Back to Journals » Journal of Pain Research » Volume 16
Thermal Grill Illusion in Post-Stroke Patients: Analysis of Clinical Features and Lesion Areas
Authors Matsuda S , Igawa Y, Uchisawa H, Iki S, Osumi M
Received 3 August 2023
Accepted for publication 8 November 2023
Published 14 November 2023 Volume 2023:16 Pages 3895—3904
DOI https://doi.org/10.2147/JPR.S433309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Soichiro Matsuda,1 Yuki Igawa,1,2 Hidekazu Uchisawa,1,2 Shinya Iki,3 Michihiro Osumi1,4
1Graduate School of Health Sciences, Kio University, Nara, Japan; 2Department of Rehabilitation, Nishiyamato Rehabilitation Hospital, Nara, Japan; 3Department of Rehabilitation, Kawaguchi Neurosurgery Rehabilitation Clinic, Osaka, Japan; 4Neurorehabilitation Research Center, Kio University, Nara, Japan
Correspondence: Soichiro Matsuda, Graduate School of Health Sciences, Kio University, 4-2-2 Umaminaka, Kitakatsuragigun, Nara, 635-0832, Japan, Tel +745-54-1601, Fax +745-54-1600, Email [email protected]
Purpose: In the thermal grill illusion, participants experience a feeling similar to burning pain. The illusion is induced by simultaneously touching warm and cool stimuli in alternating positions. In post-stroke pain, central sensitization is caused by a variety of factors, including damage to the spinothalamic tract and shoulder pain. Because the thermal grill illusion depends on central mechanisms, it has recently been suggested that it may be a useful indicator of central sensitization. Therefore, we hypothesized that post-stroke patients who are more likely to experience central sensitization may also be more likely to experience a thermal grill sensation of pain and discomfort than the likelihood among those who are less likely to experience central sensitization. However, the effects of the thermal grill illusion in post-stroke patients have not yet been reported. In this pilot study, we conducted the thermal grill illusion procedure in post-stroke patients and analyzed the relationship between clinical somatosensory functions and thermal grill sensations. We also conducted brain imaging analysis to identify brain lesion areas that were associated with thermal grill sensations.
Patients and Methods: Twenty patients (65.7 ± 11.9 years old) with post-stroke patients participated in this study. The thermal grill illusion procedure was performed as follows: patients simultaneously touched eight water-filled copper bars, with the water temperature adjusted to provide alternate warm (40°C) and cold (20°C) stimuli.
Results: Thermal grill sensation of pain and discomfort tended to be associated with the wind-up phenomenon in bedside quantitative sensory testing and thermal grill sensation of discomfort was also related to damage to the thalamic lateral nucleus.
Conclusion: These findings suggest that the thermal grill illusion might measure central sensitization, and that secondary brain hyperactivity might lead to increased thermal grill sensations.
Keywords: thermal grill illusion, post stroke pain, central sensitization, thalamus, insula
Introduction
The thermal grill illusion was first reported over 120 years ago.1 When performing this illusion, participants experience a feeling similar to burning pain. Because the thermal grill illusion depends on central mechanisms, it has recently been suggested as a useful indicator of central sensitization.2 A previous study investigated thermal grill illusion pain in patients with fibromyalgia2 and reported that individuals with high pain levels and cold hyperalgesia are more likely to experience thermal grill illusion pain. The thermal grill illusion has also been suggested to be useful for assessing the pathological mechanisms of central post-stroke pain.3 Post-stroke pain includes musculoskeletal pain, shoulder pain, and headache with a reported prevalence of 11 to 55%.4,5 Central post-stroke pain is present in 8–30% of post-stroke patients,4,6–9 and is associated with the thalamus, anterior limb of the internal capsule, internal thalamus, anterior internal parietal lobe, corona radiata (posterior), dorsolateral prefrontal cortex, and inferior parietal lobe.10–13 Symptoms of central post-stroke pain are reported to include allodynia and hyperalgesia in addition to pain and somatosensory disturbances corresponding to the region of injury.6 Allodynia refers to pain from a stimulus that does not normally cause pain, whereas hyperalgesia denotes increased pain from a stimulus that normally causes pain.14 Central disinhibition has been proposed as a pathological mechanism of central post-stroke pain;3 it refers to pain caused by an imbalance between the medial and lateral pain pathways involved in processing pain information.3,15,16 It is hypothesized that this imbalance is caused by injury to these pain pathways, resulting in the appearance of neuropathic pain caused by disinhibitory pain in the brain. For example, patients with central post-stroke pain may show allodynia to cold stimuli despite the development of cold hypoalgesia or pain hypoalgesia.13,17 In addition, Craig et al used the thermal grill illusion to quantitatively measure pain caused by this disinhibition.15 In summary, central sensitization is caused by a variety of factors, including damage to the spinothalamic tract12 and shoulder pain.5 We therefore hypothesized that post-stroke patients may be more likely to feel pain caused by the thermal grill illusion because of the central sensitization that occurs after stroke.
