Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Time To Response In Patients With Advanced Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small-Cell Lung Cancer (NSCLC) Receiving Alectinib In The Phase II NP28673 And NP28761 Studies
Authors Gadgeel S, Shaw AT, Barlesi F, Crino L , Yang JCH , Dingemans AM, Kim DW, de Marinis F , Schulz M, Liu S, Gupta R, Smoljanovic V, Ou SHI
Received 19 March 2019
Accepted for publication 29 October 2019
Published 13 November 2019 Volume 2019:10 Pages 125—130
DOI https://doi.org/10.2147/LCTT.S209231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Antonio Araujo
Shirish Gadgeel,1 Alice T Shaw,2 Fabrice Barlesi,3 Lucio Crino,4 James CH Yang,5 Anne-Marie Dingemans,6 Dong-Wan Kim,7 Filippo de Marinis,8 Mathias Schulz,8 Shiyao Liu,9 Ravindra Gupta,9 Vlatka Smoljanovic,10 Sai-Hong Ignatius Ou11
1Department of Internal Medicine, Division of Hematology and Oncology, The University of Michigan, Ann Arbor, MI 48109, USA; 2Center for Thoracic Cancers, Massachusetts General Hospital, Boston, MA, USA; 3Multidisciplinary Oncology and Therapeutic Innovations Department, Aix-Marseille University, Assistance Publique Hôpitaux de Marseille, Marseille 13005, France; 4Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) Srl I.R.C.C.S., Meldola, FC 47014, Italy; 5National Taiwan University Hospital and National Taiwan University Cancer Centre, Taipei City, Taiwan; 6Department of Pulmonology, Maastricht University Medical Centre, Maastricht 6229 HX, The Netherlands; 7Department of Internal Medicine, Seoul National University Hospital, Jongno-Gu, Seoul 03080, South Korea; 8Division of Thoracic Oncology, European Institute of Oncology IRCCS, Milan 20146, Italy; 9Genentech Inc., South San Francisco, CA, USA; 10F. Hoffmann-La Roche Ltd., Basel CH-4070, Switzerland; 11Division of Hematology-Medical Oncology, Department of Internal Medicine, University of California, Irvine School of Medicine, Orange, CA 92617, USA
Correspondence: Sai-Hong Ignatius Ou
University of California, Irvine School of Medicine, 1001 Health Sciences Road, Irvine, Orange, CA 92617, USA
Tel +1 714 456 8104
Email [email protected]
Introduction: Alectinib is a highly selective and potent ALK inhibitor, approved for the treatment of patients with metastatic ALK+ NSCLC based on results from the Phase II global NP28673 (NCT01801111) and North American NP28761 (NCT01871805) studies.
Methods: This exploratory analysis of two Phase II studies of alectinib (NP28673/NP28761) investigated time to systemic response (TTR) and time to central nervous system (CNS) response (TTCR) in patients with previously treated advanced anaplastic lymphoma kinase fusion gene-positive (ALK+) non-small-cell lung cancer. Patients (n=225) received 600 mg oral alectinib twice daily and had scans every 6/8 weeks (NP28673/NP28761).
Results: For NP28673 and NP28761, respectively: median follow-up was 21.3 months/17.0 months; most responders (72.6%/82.9%) responded by the first disease assessment; median TTR was 8 weeks (95% confidence interval [CI]: 8.00–8.14)/6 weeks (95% CI: 5.86–6.14); median TTCR in responders with measurable baseline CNS disease was 8 weeks (95% CI: 7.86–10.29)/6 weeks (95% CI: 5.71–not evaluable). Similar results were observed regardless of measurable/non-measurable disease.
Discussion: These data suggest that alectinib achieves a rapid response in patients, both systemically and in the CNS.
