validated measure of physical function and symptoms related to upper-limb musculoskeletal disorders.18 Deniset et al found that level of satisfaction with the multi-dose delivery system decline with increasing disability and age. This decrease in satisfaction is likely related to the participant’s reduced ability to open and manipulate the multi-dose bottle. Thus, while it is challenging to make direct comparisons with Denis et al and the current study because of the different study designs, age, and disability clearly have an impact on patient preferences. Disability was not analyzed in the current study because these participants were not recruited likely because participants were required to be working.
While the current study did find limited between drop dispensing system preferences, it did find that participants were more likely to indicate that they thought that the multi-dose (86%) system of Systane Hydration PF was more environmentally friendly than the unit-dose (45%) version of Systane Hydration PF. To the best of our knowledge, no other group has specifically evaluated this question, though this result is it in line with Hawes et al who determined that participants preferred products that were environmentally friendly.28 Nonetheless, the current study’s results should be verified in a future study with a more diverse group of participants.
Despite this study being strengthened by using a multi-prong approach to evaluate patient-reported outcomes (validated, VAS, and Likert questionnaires) and by using a relatively homogeneous group of participants (working age digital device users), this study is not without limitations. One limitation is the relatively young age of the included participants (28.6 ± 12.0 years). Although this mean age is in line with patients who are likely to experience DES, it is lower than the typical DED patient who will more commonly be treated with artificial tears;29 therefore, the results of this study may not directly apply to this older age group, and further studies that include an older population are needed. Additional limitations are that this study had no control group and that masking between the two dispensing systems was not possible because of the nature of the treatment. The drops were specifically challenging to mask and identify a suitable control group because one of the primary aspects of the study was to compare the unit-dose and multi-dose dispensing methods of the drops (no similar option in the market at the time). While this study did find that topical eye drops are able to improve symptoms and quality of life in digital device users, further studies with a placebo group or with an alternative brand of artificial tears are needed to better determine efficacy and if one drop is better than another for treating DES. A final limitation of this study is that it had no a priori sample size calculation due to the limited IDEEL-QoL module data in the literature. Nevertheless, if one uses the baseline IDEEL Work domain data from the current study, only 26 subjects are needed to determine if there is a significant between-visit difference when one assumes 10 units as a clinically meaningful difference (α = 0.05; power = 80%).
Conclusions
Although environmental and ergonomic modifications can be made to reduce the frequency and intensity of DES, implementing these modifications is potentially challenging, which places more emphasis on the role of artificial tears as a treatment. This study provides some of the first evidence to suggest that use of Systane Hydration PF in digital device users can provide symptomatic relief of dry eye-related symptoms in patients who self-report DES while also improving their quality of life. The participants in this study found no clear preference between the unit-dose and multi-dose Systane Hydration PF dispensing systems for most features evaluated, yet this study did clearly indicate that participants felt that the multi-dose system was more environmentally friendly. With this information in mind, these results suggest that practitioners should have a discussion with their patients to determine which artificial tears dispensing system best meets their lifestyle. This discussion may not only promote better drop compliance but it may also result in a more effective treatment.
Data Sharing Statement
No data beyond what is reported in this manuscript will be publicly shared.
Acknowledgments
Alcon Research, LLC supported this study. REDCap data collection was supported by the University of Alabama at Birmingham’s CTSA Grant (UL1TR001417).
Disclosures
The authors have received research support from AbbVie Pharmaceuticals (CL), Alcon Research, LLC (All), Allergan (CL), Art Optical (ADP), Bausch & Lomb (ADP), and Euclid Systems (ADP) and have served as consultants for Alcon Research, LLC (ADP), CooperVision (ADP), EpiTech (ADP), EyeGate Pharmaceuticals, Inc (ADP), Kala Pharmaceuticals (ADP), Lexitas (ADP), MacuLogix (CL), Nevakar Inc (ADP), Optikal Care Inc (ADP), RVL Pharmaceuticals, Inc (CL), Transitions (CL), and Transitions Optical (CL) over the past 3 years. Dr Pucker is currently an employee of Lexitas Pharma Services; however, he was employed by the University of Alabama at Birmingham during the conduct of this work. The authors report no other conflicts of interest in this work.