The mechanisms of neuropathic pain are diverse, even within a single disease pathology. Moreover, sensory abnormalities in neuropathic pain reflect changes in each pathological mechanism, such as peripheral sensitization or central sensitization. For example, the pressure pain threshold is reportedly disturbed in patients with peripheral sensitization.18 Therefore, in addition to the diagnosis of neuropathic pain itself, assessments of somatosensory function using quantitative sensory testing (QST) are also needed in clinical practice.19–21 QST using nociceptive stimulation are considered useful for classifying central post-stroke pain.17,21–23 However, QST methods using nociceptive stimulation represent a significant burden for patients in clinical practice. The thermal grill illusion is expected to be useful as a test of central sensitization produced by non-nociceptive stimuli. If an association exists between the thermal grill illusion and central sensitization, it can therefore be used clinically as a safe assessment method.
However, the effects of the thermal grill illusion have not yet been reported in post-stroke patients. Furthermore, only a few studies have investigated the response to the thermal grill illusion in chronic pain patients, either in case reports24–26 or in different diseases.27–29 In the present pilot study, we conducted the thermal grill illusion in post-stroke patients and analyzed the relationship between clinical somatosensory functions and thermal grill illusion pain. In addition, we conducted brain imaging analysis to identify areas of brain lesions that were associated with the thermal grill illusion.
Materials and Methods
Participants
Twenty post-stroke patients (66.0 ± 11.1 years) were included. Exclusion criteria were dementia (Mini Mental State Examination ≤ 23) or the presence of psychiatric, orthopedic, or central nervous system disorders before the stroke. This study was conducted in accordance with the Declaration of Helsinki, with the research plan submitted and approved by the Research Ethics Committee of Kio University (approval number: R3-8). Patients were fully informed about the study and their consent was obtained; however, the purpose of the study was not explained to the patients, and they were blinded to the experiment. Patients were recruited at Kawaguchi Neurosurgical Rehabilitation Clinic and Nishiyamato Rehabilitation Hospital. All procedures were conducted in a sitting position for about 90 minutes. Missing data included Fugl-Meyer Assessment (FMA) results in one patient. Brain imaging was excluded for one patient whose brain lesions were not drawn.
Clinical Assessments
Patients were asked to respond to questions regarding a range of motion limitations and pain during joint movements for the motor assessment. In addition, motor function was assessed using the motor components of the FMA. Although the FMA is used to measure the motor function of upper and lower limbs in stroke patients, in the present study we only used the following upper limb items: coordination and speed of the upper limb, shoulder/elbow/forearm joint function, wrist joint function, and finger function. Upper limb function was measured using the total score (0–66 points); higher scores indicate better upper limb function.