Keywords: alectinib, non-small-cell lung cancer, NP28673, NP28761, time to response
Introduction
Anaplastic lymphoma kinase fusion gene-positive (ALK+) non-small-cell lung cancer (NSCLC) is a distinct subgroup of lung cancer, occurring in approximately 5% of patients with advanced NSCLC.1 The majority of patients treated with crizotinib relapse within the first year, due to either poor penetration to the central nervous system (CNS)2 or development of secondary ALK resistance mutations.1
Alectinib is a highly selective and potent ALK inhibitor, approved by the US Food and Drug Administration for the treatment of patients with metastatic ALK+ NSCLC. The approval of alectinib in patients with metastatic ALK+ NSCLC whose disease had progressed on, or who were intolerant to, crizotinib was based on data from the global NP28673 (NCT01801111) and North American NP28761 (NCT01871805) studies. A pooled analysis of data from these studies showed that alectinib achieved high overall response rates (51.3%, 95% confidence interval [CI] 44.0–58.6; data cutoff February 1, 2016 [NP28673] and January 22, 2016 [NP28761]), and that responses were durable (median 14.9 months).3
Alectinib has also demonstrated consistent CNS efficacy. In a pooled analysis from NP28673 and NP28761, the CNS response rate was 64.0% (95% CI: 49.2–77.1) in patients with measurable CNS disease at baseline. The median duration of response in the CNS was 10.8 months.4 The CNS disease control rate was 90.0% (95% CI: 78.2–96.7) in patients with measurable CNS disease at baseline and 86% (95% CI: 79.1–91.4) in patients with measurable and non-measurable CNS disease at baseline.5
This exploratory analysis investigated how rapidly patients achieve benefit from alectinib, in terms of time to systemic response (TTR) and time to CNS response (TTCR).
Methods
NP28673 and NP28761 were Phase II, single-arm, open-label, multicenter studies, for which full methodology has been published previously.6,7 Response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Restaging scans, including brain scans, were obtained every 8 (NP28673) or 6 (NP28761) weeks. TTR and TTCR were defined, respectively, as time from date of first dose of alectinib to date of first occurrence of response in the response-evaluable (RE) population with confirmed systemic response, or in the safety population with confirmed CNS response. The studies were approved by the Institutional Review Board and the Ethics Committee of each study centre (full list available Supplementary Table 1), and were undertaken in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all patients.
The results are presented as two separate datasets rather than a pooled analysis because TTR is driven by scanning intervals. For NP28673, we present the number of patients who responded by weeks 8 and 16, and for NP28761 the number of patients who responded by weeks 6 and 12. The data cutoffs were February 1, 2016 (NP28673) and January 22, 2016 (NP28761).
Results
Patients
The dataset comprised 138 patients from NP28673 and 87 patients from NP28761; the RE populations by independent review committee (IRC) comprised 122 and 67 patients, respectively. In the RE populations, baseline CNS metastases were present in 60.7% (NP28673) and 58.2% (NP28761) of patients. In both studies, baseline characteristics were similar in confirmed responders and in the RE population (Supplementary Table 2).
Efficacy
Median follow-up was 21.3 months (range 0.6–29.7) in NP28673 and 17.0 months (range 1.1–28.6) in NP28761. Time to first response, progression, and death for the patients with confirmed responses are shown in Supplementary Figure 1A and B.
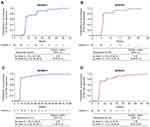
In patients with a response, median TTR by IRC was 8 weeks (95% CI: 8.00–8.14) in NP28673 (n=62; Figure 1A) and 6 weeks (95% CI: 5.86–6.14) in NP28761 (n=35; Figure 1B). Most patients achieved a response by the first assessment; 72.6% (n=45/62) in NP28673 (Week 8), and 82.9% (n=29/35) in NP28761 (Week 6). Median TTR by investigator assessment was consistent with the IRC assessment in each study (Figure 1C and D). By the second assessment (Week 16 for NP28673 and Week 12 for NP28671), 90.3% (n=56/62) of responders had achieved their response in NP28673 (Figure 1A) and 91.4% (n=32/35) in NP28671 (Figure 1B).
