Back to Journals » Cancer Management and Research » Volume 15
Therapeutic Potential of Tisotumab Vedotin in the Treatment of Recurrent or Metastatic Cervical Cancer: A Short Report on the Emerging Data
Authors Agostinelli V, Musacchio L, Camarda F, Salutari V, Carbone MV, Ghizzoni V, Nero C, Ricci C, Perri MT, Giudice E , Lardino S, Berardi R, Scambia G, Lorusso D
Received 29 April 2023
Accepted for publication 5 September 2023
Published 27 September 2023 Volume 2023:15 Pages 1063—1072
DOI https://doi.org/10.2147/CMAR.S294080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Veronica Agostinelli,1 Lucia Musacchio,2 Floriana Camarda,2 Vanda Salutari,2 Maria Vittoria Carbone,2 Viola Ghizzoni,2 Camilla Nero,2 Caterina Ricci,2 Maria Teresa Perri,2 Elena Giudice,3 Sara Lardino,3 Rossana Berardi,1 Giovanni Scambia,2,3 Domenica Lorusso2,3
1Oncologic Clinic, Università Politecnica delle Marche, Ancona, Italy; 2Department of Woman, Child and Public Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy; 3Institute of Obstetrics and Gynecology, Università Cattolica del Sacro Cuore, Rome, Italy
Correspondence: Vanda Salutari, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo Agostino Gemelli, 8, Roma, 00168, Italy, Tel +39-06-3015-3234, Email [email protected]
Abstract: Cervical cancer is the fourth most common type of cancer in women worldwide. It is associated with a high death rate, despite the fact that it is a nearly 100% preventable disease because of very effective primary and secondary preventive strategies. Advanced and recurrent disease is uncurable with a high relapse risk and the second-line therapies are limited with modest response rates and short durability. Investigating alternative mechanisms of action is crucial because of the high request for effective new therapies. Tisotumab vedotin (TV) is the first antibody-drug conjugated to target a cell surface-expressed tissue factor, and preliminary data in patients with metastatic and recurrent cervical cancer have been promising. In addition, the trials showed a favorable tolerability profile, with limited incidence of grade 3 or worse adverse events. According to the data of ENGOT-cx6/GOG-3023/innovaTV 204, the US Food and Drug Administration granted expedited approval of TV on September 20, 2021, for women with recurrent or metastatic cervical cancer. Actually, two other trials testing TV alone or in combination with other agents are ongoing. ENGOT-cx8/GOG-3024/innovaTV 205 is a Phase Ib/II trial of TV in combination with platinum or bevacizumab or pembrolizumab, in patients with recurrent or metastatic cervical cancer who have not received prior systemic therapy or who have progressed after no more than two prior systemic therapies. ENGOT-cx12/GOG-3057/InnovaTV 301 is a Phase 3 trial of TV vs investigator’s choice chemotherapy in patients with advanced or recurrent cervical cancer who had received no more than 2 prior chemotherapy lines. The outcomes of these two trials will potentially confirm and reinforce the use of TV as a new standard of care in advanced or recurrent cervical cancer.
Keywords: cervical cancer, gynecological cancer, antibody–drug conjugate, tissue factor
Introduction
Cervical cancer remains the fourth most frequent type of cancer in women worldwide1 with an estimated 14.100 new cases in 2022.