Pain intensity was assessed using average values: 0 (no pain) to 10 (maximum imaginable pain). In addition, pain characteristics were assessed using the short-form McGill Pain Questionnaire-2 (SF-MPQ-2)30 to analyze 18 sensory factor items (four items were excluded: exhausting, sickening, fearful, and cruel). Neuropathic pain was assessed using the Pain DETECT31 and the Neuropathic Pain Symptom Inventory (NPSI),32 catastrophic thoughts were evaluated using the short-form Pain Catastrophizing Scale (PCS-6),33 and kinesiophobia was assessed using the short-form Tampa Scale of Kinesiophobia (TSK-11).34
Somatosensory function was assessed using bedside-QST.35 The testing accuracy of bedside-QST is slightly less than that of conventional QST, but it can be used to identify sensory phenotypes.35 In post-stroke pain patients, QST was evaluated at painful sites (eg, shoulders and palms). Previous study suggests that the dorsal hand should be used for QST.36 Therefore, QST was performed on the bilateral dorsal hands in the post-stroke patients. The bedside-QST included: warm detection threshold (WDT), heat pain threshold (HPT), cold detection threshold (CDT), cold pain threshold (CPT), mechanical pain sensitivity (MPS), pressure pain threshold (PPT), dynamic mechanical allodynia (DMA), and wind-up ratio (WUR). The tests were performed according to a previous study;35 however, in contrast to the previous study, Peltier was used for the thermal sensation test. Patients were asked to score the pain of each item after stimulation on an 11-point Likert scale from 0 (no pain) to 10 (maximum imaginable pain). The tests were performed using a Peltier temperature control set (VPE 20–30, VICS, Tokyo, Japan); cotton swabs, cotton balls, and brushes (Somedic, Sösdala, Sweden); a Chicago Medical Supply (CMS) 0.7 mm Rotating Weinstein Foot Filament Wheel filament (Chicago Medical Supply, Chicago, IL, USA); and a 10 mL syringe.
Specific procedures are described herein (see details in35). Patients were asked to make contact with a Peltier temperature controller for 3 seconds; the controller was set at 37°C for WDT, 45°C for HPT, 22°C for CDT, and 8°C for CPT. MPS was measured by stimulating the patient with a CMS 0.7 mm filament and asking the patient to score their pain on an 11-point Likert scale. In the PPT, patients scored pain intensity while pushing a syringe filled with 10 mL of air down to the 4 mL mark. DMA was assessed by scoring the intensity of pain when a brush, cotton swab, or cotton wool was moved back and forth across the pain site twice in a cross pattern. The average pain score was calculated from the items of brush, cotton swab, and cotton wool for the analysis. The WUR was evaluated by repeating the stimulation 10 times at a frequency of once per second with a CMS 0.7 mm filament and having the subjects respond to the intensity of pain at the first and tenth repetitions. The WUR was then calculated by subtracting the pain intensity of the first stimulus from the pain intensity of the tenth stimulus. All items were answered on an 11-point Likert scale and standardized for analysis.
Thermal Grill Illusion
The thermal grill illusion procedure was performed in a room with a temperature of 24.8°C ± 0.8°C. Water flowed through a 1 cm diameter copper bar and a plastic tube, and water temperatures were adjusted to provide warm (40 °C) and cold (20°C) stimuli to the patient’s contact surface. The temperature settings for the warm and cold stimuli were adjusted within ±1.0°C. Four warm and four cool copper bars (20 cm long, 0.8 cm diameter, 0.1 cm thick) were arranged alternately and were touched simultaneously by the patients to create the thermal grill illusion. Each copper bar was separated from its neighbor by 5 mm. Four copper bars were connected to a circulating thermostatic chamber (LBX-300, AS ONE, Osaka, Japan) via plastic tubing for warming, while the other four copper bars were connected to another thermostatic chamber for cooling. The surface temperature of each copper bar was monitored during the experiment using a thermometer probe (Unique Medical Co., Tokyo, Japan) attached to each bar (Figure 1).
To test the response to the thermal grill illusion, patients placed their palm on the copper bars for up to 30 seconds in the following order: unaffected side, affected side. If the pain was severe, the painful area was removed from the copper bar at that point and a questionnaire was administered to assess the patient’s experience of the thermal grill illusion. Responses for pain and discomfort experienced during the thermal grill illusion ranged from 0 (no pain) to 10 (maximum imaginable pain) and 0 (no discomfort) to 10 (maximum imaginable discomfort), respectively.28
Statistical Analysis
Thermal grill sensations (pain and discomfort) on the unaffected and affected sides were analyzed using the Bayesian Wilcoxon signed-rank test with four chains and 4000 iterations. Pain intensity and thermal grill sensations (pain and discomfort) were analyzed by Bayesian correlation. The results were confirmed by the Bayes factor, which provides evidence in support of the alternative hypothesis. A Bayes factor of 1–3 was interpreted as providing weak evidence, 3–10 as moderate evidence, 10–30 as strong evidence, 30–100 as very strong evidence, and >100 as extremely strong evidence.37,38 The thermal grill sensation index was used in the analysis of generalized linear model analysis and voxel-based lesion–symptom mapping by calculating thermal grill sensation of pain and discomfort by subtracting the score of the unaffected side from the score of the affected side.