References
1. Damant J, Knapp M, Freddolino P, Lombard D. Effects of digital engagement on the quality of life of older people. Health Soc Care Community. 2017;25:1679–1703. doi:10.1111/hsc.12335
2. Coles-Brennan C, Sulley A, Young G. Management of digital eye strain. Clin Exp Optom. 2019;102:18–29. doi:10.1111/cxo.12798
3. Segui Mdel M, Cabrero-Garcia J, Crespo A, Verdu J, Ronda E. A reliable and valid questionnaire was developed to measure computer vision syndrome at the workplace. J Clin Epidemiol. 2015;68:662–673. doi:10.1016/j.jclinepi.2015.01.015
4. Sheppard AL, Wolffsohn JS. Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018;3:e000146. doi:10.1136/bmjophth-2018-000146
5. Talens-Estarelles C, Sanchis-Jurado V, Esteve-Taboada JJ, Pons AM, Garcia-Lazaro S. How do different digital displays affect the ocular surface? Optom Vis Sci. 2020;97:1070–1079. doi:10.1097/OPX.0000000000001616
6. Sanchez-Valerio MDR, Mohamed-Noriega K, Zamora-Ginez I, Baez Duarte BG, Vallejo-Ruiz V. Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin Ophthalmol. 2020;14:4311–4317. doi:10.2147/OPTH.S252889
7. Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. 2021;15:3811–3820. doi:10.2147/OPTH.S321591
8. Duong K, McGwin G, Franklin QX, Cox J, Pucker AD. Treating uncomfortable contact lens wear with orthokeratology. Eye Contact Lens. 2021;47:74–80. doi:10.1097/ICL.0000000000000690
9. Duong K, Pucker AD, McGwin G, Franklin QX, Cox J. Established soft contact lens wearers’ awareness of and initial experiences with orthokeratology. Ophthalmic Physiol Opt. 2021;41:673–682. doi:10.1111/opo.12828
10. Meyer D, Rickert M, Kollbaum P. Ocular symptoms associated with digital device use in contact lens and non-contact lens groups. Cont Lens Anterior Eye. 2021;44:42–50. doi:10.1016/j.clae.2020.07.007
11. Pouyeh B, Viteri E, Feuer W, et al. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol. 2012;153:1061–1066 e1063. doi:10.1016/j.ajo.2011.11.030
12. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi:10.1001/archopht.118.5.615
13. Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the Ocular Surface Disease Index (OSDI). Investig Ophthalmol Vis Sci. 2011;52:8630–8635. doi:10.1167/iovs.11-8027
14. Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31:1000–1008. doi:10.1097/ICO.0b013e318242fd60
15. Fairchild CJ, Chalmers RL, Begley CG. Clinically important difference in dry eye: change in IDEEL-symptom bother. Optom Vis Sci. 2008;85:699–707. doi:10.1097/OPX.0b013e3181824e0d
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi:10.1016/j.jbi.2008.08.010
18. Denis P, Duch S, Chen E, et al. European real-world data about the use of a new delivery system containing a preservative-free multi-dose glaucoma treatment. Eur J Ophthalmol. 2020;1:1120672120919342.
19. Okumura Y, Inomata T, Iwata N, et al. A Review of Dry Eye Questionnaires: measuring Patient-Reported Outcomes and Health-Related Quality of Life. Diagnostics. 2020;11:10. doi:10.3390/diagnostics11010010
20. Grubbs JR, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33:215–218. doi:10.1097/ICO.0000000000000038
21. Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005;8:168–174. doi:10.1111/j.1524-4733.2005.03074.x
22. Guillon M, Maissa C, Pouliquen P, Delval L. Effect of povidone 2% preservative-free eyedrops on contact lens wearers with computer visual syndrome: pilot study. Eye Contact Lens. 2004;30:34–39. doi:10.1097/01.ICL.0000101489.13687.9A
23. Reddy SC, Low CK, Lim YP, Low LL, Mardina F, Nursaleha MP. Computer vision syndrome: a study of knowledge and practices in university students. Nepal J Ophthalmol. 2013;5:161–168. doi:10.3126/nepjoph.v5i2.8707
24. Acosta MC, Gallar J, Belmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Exp Eye Res. 1999;68:663–669. doi:10.1006/exer.1998.0656
25. Skilling FC, Weaver TA, Kato KP, Ford JG, Dussia EM. Effects of two eye drop products on computer users with subjective ocular discomfort. Optometry. 2005;76:47–54. doi:10.1016/S1529-1839(05)70254-2
26. Aptel F, Villemont AS, Cunnac P, et al. Comparison of Topical Instillation From Single-dose and Multidose Eye Drop Containers in Glaucoma: a Multicenter Randomized Cross-sectional Trial. J Glaucoma. 2021;30:718–724. doi:10.1097/IJG.0000000000001867
27. Dietlein TS, Jordan JF, Luke C, Schild A, Dinslage S, Krieglstein GK. Self-application of single-use eyedrop containers in an elderly population: comparisons with standard eyedrop bottle and with younger patients. Acta Ophthalmol. 2008;86:856–859. doi:10.1111/j.1755-3768.2007.01155.x
28. Haws KL, Winterich KP, Naylor RW. Seeing the world through GREEN-tinted glasses: green consumption values and responses to environmentally friendly products. J Consumer Psychol. 2014;24:336–354. doi:10.1016/j.jcps.2013.11.002
29. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