In the US cervical cancer is also the fourth leading cause of cancer death with 4.280 death in 2022.2 The most significant prognostic factor is the stage of disese3 and the 5-years overall survival (OS) is approximately 92%, 65% and 17% for early stage, locally advanced and metastatic disease respectively.4
Cervical cancer is thought to be a nearly 100% preventable disease due to very effective primary (human papillomavirus (HPV) vaccine) and secondary (PAP test and HPV test screening) preventive strategies.5
HPV is the major cause of cervical cancer,6 although other significant cofactors include sexually transmittable infections (HIV and Chlamydia trachomatis), smoking, a higher number of childbirths and sexual partners and long-term use of oral contraceptives.7
Actually, the standard of care for locally advanced cervical cancer is represented by concomitant chemoradiation,8 while platinum-based (cisplatin or carboplatin) chemotherapy plus paclitaxel and with or without bevacizumab represents the standard option treatment in patients with metastatic disease.9 Unfortunately, after the failure of the first line, no effective therapeutic strategies are available: standard chemotherapies in fact present with a poor response rate of about 10% and an estimated progression free survival of about 3–6 months.10
In recent years, there has been a considerable interest in the use of immune-checkpoint inhibitors for the treatment of advanced/metastatic cervical cancer with trials showing encouraging results.11
Based on KEYNOTE-158 US Food and Drug Administration (FDA) approved pembrolizumab in PD-L1 positive cervical cancer patients progressing after platinum-based chemotherapy.12 Similarly, the Phase III EMPOWER-Cervical-1/GOG3016/ENGOT-cx-9 trial demonstrated that anti PD-1 inhibitor cemiplimab significantly improved progression free survival (PFS) and OS in the same patients setting regardless PD-L1 expression.13
In addition, the randomized phase III KEYNOTE-826 trial, which investigated the efficacy of pembrolizumab in combination with platinum-based chemotherapy with or without bevacizumab vs standard of care in the first-line treatment of persistent, recurrent, or metastatic cervical cancer showed an improvement in OS, PFS, and objective response rate (ORR) for the experimental arm defining immunotherapy as the new standard of care for patients with combined-positive score (CPS) ≥ 1 in the treatment of advanced disease.11
Despite immunotherapy improving the outcome of metastatic/advanced disease, the absence of a standard second line treatment for women previously treated with platinum and immunotherapy represents a great unmet clinical need, suggesting that the investigation of treatments with alternative mechanisms of action is crucial for searching effective new therapies.
Tisotumab vedotin (TV) is the first antibody-drug conjugate (ADC) targeting cell surface-expressed tissue factor (TF), a protein that is abnormally expressed in several solid tumors, including cervical cancer.14 In physiological condition TF is central to the coagulation cascade, while in oncogenesis it is a major component in tumor-associated angiogenesis and it plays a role in tumor progression and metastasis.14
TV is conjugated with microtubule-disrupting agent monomethyl auristatin E (MMAE) through a protease-cleavable linker. TV targets TF-expressing cells with MMAE, causing direct cytotoxicity and indirect death of adjacent cells. According to in vitro studies, TV induces immunogenic cell death and efficiently engages immune cells to promote tumor cell death via Fcg receptor-mediated effector functions such as antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. Furthermore, TV was discovered to suppress TF-activated factor VII (FVIIa)-dependent intracellular signaling while having no effect on procoagulant activity.14
FDA granted expedited approval of TV on September 20, 2021, following the readout of pivotal trial, ENGOT-cx6/GOG-3023/innovaTV 204 results, reporting 24% ORR with long response duration in women with recurrent or metastatic cervical cancer progressing after platinum-based regimen.15
The present review reports the existing evidence and future perspectives on TV in the treatment of recurrent or advanced cervical cancer in order to clarify its potential role in the treatment of advanced cervical cancer.
Materials and Methods
The studies included in the present review were selected from PUBMED/GoogleScholar/ ScienceDirect databases. We used the terms “Cervical Cancer”, “Gynecological Cancer”, “Metastatic Cervical Cancer” and “Recurrence Cervical Cancer” for tumour location and setting of disease; the terms “Tisotumab Vedotin”, “Second-line Chemotherapy”, “Recurrence Cervical Treatment” and “Metastatic Cervical Treatment” to select papers focused on the therapy.
The following inclusion criteria were used to select the studies included in the present review:
- Type of published study: clinical trial, review articles, guidelines, conference abstracts;
- Selected population: patients affected by cervical cancer with an advanced and/or recurrence disease;
- No limitation regarding the year of the publication of papers was used.
Conversely, exclusion criteria are reported below:
- Duplicate papers;
- Articles not written in English language.
Based on the inclusion and exclusion criteria, some of the selected articles are those whose results have allowed an accelerated approval of the drug by the FDA.