A generalized linear model (GLM) analysis was performed using the thermal grill sensation score as the response variable and the bedside-QST as the explanatory variable. We created four models. First model: the thermal grill sensation of pain as the response variable; bedside-QST subgroups of WDT, HPT, CDT, and CPT as the explanatory variables. Second model: the thermal grill sensation of pain as the response variable; bedside-QST subgroups of MPS, PPT, DMA, and WUR as the explanatory variables. Third model: the thermal grill sensation of discomfort as the response variable; bedside-QST subgroups of WDT, HPT, CDT, and CPT as the explanatory variables. Fourth model: the thermal grill sensation of discomfort as the response variable; bedside-QST subgroups of MPS, PPT, DMA, and WUR as the explanatory variables.
Given the small sample size of this study, the GLM analysis was performed using the Bayesian method, which is capable of reasonable estimation even with a small sample size. The Bayesian approach was used to investigate the Markov chain Monte Carlo method fitting of the GLM, which is a method of generating samples with distributional characteristics consistent with the posterior distribution by means of a Markov chain using the Bayesian method and calculating estimates of the target variable using these samples. The prior distribution was assumed to be normal; the total number of iterations was 16,000, the burn-in sample was 12,000, and the number of chains was four. The Rhat value was used to confirm the modeling hypotheses; a Rhat of less than 1.1 for all parameters indicates that the model is well estimated.39 All confidence interval (CI) is presented as 95% CI. All values are given as the mean ± standard deviation unless otherwise indicated. JASP 16.3,40 R 4.2.0 was used for the statistical analysis.
Analysis of Lesion Areas
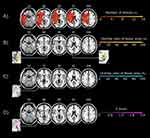
The analysis of lesion areas was performed using magnetic resonance imaging (MRI) scans from the 20 patients. To standardize the MRI scans, we first manually indicated the extent of ischemic lesions on anonymized MRI scans using MRIcron.41 Next, the MRI scans were further standardized using the MR normalization algorithm42 from the Clinical Toolbox in SPM8 of MATLAB (MathWorks, Natick, MA, USA). The participant’s MRI images were selected using anatomical scans, and MRI scans with lesion maps and pathological scans indicating ischemic lesions were selected. Modality was set to match the MRI scan; T1 or T2 MRI scans were selected. The brain images and lesion areas were converted to T1 templates based on data from older adults. A template mask was not set, the bounding box was 2×3 (−90, −126, −72; 90, 90, 108), the voxel size was 1 × 1×1 mm, and intermediate images were set to False.42–44 Brain images were analyzed to identify the lesion regions related to the experiences with the thermal grill illusion. MATLAB R2020a was used for the analysis. Those with thermal grill illusion pain and discomfort scores higher than the median were classified as having thermal grill sensation; all others were classified as having non-thermal grill sensation. Subtraction mapping was performed by subtracting the scores at the affected side of the non-thermal grill sensation group from the scores at the affected side of the thermal grill sensation group. Patients with values higher than the median were compared with patients with values below the median.45 Pain was reported by six patients with values above the median and 13 patients below the median. Discomfort was reported by seven patients with values above the median and 12 patients below the median. Subtraction mapping results are shown as the percentage overlap of the lesion area for those who reported values above the median (ie, thermal grill sensations of pain and discomfort).
As a secondary approach, we performed voxel-based lesion–symptom mapping analysis using non-parametric mapping41 to investigate the relationship between thermal grill sensations (pain and discomfort) and lesion areas. Voxel-based lesion–symptom mapping analysis was performed to identify the lesion areas that were significantly associated with thermal grill sensation. Voxels damaged in at least 1% of patients were included. The Brunner Munzel test was used for statistical analysis. Family-wise errors were adjusted for significance level by performing a permutation test (4000 permutations).