In responders with measurable CNS disease at baseline, median TTCR by IRC was 8 weeks (95% CI: 7.86–10.29) in NP28673 (n=20; Figure 2A) and 6 weeks (5.71–not estimable [NE]) in NP28761 (n=12; Figure 2B). Overall, 75% of patients with measurable CNS disease at baseline in both NP28673 (n=15/20 patients) and NP28761 (n=9/12 patients) achieved a CNS response by the first assessment (Week 8 and Week 6, respectively). Similar results were observed in patients with measurable and/or non-measurable CNS disease at baseline (Figure 2C and D).
In responders with measurable CNS disease at baseline and no prior radiotherapy treatment, median TTCR by IRC was 7.86 weeks (95% CI: 7.86–NE) in NP28673 (n=6; Supplementary Figure 2A) and 5.71 weeks (95% CI: 5.71–NE) in NP28761 (n=5; Supplementary Figure 2B). Most CNS responses were achieved by the first assessment (83.3% [n=5/6 patients] by Week 8 in NP28763 and 80.0% [n=4/5 patients] by Week 6 in NP28761). Similar results were observed in patients with measurable and/or non-measurable CNS disease at baseline who had not received prior radiotherapy (Supplementary Figure 2C and D).
Discussion
In pooled analyses from NP28673 and NP28761, alectinib showed activity against systemic and CNS disease in patients previously treated with crizotinib.3,4 Alectinib demonstrates effective CNS penetration and is not a substrate for P-glycoprotein, which promotes efflux at the blood–brain barrier.8 Responses in the alectinib Phase II studies were durable, lasting for longer than 1 year.3 In the exploratory analyses presented here, we investigated how quickly patients can achieve benefit from alectinib. Systemic and CNS responses were rapid (median 6–8 weeks), and most patients achieved a response by the time they underwent their first scan. This trend was consistent when patients were analyzed by measurable and/or non-measurable baseline CNS disease or measurable baseline disease only, indicating that the onset of clinical activity is not impacted by the lesion being measurable or non-measurable. It is important to note that TTR also included patients with CNS disease for whom quick response in the CNS contributed to the overall rapid systemic response.
The previously published pooled analysis from NP28673 and NP28761 showed that alectinib is also effective in patients with CNS metastases at baseline who had not received prior radiotherapy.4 Our exploratory analyses identified a rapid TTCR in patients with baseline CNS metastases who had not received prior radiotherapy, indicating that a lack of prior radiotherapy does not impact how rapidly these patients respond to alectinib. TTCR is critical in determining the appropriate therapy, especially for symptomatic CNS disease where radiation is considered a standard of care and is associated with rapid symptomatic improvement. While our analysis is limited by the timing of radiographic assessments, future studies incorporating both clinical symptom assessment and imaging at earlier time points may be helpful to determine whether alectinib TTR can be confirmed within an earlier timeframe, which would aid the management of symptomatic CNS metastases. The documented penetration and activity of alectinib in the CNS suggest that it may be possible to substitute alectinib for radiation in some circumstances.
These exploratory analyses have some limitations, which should be considered when interpreting the data. Patient numbers for some of the subgroup analyses are small, and the data should therefore be interpreted with caution. In addition, pseudoprogression is a well-defined phenomenon that can occur within 3 months of radiotherapy completion due to radiation necrosis. However, it is not possible to account for pseudoprogression in NSCLC metastases in the CNS, as the current RECIST criteria lack an outline for determining pseudoprogression in non-primary CNS solid tumors. In the NP28673 and NP28761 studies, 20% and 53% of patients, respectively, were enrolled less than 6 months after completing radiotherapy,4,6,7 so it is possible that some patients identified as having disease progression may actually have had pseudoprogression.