Results
How Tisotumab Vedotin Works
TV, or HuMax-TF-ADC or TIVAK, is a human TF (TF-001) directed ADC. It is formed by a human monoclonal immunoglobulin G1 (subtype κ) linked to the MMAE through a protease-cleavable linker.16
TF, also known as coagulation factor III or thromboplastin, has a significant impact on cancer biology; it is a transmembrane glycoprotein expressed on the cell membranes of several cancers, including breast cancer, gastrointestinal tumors, gliomas, melanomas, lung tumours, and also cervical cancer, with limited expression in healthy tissue cells.17 TF is expressed in about 94–100% of cervical cancer cells.18
TF may activate an intracellular signaling cascade, and its expression is increased in cancer through a variety of mechanisms.19 It appears to have a pro-tumor impact and has been related to cancer pathogenesis, including thrombogenicity, angiogenesis, tumor growth, proliferation, and metastasis.20 Tumor cells produce TF, which in turn induces the production of interleukin-1 and vascular endothelial growth factor (VEGF), both of which increase hypercoagulability while interacting with platelets and endothelial cells.21
Furthermore, TF may promote tumor progression and metastasis by intracellular signal transduction. In fact, TF, by prothrombin hydrolyzation, may enhance the synthesis and release of cytokines such as interleukin-1 and interleukin-8, which control inflammation and promote tumor cell invasion. Enhanced tumor spread is also favored by extracellular matrix degradation caused by activated plasmin’s proteolytic activity, which is promoted by TF’s increased expression of plasminogen activator receptors. Finally, VEGF secretion, in combination with the aforementioned mechanisms, stimulates angiogenesis by promoting the development of new blood vessels.22
TV has several mechanisms that contribute to its therapeutic efficacy, the most impacting being represented by the MMAE-mediated tumor cell death. MMAE is a strong anti-microtubule agent that binds to tubulin and interrupts microtubule polymerization, inducing cycle arrest during the G2/M phase and, as a result, apoptosis.23
When ADC interacts to TF-positive tumor cells, it is internalized and undergoes lysosomal degradation, releasing MMAE within the tumor cell; MMAE can also be released outside the cell in the tumor microenvironment by causing the death of adjacent cells (bystander effect).14
Additionally, TV has the ability to induce immunogenic cell death in tumor antigen-expressing cells by stimulating both innate and adaptive immune responses23 (Figure 1).
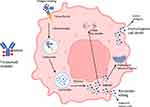 |
Figure 1 Mechanism of action of tisotumab vedotin. |
In vitro, TV treatment resulted in tumor cell death by antibody-dependent cellular cytotoxicity, phagocytosis, and immunogenic cell death.24
First Trials in Solid Tumours and Cervical Cancer
In phase I/II studies in patients with metastatic solid tumors expressing TF, TV demonstrated clinically relevant and durable anticancer efficacy with a manageable and tolerable toxicity profile.14
TV’s pharmacokinetic (PK) profile was evaluated during the dose-escalation phase of the first-in-human, open-label, dose-escalation/expansion innovaTV 201 phase I/II trial. TV used to have a manageable safety profile in the dose escalation phase including 27 patients, and the recommended Phase II dose of 2.0 mg/kg every 3 weeks was determined.14
The most common adverse events of any grade were: epistaxis (69%), fatigue (56%), nausea (52%), alopecia (44%), conjunctivitis (43%), decreased appetite (36%), constipation (35%), diarrhea (30%), vomiting (29%), peripheral neuropathy (22%), and dry eye (22%).14
Patients in the cervical cancer cohort received an intravenous infusion of TV 2.0 mg/kg every three weeks for four cycles. Patients who had clinical benefit (stable disease or partial response) after four cycles had the option of continuing therapy for an additional eight cycles (up to a total of 12 cycles), or until disease progression or unacceptable toxicity25 (NCT03245736).