Results
Clinical Assessments
Results from the clinical assessments of motor function and pain are given in Table 1. Pain was reported from 11 patients, their pain intensity was 2.8 ± 2.8 (Mean ± SD). Three patients had pain spreading from the forearm to the hand, two patients had pain spreading from the shoulder to the forearm, and six patients had pain in the upper limb. Bedside QST scores are given in Table 2.
 |
Table 1 Participants Characteristics |
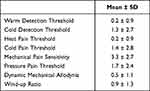 |
Table 2 Bedside QST Scores |
The intensity of the thermal grill sensation of pain was 1.1 ± 2.3 for the affected side, 0.8 ± 1.8 for the unaffected side. The intensity of the thermal grill sensation of discomfort was 2.6 ± 2.8 for the affected side, 2.4 ± 2.1 for the unaffected side. No thermal grill sensation of pain or discomfort was evident between the unaffected and affected sides. Moreover, the pain intensity and patient characteristics did not significantly correlate with the thermal grill sensation.
Generalized Linear Model Analysis
GLM analysis was performed to confirm the relationship between thermal grill sensations (pain and discomfort) and bedside-QST. There was no association between the thermal grill sensation (pain and discomfort) and the temperature stimulus because the model was not validated. There was a positive association between thermal grill sensations of pain and WUR. Thermal grill sensation of discomfort and WUR also tended to be positively correlated, but negatively associated with DMA in Figure 2.
Analysis of Lesion Areas
The lesion areas in the 20 post-stroke patients are indicated in Figure 3A. Post-stroke patients with more damage to the insular cortex and parietal lobes were more likely to feel pain during the thermal grill illusion in Figure 3B. Post-stroke patients with more damage to the thalamic lateral nucleus and parietal lobes were more likely to feel discomfort during the thermal grill illusion in Figure 3C. In the voxel-based lesion–symptom mapping analysis, which was conducted to assess the relationship between thermal grill sensations and lesion areas, no voxels were significantly correlated with thermal grill sensations of pain. However, thermal grill sensations of discomfort were significantly associated with voxels in lesion areas around the crus posterius of the internal capsule and the lateral nucleus of the thalamus in Figure 3D. It indicates that subjects more likely to experience discomfort had damaged lesions around the crus posterius of the internal capsule and the lateral nucleus of the thalamus. The results are shown at a false discovery rate-corrected threshold of p < 0.05, z > 1.92.
Discussion
Thermal grill sensations of pain and discomfort were tended to be associated with the wind-up phenomenon. In addition, thermal grill sensations of pain and discomfort were not associated with thermal sensory stimulation in the bedside-QST. Voxel-based lesion–symptom mapping analysis revealed that patients with lesions of the thalamus were more likely to experience thermal grill sensations of discomfort.
Pain evoked by thermal stimulation in the bedside-QST was not associated with thermal grill sensations of pain and discomfort; this finding suggests that the mechanisms of simple heat/cold pain might be different from the mechanism underlying the thermal grill illusion. The results are therefore in line with a previous proposal3 that the thermal grill illusion should be included in the QST as a new clinical assessment.
Post-stroke patients who experienced the thermal grill illusion on the affected side more than on the unaffected side were also more likely to experience the wind-up phenomenon, in which pain is amplified by temporal summation when it is repeatedly stimulated at short intervals.46–50 This phenomenon has been suggested to be enhanced by central sensitization, and the QST quantifies the phenomenon as the WUR.51 An enhancement of the wind-up phenomenon is relatively common in chronic pain patients52–57 such as those with fibromyalgia58 and chronic musculoskeletal pain.59,60 Together, these findings suggest that central sensitization may be evaluated in post-stroke patients by characterizing thermal grill sensations of pain and discomfort. A previous study also supports this concept, reporting that central sensitization in fibromyalgia can be quantified using the thermal grill illusion.2 Because the thermal grill illusion is not a nociceptive stimulus, it is safer and easier to use in clinical practice than the currently used procedure of the wind-up phenomenon. Post-stroke pain includes central post-stroke pain, headache, and pain caused by musculoskeletal pain and spasticity.4,9 In the current study, nearly half of all patients experienced pain during joint movement; thus, many patients had musculoskeletal pain. Therefore, it is possible that in the present cohort, problems with the somatosensory pathways were not the cause of the pain, and there was no relationship between pain intensity and the thermal grill sensation intensity, and the low association with allodynia. Future studies are needed to further identify pain types in post-stroke patients, and to investigate which post-stroke patients are most likely to experience thermal grill sensations of pain.