Three Phase III studies have demonstrated superiority of alectinib compared with crizotinib in patients with either treatment-naïve or ALK inhibitor-naïve ALK+ NSCLC (treatment-naïve in ALEX [NCT02075840] and ALESIA [NCT02838420]; ALK inhibitor-naïve in J-ALEX [JapicCTI-132316]).9–11 All three studies included patients with untreated baseline CNS metastases, and all demonstrated prolonged PFS with alectinib versus crizotinib (median PFS ALEX, 34.8 months vs 10.9 months;9 ALESIA, NE months vs 11.1 months;10 J-ALEX, 34.1 months vs 10.2 months).11
Alectinib also consistently demonstrated superior efficacy in the CNS versus crizotinib across all three first-line studies. In ALEX, the hazard ratio (HR) for time to CNS progression, without prior non-CNS progression, was significantly longer with alectinib versus crizotinib; HR 0.18 (95% CI: 0.09–0.36) in patients with baseline CNS metastases, and 0.14 (95% CI: 0.06–0.33) in patients without baseline CNS metastases.12 In ALESIA, alectinib significantly decreased the risk of CNS progression without prior non-CNS progression compared with crizotinib (cause-specific HR 0.14; 95% CI 0.06–0.30).10
In J-ALEX, alectinib demonstrated superiority to crizotinib in preventing the onset of CNS metastases (HR 0.19, 95% CI 0.07–0.53) and in patients with brain metastases at baseline, prevented CNS progression compared with crizotinib (HR 0.51, 95% CI: 0.16–1.64).13
Median PFS in patients with CNS metastases at baseline was superior for alectinib versus crizotinib in all three trials (ALEX, 27.7 months [95% CI: 9.2–NE] for alectinib versus 7.4 months [95% CI: 6.6–9.6] for crizotinib [HR 0.35; 95% CI: 0.22–0.56];9 ALESIA, NE months for alectinib versus 9.2 months for crizotinib [HR 0.11; 95% CI: 0.05–0.28];10 J-ALEX, 25.9 months [95% CI: 17.5–NE] for alectinib versus 10.3 months [95% CI: 6.5–14.2] for crizotinib [HR 0.47; 95% CI: 0.19–1.18]).13 In patients without baseline CNS metastases, median PFS for alectinib was also superior for alectinib in all three studies (ALEX, 34.8 months [95% CI: 22.4–NE] for alectinib versus 14.7 months [95% CI: 10.8–20.3] for crizotinib [HR 0.47; 95% CI: 0.32–0.71];9 ALESIA, 20.3 months for alectinib versus 12.7 months for crizotinib [HR 0.34; 95% CI: 0.18–0.65];10 J-ALEX, NE months [95% CI: 20.3–NE] for alectinib versus 10.2 months [95% CI: 8.3–12.1] for crizotinib [HR 0.36; 95% CI: 0.23–0.56]).13
These data suggest that many patients could be spared the toxicity of radiation by using a targeted therapy, such as alectinib, that is effective both systemically and in the CNS.
In summary, the data reported here demonstrate that alectinib can achieve a rapid response in both untreated and previously treated patients with ALK+ NSCLC, both systemically and in the CNS. Further investigation into the early clinical benefit (<6 weeks) is warranted to evaluate alectinib for the initial treatment of CNS metastases and the potential for sparing radiation therapy.
Data Sharing
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here.