During dose expansion, InnovaTV 201 had included 147 patients with bladder cancer (n = 15), cervical cancer (n = 34), endometrial cancer (n = 14), non-small-cell lung cancer (n = 15), ovarian cancer (n=36), and a few others. The objective response rate (ORR) for all treated patients was 15.6%, with bladder cancer and cervical cancer patients showing the highest response (26.7% and 26.5%, respectively).14
These results encouraged the continuation of the clinical research of TV in the treatment of TF-expressing solid tumours.26
ENGOT-cx6/GOG-3023/innovaTV 204 is an open-label, multicentre, single-arm, phase-2 trial27 (NCT03438396) which enrolled 101 patients with recurrent or advanced cervical cancer (squamous cell, adenocarcinoma, or adenosquamous histology) who had a disease progressing after platinum based chemotherapy plus or minus bevacizumab, and had received no more than 2 previous systemic regimens for recurrent or metastatic disease.28
The primary endpoint of the study was confirmed ORR by RECIST (version 1.1). Secondary efficacy endpoints included duration of response (DOR), time to response, PFS, OS and safety. The exploratory endpoints were to determine TF expression in pre-treatment tumor biopsies and to assess the clinical responses according to TF expression level.28
Patients received TV 2 mg/kg every 3 weeks until disease progression or unacceptable toxicity. After a median follow up of 4.2 months, the ORR was 24% (95% CI: 15.9%, 33.3%) with 7% complete responses and 17% partial responses. The trial showed a median DOR of 8.3 months (95% CI: 4.2, not reached), median PFS of 4.2 months (95% CI: 3.0, 4.4) and OS of 12.1 months (95% CI: 9.6, 13.9).28
Adverse events occurred in 92% of patients with 65% reporting grade 1–2 toxicities and 25% grade 3. The most common grade 1–2 adverse events were: alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (24%), and dry eye (23%) while the most common grade 3 adverse events were: peripheral neuropathies (8%); sensory neuropathy (2%); sensorimotor neuropathy (2%); motor neuropathy (4%); neutropenia (3%); fatigue (2%); and ulcerative keratitis (2%).28
A pre-specified exploratory analysis aiming at evaluation of TF expression on tumour tissue samples reported the presence of the receptor on 77 (96%) out of 80 patients but, interestingly, the response to TV were observed regardless of membrane TF expression.28
On September 20, 2021, based on the results of InnovaTV-201 and InnovaTV-204 the FDA grants TV accelerated approval for patients with recurrent or metastatic cervical cancer who have disease progression during or after platinum-based chemotherapy.16
ENGOT-cx12/GOG-3057/InnovaTV 301 (NCT04697628) is a randomized, open-label, phase 3 trial of TV vs investigator’s choice chemotherapy (topotecan, vinorelbine, gemcitabine, pemetrexed, or irinotecan) in second- or third-line recurrent or metastatic cervical cancer.29
The trial has concluded the recruitment and the primary end point data readout is estimated for Q2 2024 and will potentially represent, if positive, the registration trial for TV in Europe.
Tisotumab Vedotin Combinations
The encouraging results of these two studies, as well as the manageable toxicity profile of the drug, prompted the development of other studies with TV combinations in metastatic or recurrent cervical cancer.
ENGOT-cx8/GOG-3024/innovaTV 205 (NCT0378608) is a multicentre, open-label phase Ib/II trial of TV in combination with bevacizumab (arm A) or pembrolizumab (arm B, E, F) or carboplatin (arm C and D) or in monotherapy (arm G), in patients with recurrent or metastatic cervical cancer. Participants enrolled to Arm G will receive tisotumab vedotin weekly.
Arm H is the combination of TV with carboplatin and pembrolizumab with/without bevacizumab in previously untreated patients. The trial consists of a dose escalation part with patients progressing during or after standard chemotherapy, and an expansion phase that included both cervical cancer patients who have not received prior systemic therapy for recurrent or metastatic disease and patients who had progressed on or after at least one but no more than two prior systemic therapies.30
The trial is still ongoing, but the first results of dose escalation phase had shown encouraging activity of TV’ combinations: in arm A (TV + bevacizumab) 5/15 patients, in arm B (TV + pembrolizumab) 2/12 and in arm C (TV + carboplatin) 4/13 had confirmed objective response (cOR).27 The activity was corroborated by first results of dose expansion cohort: the confirmed ORR was 54.5%, 40.6% and 38.3% in patients treated with first line TV + carboplatin (arm D), first line TV + pembrolizumab (arm E) and second/third line TV + pembrolizumab (arm F), respectively.31
The recommended phase II dosing of TV was 2.0 mg/kg every three weeks in the three arms.27
The most common adverse events were grade 1 and 2 and they predominantly are: alopecia, diarrhoea, epistaxis, conjunctivitis, and nausea.31 Individual percentages differ based on the study arm.