This study was the first report of the relationship between the thermal grill illusion and the brain lesion area. Our brain imaging analysis findings suggest that the thalamus is associated with thermal grill sensations of discomfort. In the present study, these lesion areas were similar to the brain areas that have been previously reported as activated by the thermal grill illusion.61 Previous studies have reported that the thalamus is responsible for processing and integrating thermal and pain information.3,16,62,63 Together, these findings indicate that experiences of the thermal grill illusion are likely to be enhanced by problems in pain or thermal perception processing. Post-stroke patients with lesions of the thalamus are therefore relatively prone to perceiving discomfort in the thermal grill illusion because their somatosensory functions of pain or thermal sensation are deficient. The results of this study may help identify the mechanism underlying the thermal grill illusion and its relevance to central sensitization in post-stroke patients. Although the present study did not observe brain activity using functional MRI, secondary brain hyperactivity might also lead to enhancement of the thermal grill illusion. A previous study in monkeys with central post-stroke pain revealed that experimental damage to the ventral posterolateral nucleus of the thalamus results in the hyperactivity of brain areas such as the posterior insula and secondary somatosensory cortex, which secondarily causes central pain.64,65 These results suggest that primary thalamic damage causes hyperactivity of the pain matrix (ie, central sensitization), thus causing the clinical symptoms of central pain. However, the process of how the pain matrix becomes overactive and affects the thermal grill illusion needs clarifying in future studies. Moreover, further investigation into the relationship between the effects of the thermal grill illusion and lesion areas in post-stroke patients may aid differential diagnoses in post-stroke patients. More data from post-stroke patients are required to clarify our findings beyond those of the present pilot study because of its limited sample size.
Conclusion
It was suggested that thermal grill sensation of pain and discomfort may be useful as an evaluation of central sensitization using non-nociceptive stimulation in post-stroke patients.
Acknowledgments
We thank Bronwen Gardner, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. There was no research support. However, the research is funded by Kio University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alrutz S. On the temperature-senses. Mind. 1897;VI(3):445–448. doi:10.1093/mind/vi.3.445
2. Adam F, Jouët P, Sabaté JM, et al. Thermal grill illusion of pain in patients with chronic pain: a clinical marker of central sensitization? Pain. 2022;164(3):638–644. doi:10.1097/j.pain.0000000000002749
3. Forstenpointner J, Berry D, Baron R, Borsook D. The cornucopia of central disinhibition pain–an evaluation of past and novel concepts. Neurobiol Dis. 2020;145:105041. doi:10.1016/j.nbd.2020.105041
4. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8(9):857–868. doi:10.1016/S1474-4422(09)70176-0
5. Roosink M, Renzenbrink GJ, Buitenweg JR, van Dongen RTM, Geurts ACH, Ijzerman MJ. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. J Pain. 2011;12(4):476–485. doi:10.1016/j.jpain.2010.10.009
6. Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995;61(2):187–193. doi:10.1016/0304-3959(94)00144-4
7. Widar M, Samuelsson L, Karlsson-Tivenius S, Ahlström G. Long-term pain conditions after a stroke. J Rehabil Med. 2002;34(4):165–170. doi:10.1080/16501970213237
8. Kong KH, Woon VC, Yang SY. Prevalence of chronic pain and its impact on health-related quality of life in stroke survivors. Arch Phys Med Rehabil. 2004;85(1):35–40. doi:10.1016/s0003-9993(03)00369-1
9. Klit H, Finnerup NB, Andersen G, Jensen TS. Central poststroke pain: a population-based study. Pain. 2011;152(4):818–824. doi:10.1016/j.pain.2010.12.030
10. Bassetti C, Bogousslavsky J, Regli F. Sensory syndromes in parietal stroke. Neurology. 1993;43(10):1942–1949. doi:10.1212/wnl.43.10.1942