Acknowledgments
The authors would like to acknowledge Dr Leena Gandhi for her contributions to this analysis. The authors thank the patients, their families, and the participating study centers. Third-party medical writing assistance, under the direction of the authors, was provided by Emma Evans, PhD, of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd. This work was supported by F. Hoffmann-La Roche Ltd.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SG has received consultancy fees from Araid, Genentech/Roche, and AstraZeneca and personal fees from Genentech/Roche, Takeda, AstraZeneca, Xcovery, and Boehringer-Ingelheim, during the conduct of the study. ATS has received fees for consulting and advisory boards from Pfizer, Novartis, Chugai, Genentech/Roche, Ariad, Daiichi-Sankyo, and Blueprint Medicines; consultancy fees from Ignyta, Taiho, and Foundation Medicine; and advisory board fees from Loxo, EMD Serono, and Natera. FB has received consulting fees and honorarium from F. Hoffmann-La Roche Ltd.; and consultancy fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Clovis Oncology, Eli Lilly Oncology, Novartis, Merck, MSD, Pierre Fabre, Takeda, and Pfizer. JCHY has received fees for advisory board/speech from Boehringer Ingelheim, AstraZeneca, Roche/Genentech, Chugai, BMS, Ono Pharmaceuticals, and Pfizer; and advisory board fees from Bayer, Eli Lilly, MSD, Merck Serono, Novartis, Celgene, Merrimack, Yuhan Pharmaceuticals, Hansoh Pharmaceuticals, Takeda Pharmaceuticals, Blueprint Medicines, G1 Therapeutics, and Daiichi Sankyo. AMD has received consulting fees and honorarium from Roche; and consulting fees from BMS, Eli Lilly, AstraZeneca, Clovis, MSD, Takeda, and Boehringer Ingelheim. DWK has received non-financial support from F. Hoffmann-La Roche Ltd. for travel to meetings for the study or other purposes, and provision of writing assistance, medicines, equipment, or administrative support; and non-financial support from Novartis Oncology for travel to advisory meetings. FDM has received personal fees from AstraZeneca, MSD, Bristol-Myers Squibb, and Roche. MS is an employee of Genentech and holds Roche shares and Settled Stock Appreciation Rights. SL was an employee of Genentech during the study conduct. RG is an employee of Genentech. VS is an employee of F. Hoffmann-La Roche Ltd. SHIO has received personal fees from Pfizer, Roche, AstraZeneca, Merck, and Takeda/ARIAD, Foundation Medicine Inc., owns stock from and a member of the Scientific Advisory Board of Turning Point Therapeutics Inc., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi:10.1158/2159-8290.CD-16-0596
2. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi:10.1200/JCO.2010.34.1313
3. Yang JC, Ou SI, De Petris L, et al. Pooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancer. J Thorac Oncol. 2017;12:1552–1560. doi:10.1016/j.jtho.2017.06.070
4. Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–4085. doi:10.1200/JCO.2016.68.4639
5. Ou SH, Gandhi L, Shaw AT, et al. Updated pooled analysis of CNS endpoints in two phase II studies of alectinib in ALK+ NSCLC. J Thorac Oncol. 2017;12(Suppl.1):
6. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–668. doi:10.1200/JCO.2015.63.9443
7. Camidge DR, Gadgeel S, Ou SH, et al. Updated efficacy and safety data from the phase 2 NP28761 study of alectinib in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2017;12(Suppl.1):
8. Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–1028. doi:10.1007/s00280-014-2578-6
9. Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global Phase III ALEX study. J Thorac Oncol. 2019;14(7):1233–1243. [Epub ahead of print].
10. Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437–446. doi:10.1016/S2213-2600(19)30053-0
11. Seto T, Nishio M, Hida T, et al. Final PFS analysis and safety data from the phase III J-ALEX study of alectinib (ALC) vs. crizotinib (CRZ) in ALK-inhibitor naïve ALK-positive non-small cell lung cancer (ALK+ NSCLC). J Clin Oncol. 2019;37(15_suppl):9092–9092S.
12. Gadgeel S, Peter S, Mok T, et al. Alectinib vs crizotinib in treatment-naïve ALK+ NSCLC: CNS efficacy results from the ALEX study. Ann Oncol. 2017;28(suppl_5):v605–v649. doi:10.1093/annonc/mdx440.057
13. Takiguchi Y, Hida T, Nokihara H, et al. Updated efficacy and safety of the J-ALEX study comparing alectinib (ALC) with crizotinib (CRZ) in ALK-inhibitor naïve ALK fusion positive non-small cell lung cancer (ALK+ NSCLC). J Clin Oncol. 2017;35(Suppl.15):Abs9064. doi:10.1200/JCO.2017.35.15_suppl.9064
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.