Table 1 and Table 2 summarizes clinical trials of TV in patients with recurrent or metastatic cervical cancer.
 |
Table 1 Completed Clinical Trials of TV in Patients with Recurrent or Advanced Cervical Cancer |
 |
Table 2 Ongoing Clinical Trials of TV in Patients with Recurrent or Advanced Cervical Cancer |
Safety and Adverse Events of Tisotumab Vedotin
According to results of InnovaTV 201 the most common adverse events of any grade were epistaxis (69%), fatigue (56%), nausea (52%), alopecia (44%), conjunctivitis (43%), decreased appetite (36%), constipation (35%), diarrhoea (30%), vomiting (29%), peripheral neuropathy (22%), dry eye (22%), and abdominal pain (20%). Serious adverse events occurred in 46% of patients and 22% of patients discontinued treatment due to adverse events.14
The same adverse events were also found in InnovaTV 204 where 92% of patients reported treated-related adverse events (65% grade 1 or 2), the most common being alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (24%), and dry eye (23%).28
The most common grade 3 or worse adverse events were: peripheral neuropathies (8%); Neuropathy peripheral (2%); Peripheral sensory neuropathy (2%); Peripheral sensorimotor neuropathy (2%); Peripheral motor neuropathy (2%); neutropenia (3%); fatigue (2%); ulcerative keratitis (2%); occurring in 28% of patients.28
Of interest, InnovaTV 205 trial showed that the most common adverse events (grade 1–2/grade ≥3) were ocular events (55%/3%), peripheral neuropathy (27%/12%) and bleeding events (48%/6%).32
The onset of adverse events in this combination trial could be associated with the other drugs too.
In arm D the most common grade 1–2 adverse events were: nausea (64%), alopecia (55%), fatigue (49%), epistaxis (46%), dry eye (42%), and conjunctivitis (30%); while the most common grade 3 or worse were anaemia (39%), nausea and diarrhoea (15%), and neutropenia (12%).31
In arm E the most common grade 1–2 adverse events were: alopecia (58%), diarrhoea (52%), epistaxis (48%), nausea (45%), conjunctivitis (42%), and dry eye (39%); and the most common grade 3 was anaemia (12%).31
In arm F the most common grade 1–2 adverse events were: diarrhoea (49%), nausea (46%), and epistaxis (37%), while conjunctivitis and dry eye only 26% while the most common grade 3 was anaemia (29%).31
Ocular events and neuropathy are common toxicities in MMAE-containing drugs while haemorrhage is associated with TF coagulation properties and its effect on coagulation and haemostasis.
Based on the safety profile of the drug in InnovaTV 201 trial, both the drug manufacturer and FDA developed some mitigation strategies to anticipate and manage these adverse events.33
Ocular toxicities can be anticipated with TV as TF is expressed also in the conjunctiva.34
In InnovaTV 201 conjunctivitis affected 39% (grade 1–2) and 3% (grade 3) of patients14 and in the InnovaTV 204, 26% of patients had grade 1–2 conjunctivitis.28
The implementation of an eye care plan in the studies was of utmost importance to reduce ocular adverse events.28 Patients were instructed to administer adequate ocular premedication with steroid eye drops (prior to start treatment until 72 h after each infusion), vasoconstrictor eye drops (prior to infusion), lubricating eye drops (from the first dose to 30 days after the last TV dose). Moreover, refrigerator-based eye cooling pads were maintained throughout the infusion, and patients were instructed to avoid using contact lenses for the entire duration of the treatment.14,28
These mitigation strategies revealed to be very effective in patients participating in the innovaTV 201 trial, where the percentage of grade 1–2 ocular events dropped down from 80% to 25% after the introduction of preventive measures.25
In InnovaTV 204 peripheral neuropathy was reported in 42% of patients and the most common adverse events were peripheral neuropathy (10%), peripheral sensory neuropathy (9%), and peripheral motorial neuropathy (5%).28
Haemorrhage occurring in 39% of patients and the most common reported were epistaxis (30%), vaginal haemorrhage (7%), and haematuria (3%).28
The presence of TF in the nasal mucosa is associated with the high prevalence of epistaxis.35
Discussion
Despite screening and vaccination, cervical cancer continues to be a leading cause of death worldwide. Moreover, after first-line, almost all patients with metastatic cervical cancer will recur and all the second-line available single agent chemotherapies (such as docetaxel, gemcitabine, pemetrexed, topotecan, or vinorelbine) have quite low response rates with short response duration.36
To meet this high unmet clinical need, new drugs with new and different mechanism of action are required and ADCs represent, in our opinion, the most interesting class of agents in this setting.