11. Garcia-Larrea L, Perchet C, Creac’H C, et al. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain. 2010;133(9):2528–2539. doi:10.1093/brain/awq220
12. Elias GJB, De Vloo P, Germann J, et al. Mapping the network underpinnings of central poststroke pain and analgesic neuromodulation. Pain. 2020;161(12):2805–2819. doi:10.1097/j.pain.0000000000001998
13. Delboni Lemos M, Faillenot I, Tavares Lucato L, et al. Dissecting neuropathic from poststroke pain: the white matter within. Pain. 2022;163(4):765–778. doi:10.1097/j.pain.0000000000002427
14. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi:10.1016/S1474-4422(14)70102-4
15. Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265(5169):252–255. doi:10.1126/science.8023144
16. Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384(6606):258–260. doi:10.1038/384258a0
17. Boivie J, Leijon G, Johansson I. Central post-stroke pain--a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989;37(2):173–185. doi:10.1016/0304-3959(89)90128-0
18. Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi:10.1016/j.pain.2010.04.003
19. Bowsher D. Allodynia in relation to lesion site in central post-stroke pain. J Pain. 2005;6(11):736–740. doi:10.1016/j.jpain.2005.06.009
20. Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138(2):343–353. doi:10.1016/j.pain.2008.01.006
21. Barbosa LM, da Silva VA, de Lima Rodrigues AL, et al. Dissecting central post-stroke pain: a controlled symptom-psychophysical characterization. Brain Commun. 2022;4(3):fcac090. doi:10.1093/braincomms/fcac090
22. de Oliveira RAA, de Andrade DC, Machado AGG, Teixeira MJ. Central poststroke pain: somatosensory abnormalities and the presence of associated myofascial pain syndrome. BMC Neurol. 2012;12(1):89. doi:10.1186/1471-2377-12-89
23. Kalita J, Kumar B, Misra UK, Pradhan PK. Central post stroke pain: clinical, MRI, and SPECT correlation. Pain Med. 2011;12(2):282–288. doi:10.1111/j.1526-4637.2010.01046.x
24. Harper DE, Hollins M. Conditioned pain modulation dampens the thermal grill illusion. Eur J Pain. 2017;21(9):1591–1601. doi:10.1002/ejp.1060
25. Heavner JE, Calvillo O, Racz G. Thermal grill illusion and complex regional pain syndrome type I (reflex sympathetic dystrophy). Reg Anesth. 1997;22(3):257–259. doi:10.1016/s1098-7339(06)80011-8
26. Morin C, Bushnell MC, Luskin MB, Craig AD. Disruption of thermal perception in a multiple sclerosis patient with central pain. Clin J Pain. 2002;18(3):191–195. doi:10.1097/00002508-200205000-00008
27. Rivel M, Achiron A, Dolev M, Stern Y, Zeilig G, Defrin R. Central neuropathic pain in multiple sclerosis is associated with impaired innocuous thermal pathways and neuronal hyperexcitability. Pain Med. 2021;22(10):2311–2323. doi:10.1093/pm/pnab103
28. Sumracki NM, Buisman-Pijlman FTA, Hutchinson MR, Gentgall M, Rolan P. Reduced response to the thermal grill illusion in chronic pain patients. Pain Med. 2014;15(4):647–660. doi:10.1111/pme.12379
29. Ventzel L, Madsen CS, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Assessment of acute oxaliplatin-induced cold allodynia: a pilot study. Acta Neurol Scand. 2015;133(2):152–155. doi:10.1111/ane.12443
30. Maruo T, Nakae A, Maeda L, et al. Validity, reliability, and assessment sensitivity of the Japanese version of the short-form McGill pain questionnaire 2 in Japanese patients with neuropathic and non-neuropathic pain. Pain Med. 2014;15(11):1930–1937. doi:10.1111/pme.12468
31. Matsubayashi Y, Takeshita K, Sumitani M, et al. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One. 2013;8(9):e68013. doi:10.1371/journal.pone.0068013
32. Matsubayashi Y, Takeshita K, Sumitani M, et al. Psychometric validation of the Japanese version of the Neuropathic Pain Symptom Inventory. PLoS One. 2015;10(11):e0143350. doi:10.1371/journal.pone.0143350
33. Nishigami T, Mibu A, Tanaka K, Yamashita Y, Watanabe A, Tanabe A. Psychometric properties of the Japanese version of short forms of the Pain Catastrophizing Scale in participants with musculoskeletal pain: a cross-sectional study. J Orthop Sci. 2017;22(2):351–356. doi:10.1016/j.jos.2016.11.015
34. Kikuchi N, Matsudaira K, Sawada T, Oka H. Psychometric properties of the Japanese version of the Tampa Scale for Kinesiophobia (TSK-J) in patients with whiplash neck injury pain and/or low back pain. J Orthop Sci. 2015;20(6):985–992. doi:10.1007/s00776-015-0751-3
35. Reimer M, Forstenpointner J, Hartmann A, et al. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Rep. 2020;5(3):e820. doi:10.1097/PR9.0000000000000820
36. Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. doi:10.1016/j.pain.2010.07.026
37. Wagenmakers EJ, Marsman M, Jamil T, et al. Bayesian inference for psychology. Part I: theoretical advantages and practical ramifications. Psychon Bull Rev. 2018;25(1):35–57. doi:10.3758/s13423-017-1343-3
38. Wagenmakers EJ, Love J, Marsman M, et al. Bayesian inference for psychology. Part II: example applications with JASP. Psychon Bull Rev. 2018;25(1):58–76. doi:10.3758/s13423-017-1323-7
39. Cowles MK, Carlin BP. Markov chain monte carlo convergence diagnostics: a comparative review. J Am Stat Assoc. 1996;91(434):883–904. doi:10.1080/01621459.1996.10476956
40. Love J, Selker R, Marsman M, et al. JASP: graphical statistical software for common statistical designs. J Stat Softw. 2019;88(2):1–17. doi:10.18637/jss.v088.i02
41. Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081–1088. doi:10.1162/jocn.2007.19.7.1081
42. Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi:10.1016/j.neuroimage.2012.03.020
43. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi:10.1016/j.neuroimage.2005.02.018
44. Winder K, Seifert F, Köhrmann M, et al. Lesion mapping of stroke-related erectile dysfunction. Brain. 2017;140(6):1706–1717. doi:10.1093/brain/awx080
45. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(Pt 4):835–843. doi:10.1093/brain/awh098
46. Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139(2):315–323. doi:10.1016/j.pain.2008.04.024
47. Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66(2–3):105–108. doi:10.1097/00006396-199608000-00001
48. Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22(3):261–269. doi:10.1016/0304-3959(85)90026-0
49. Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59(2):165–174. doi:10.1016/0304-3959(94)90069-8
50. McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993;3(4):602–610. doi:10.1016/0959-4388(93)90062-4
51. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi:10.1016/j.pain.2006.01.041
52. Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi:10.1016/j.pain.2004.12.032
53. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi:10.1016/j.neuron.2006.09.021
54. Mohr Drewes A, Pedersen J, Reddy H, et al. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol. 2006;41(6):640–649. doi:10.1080/00365520500442559
55. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
56. Nijs J, Torres-Cueco R, van Wilgen CP, et al. Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician. 2014;17(5):447–457. doi:10.36076/ppj.2014/17/447
57. Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(7):1043–1056. doi:10.1016/j.joca.2015.02.163
58. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi:10.1186/ar3306
59. Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain. 2013;29(7):625–638. doi:10.1097/AJP.0b013e31826f9a71
60. Lluch E, Nijs J, Courtney CA, et al. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disabil Rehabil. 2018;40(23):2836–2845. doi:10.1080/09638288.2017.1358770
61. Uragami S, Osumi M. Cortical oscillatory changes during thermal grill illusion. Neuroreport. 2023;34(4):205–208. doi:10.1097/WNR.0000000000001874.
62. Vartiainen N, Perchet C, Magnin M, et al. Thalamic pain: anatomical and physiological indices of prediction. Brain. 2016;139(3):708–722. doi:10.1093/brain/awv389
63. Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and Function of the Human Insula. J Clin Neurophysiol. 2017;34(4):300–306. doi:10.1097/WNP.0000000000000377
64. Nagasaka K, Takashima I, Matsuda K, Higo N. Late-onset hypersensitivity after a lesion in the ventral posterolateral nucleus of the thalamus: a macaque model of central post-stroke pain. Sci Rep. 2017;7(1):1–12. doi:10.1038/s41598-017-10679-2
65. Nagasaka K, Takashima I, Matsuda K, Higo N. Brain activity changes in a monkey model of central post-stroke pain. Exp Neurol. 2020;323:113096. doi:10.1016/j.expneurol.2019.113096
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.