TV is the first ADC to demonstrate remarkable clinical activity in advanced and recurrent cervical cancer by targeting TF, a receptor overexpressed in many solid cancers and related with poor outcomes.
Early clinical data on TV were reported in the innovaTV201 trial, where antitumor activity was demonstrated in patients with pretreated, advanced bladder, cervical, endometrial, esophageal, lung, and ovarian cancers, despite the fact that the trial was not designed to evaluate anticancer activity.14
The results of the phase II InnovaTV 204 trial demonstrated strong and durable antitumor activity of TV in advanced and recurrent cervical cancer with a 24% ORR, a mDOR of 8.3 months, a mPFS of 4.2 months and a mOS of 12.1 months.28 Of note, the responses to TV were rapid with a median time to response of 1.4 months suggesting a possible antitumor activity within the first two cycles; moreover a reduction in tumor lesion sizes from baseline was seen in 79% of patients.28
Overall, TV had a manageable safety profile: both in InnovaTV 201 and in InnovaTV 204 there was reported a limited number of grade 3 or higher adverse events and the most common all grades adverse events were peripheral neuropathy, ocular toxicity and bleeding events. Almost all epistaxis and ocular events were grade 1/2, and none required clinical intervention.14,28 Moreover, the frequency of ocular events, including conjunctivitis, was further reduced after the implementation of mitigation measures.26
The interim analysis of the phase I/II InnovaTV 205 study showed promising activity when TV was used in combination with platinum and pembrolizumab with manageable and non overlapping toxicity profiles31,32 ORR was 54.5%, 40.6% and 38.3% in patients treated with first line TV + carboplatin, first line TV + pembrolizumab and second/third line TV + pembrolizumab, respectively.31
In the last year, the results of KEYNOTE-826 changed the paradigm of first line treatment of advanced/recurrent cervical cancer and pembrolizumab has been approved for patients with CPS ≥ 1 in combination with carboplatin-paclitaxel ± bevacizumab for advanced or recurrent, chemo-naïve, cervical cancer patients.11 The anticipation in first line of immunotherapy treatment, raises the question about what treatments can be offered to immune-resistant patients in second and later lines and in this setting ADC will play a major role. In addition, patients who are not candidates for checkpoint inhibitor therapy can still benefit from TV.
Moreover, KEYNOTE-826 data prompted the addition of another arm in InnovaTV 205 trial: in the arm H previously untreated advanced or metastatic cervical cancer patients will receive the combination of TV, carboplatin, and pembrolizumab with/without bevacizumab.
The results of arm H are currently greatly expected. If this combination will demonstrate to be feasible in terms of safety and active in terms of preliminary efficacy data, it would be of interest to compare it to standard of care in the first-line setting.
Conclusion
TV appears to be an attractive therapeutic option in patients with metastatic or recurrent cervical cancer due to the advantageous reported results in terms of clinical responses and also with a modest safety profile, particularly with devices learnt to use over time.
Additional data from the ongoing clinical trials will further clarify the role of TV in the treatment algorithm both in terms of line of treatment and optimal combination partner.
Abbreviations
ADC, antibody-drug conjugate; CR, complete response; DOR, duration of response; FDA, US Food and Drug Administration; HPV, human papillomavirus; MMAE, microtubule-disrupting agent monomethyl auristatin E; ORR, objective response rate; OS, overall survival; PFS progression free survival; PK, pharmacokinetic; PR, partial response; TF, tissue factor; TV, tisotumab vedotin; VEGF, vascular endothelial growth factor.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
V.S. reports grants and personal fees from: Clovis Oncology, GlaxoSmithKline, AstraZeneca, Merck Sharp & Dohme, PharmaMar, Novocure, outside the submitted work. R.B. reports personal fees from Boehringer, EISAI, Otsuka, Lilly, GSK, PharmaMar, Novartis, Amgen, GSK, and Gilead; grants from MSD, Roche, and Astra Zeneca, outside the submitted work. G.S. reports personal fees from: ROCHE, Clovis Oncology, AstraZeneca, PharmaMar, GlaxoSmithKline, outside the submitted work. He also reports grants from MSD Italia S.r.l.; personal fees from TESARO Bio Italy S.r.l, Johnson & Johnson, outside the submitted work. D.L. reports research funding from: Clovis Oncology, AstraZeneca, Corcept Therapeutics, GlaxoSmithKline, Genmab, ImmunoGen, Merck Sharp & Dohme, Novartis, PharmaMar, Roche, and Seagen; reports consulting fees from: Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Oncoinvest, Corcept, Sutro, and PharmaMar; reports payment or honoraria for lectures from: Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and Seagen; reports support for attending meetings and/or travel from: AstraZeneca, PharmaMar, and Roche; Participation on a data safety monitoring board or advisory board: Clovis Oncology, Agenus, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Roche; and serves on the board of directors for GCIG and as chair of GCA for ENGOT, outside the submitted work; The authors report no other conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Cancer Stat Facts: Cervical Cancer. National cancer institute. Available from: https://seer.cancer.gov/statfacts/html/cervix.html.
3. Neoplasie dell’utero: endometrio e cervice [Neoplasia of the uterus: endometrium and cervix]. September, 2022. Available from: https://www.iss.it/documents/20126/8403839/LG-486-AIOM_Ca-Cervice-Endometrio.
4. Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer. 2018;28(4):641–655. doi:10.1097/IGC.0000000000001216
5. PATH. Global HPV vaccine introduction overview: projected and current national introductions, demonstration/pilot projects, gender-neutral vaccination programs, and global HPV vaccine introduction maps (2006–2023) PATH; 2022. Available from: https://www.path.org/resources/global-hpv-vaccine-introduction-overview/.
6. Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
7. Herrero R, Murillo R. Cervical cancer. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, editors. Cancer Epidemiology and Prevention.
8. Gupta S, Maheshwari A, Parab P, et al. neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36(16):1548–1555. PMID: 29432076. doi:10.1200/JCO.2017.75.9985
9. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240). Lancet. 2017;390(10103):1654–1663. doi:10.1016/S0140-6736(17)31607-0
10. McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol. 2017;29(3):153–160. doi:10.1016/j.clon.2016.10.002
11. Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–1867. doi:10.1056/NEJMoa2112435
12. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–1478. PMID: 30943124. doi:10.1200/JCO.18.01265
13. Tewari KS, Monk BJ, Vergote I, et al. VP4-2021: EMPOWER-cervical 1/GOG-3016/ENGOT-cx9: interim analysis of phase III trial of cemiplimab vs. investigator’s choice chemotherapy in recurrent/metastatic cervical carcinoma. ESMO J. 2021;32(7):940–941.
14. De Bono JS, Concin N, Hong DS, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, Phase 1–2 trial. Lancet Oncol. 2019;20(3):383–393. doi:10.1016/S1470-2045(18)30859-3
15. Food and Drug administration. FDA Grants Accelerated Approval to Tisotumab Vedotin-Tftv for Recurrent or Metastatic Cervical Cancer. U.S: Food and Drug administration; 2021.
16. Markham A. Tisotumab vedotin: first approval. Drugs. 2021;81(18):2141–2147. doi:10.1007/s40265-021-01633-8
17. Theunissen JW, Cai AG, Bhatti MM, et al. Treating tissue factor-positive cancers with antibody-drug conjugates that do not affect blood clotting. Mol Cancer Ther. 2018;17(11):2412–2426. doi:10.1158/1535-7163.MCT-18-0471
18. Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284. doi:10.4061/2011/367284
19. van den Berg YW, Osanto S, Reitsma PH, et al. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119(4):924–932. doi:10.1182/blood-2011-06-317685
20. Eisenreich A, Bolbrinker J, Leppert U. Tissue factor: a conventional or alternative target in cancer therapy. Clin Chem. 2016;62(4):563–570. doi:10.1373/clinchem.2015.241521
21. Yu Y, Hou X, Li Y. Effect of tissue factor knockdown on the growth, invasion, chemoresistance and apoptosis of human gastric cancer cells. Exp Ther Med. 2014;7(5):1376–1382. doi:10.3892/etm.2014.1591
22. Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73(2):145–150. doi:10.1007/BF02981930
23. Tisotumab vedotin. Seagen. Available from: https://www.seagen.com/science/pipeline/tisotumab-vedotin.
24. Breij EC, de Goeij BE, Verploegen S, et al. An antibody–drug conjugate that targets tissue factor exhibits potent therapeu- tic activity against a broad range of solid tumors. Cancer Res. 2014;74(4):1214–1226. doi:10.1158/0008-5472.CAN-13-2440
25. Hong DS, Concin N, Vergote I, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin Cancer Res. 2020;26(6):1220–1228. PMID: 31796521. doi:10.1158/1078-0432.CCR-19-2962
26. Luu K, Chu A, Chang B. A review of the novel tissue factor antibody-drug conjugate: tisotumab vedotin. J Oncol Pharm Pract. 2023;29(2):441–449. PMID: 36415085. doi:10.1177/10781552221139775
27. Monk B, Van Gorp T, Lorusso D, et al. Tisotumab vedotin (TV) þ bevacizumab or pembrolizumab or carboplatin in recurrent/metastatic cervical cancer (R/MCC): phase 1B/2 engot-CX8/GOG-3024/innovaTV 205 study dose-escalation results. Int J Gynecol Cancer. 2021;31(suppl 4):A7–A8.
28. Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ ENGOT-cx6): a multicentre, open-label, single-arm, Phase 2 study. Lancet Oncol. 2021;22:609–619. doi:10.1016/S1470-2045(21)00056-5
29. Clinicaltrial.gov. Tisotumab Vedotin vs Chemotherapy in Recurrent or Metastatic Cervical Cancer (innovaTV 301). Available from: https://clinicaltrials.gov/ct2/show/NCT04697628.
30. Clinicaltrial.gov. Safety and Efficacy of Tisotumab Vedotin Monotherapy & in Combination With Other Cancer Agents in Subjects With Cervical Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03786081.
31. Lorusso D, Vergote I, O’Cearbhaill RE, et al. Tisotumab vedotin (TV) þ pembrolizumab (pembro) in first-line (1L) recurrent or metastatic cervical cancer (r/mCC): interim results of ENGOT Cx8/GOG 3024/ innovaTV 205. J Clin Oncol. 2022;40(16_suppl):5507. doi:10.1200/JCO.2022.40.16_suppl.5507
32. Vergote I, Monk B, O’Cearbhaill RE, et al. 723MO - Tisotumab vedotin (TV) + carboplatin (Carbo) in first-line (1L) or + pembrolizumab (Pembro) in previously treated (2L/3L) recurrent or metastatic cervical cancer (r/mCC): interim results of ENGOT-Cx8/GOG-3024/innovaTV 205 study. Ann Oncol. 2021;32(suppl_5):S725–S772. doi:10.1016/annonc/annonc703
33. TIVDAK (Tisotumab Vedotin-Tftv) [Package Insert]. Bothell, WA: Seagen Inc; 2021.
34. Kim SK, Ursell P, Coleman RL, et al. Mitigation and management strategies for ocular events associated with tisotumab vedotin. Gynecol Oncol. 2022;165(2):385–392. doi:10.1016/j.ygyno.2022.02.010
35. Shimizu S, Ogawa T, Takezawa K, et al. Tissue factor and tissue factor pathway inhibitor in nasal mucosa and nasal secretions of chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy. 2015;29(4):235–242. doi:10.2500/ajra.2015.29.4183
36. Cervical Cancer. National comprehensive cancer network clinical practice guidelines in oncology. Version 1.2023; 2023.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
