Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Therapeutic Potential of Fingolimod in Diabetes Mellitus and Its Chronic Complications
Authors Li J, Nan X, Ma Y, Wang Z, Fang H
Received 23 October 2023
Accepted for publication 19 January 2024
Published 1 February 2024 Volume 2024:17 Pages 507—516
DOI https://doi.org/10.2147/DMSO.S385016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Jie Li,1,2 Xinyu Nan,1 Yixuan Ma,3 Zhen Wang,4 Hui Fang1,2
1Department of Internal Medicine, Hebei Medical University, Shijiazhuang, 050000, People’s Republic of China; 2Department of Endocrinology, Tangshan Gongren Hospital, Tangshan, 063000, People’s Republic of China; 3Graduate School, Hebei North University, Zhangjiakou, 075000, People’s Republic of China; 4Department of Orthopedics, Handan First Hospital, Handan, 056000, People’s Republic of China
Correspondence: Hui Fang, Department of Endocrinology, Tangshan Gongren Hospital, Tangshan, 063000, People’s Republic of China, Tel +86 13831581838, Email [email protected]
Abstract: Diabetes mellitus is a metabolic disease characterized by elevated blood glucose due to a deficiency of insulin secretion and/or action. Long-term poor blood glucose control may lead to chronic damage and dysfunction of the heart, kidneys, eyes, and other organs. Therefore, it is important to develop treatments for diabetes and its chronic complications. Fingolimod is a structural sphingosine analogue and sphingosine-1-phosphate receptor modulator currently used for the treatment of relapsing-remitting multiple sclerosis. Several studies have shown that it has beneficial effects on the improvement of diabetes and its chronic complications. This paper reviews the therapeutic potential of Fingolimod in diabetes and its chronic complications, aiming to further guide future treatment strategies.
Keywords: fingolimod, diabetes mellitus, diabetes-related complications, treatment
Introduction
Diabetes mellitus(DM) is one of the most common diseases in the modern world, caused by many factors such as environment and heredity. With the changes in people’s social lifestyles, DM is expected to affect 783 million adults by 2045.1 DM is characterized by high blood glucose, and has complex and diverse pathogenesis. It is divided into two main types, including type 1 diabetes mellitus(T1DM) and type 2 diabetes mellitus(T2DM). T1DM occurs as a result of the autoimmune destruction of the islet β cells resulting in an absolute insufficiency of insulin secretion. T2DM is caused by insulin resistance or dysfunction of islet β cells.2 Long-term chronic hyperglycemia can lead to diabetes-related complications, causing dysfunction and damage to many organs and tissues. Chronic complications of DM include diabetic macrovascular complications mainly including cerebral infarction and coronary heart disease, diabetic microvascular complications mainly including diabetic retinopathy(DR) and diabetic nephropathy(DN), and so on.3,4 With the increasing trend of patients with DM around the world, the incidence and mortality of diabetes-related chronic complications are increasing, which has become a great challenge that seriously endangers public health.2,5,6 Therefore, the search for active treatment is very urgent.
Sphingosine-1-phosphate(S1P), as an important metabolite of sphingomyelin, has various biological activities and has gotten much attention in recent years.7 By binding to five S1P receptors(S1PRs) that belong to G-protein-coupled receptors, S1P can have effects on many physiological and pathological processes. Abnormal S1P signaling pathways are involved in the occurrence and development of many diseases such as DM, multiple sclerosis(MS), cancers, and neurodegenerative diseases.8–11 The S1PR modulator Fingolimod(FTY720) is the first drug approved orally for the treatment of MS by the United States Food and Drug Administration(FDA) and the European Medical Agency(EMA).9 More and more evidence shows that FTY720 has an anti-diabetic effect and a good therapeutic effect in DM complications, so it is expected to become a new strategy for the treatment of DM and its chronic complications.8,12–14 In this article, we summarize the application of FTY720 in the treatment of DM and its related chronic complications. In addition, the review elucidates potential mechanisms and discusses the challenges associated with its application.
Introduction to FTY720
S1P is a bioactive metabolite of sphingomyelin. Its formation and metabolism are regulated by many enzymes. Sphingomyelin produces ceramide under the action of intracellular synthetase, and subsequently ceramide produces sphingosine under the action of ceramidase. Sphingosine is phosphorylated to produce S1P by sphingosine kinases 1(SphK1) and sphingosine kinases 2(SphK2). S1P can be irreversibly degraded by sphingosine-1-phosphate lyase(S1PL) to hexadecenal and phosphoethanolamine. Intracellular S1P can directly participate in signal transduction as a second messenger. S1P expressed on the cell surface specifically binds to different subtypes of S1PRs. S1PRs can be coupled with G proteins and activate a variety of downstream signaling pathways(Figure 1). Thus they play important roles in regulating the proliferation, differentiation, migration, and apoptosis of cells in different tissues.15 At present, five types of S1PRs have been confirmed. They are widely distributed throughout the body, but their expression in various tissues and organs is different. S1PR1, S1PR2, and S1PR3 are widely distributed in tissues. However S1PR4 is mainly expressed in immune cells, and S1PR5 is mainly expressed in the spleen and central nervous system.16 Due to the multiple effects and ubiquitously expression of S1PRs, S1P is essential for the normal function of cells in cardiovascular, nervous, immune, and other systems.
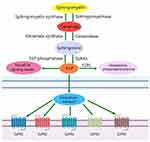 |
Figure 1 S1P metabolic pathway. |
FTY720 is a derivative of myristin and a structural analogue of sphingosine. FTY720 is phosphorylated by SphK2 in vivo to form the activated metabolite FTY720 phosphate(FTY720-P). In addition to S1PR2, it can bind and activate the other four S1PRs. The binding affinity of FTY720-P to S1PR1, S1PR3 and S1PR5 is similar to S1P, but the binding to S1PR4 is superior to S1P.17 However, the long-term excitatory effect of FTY720 on S1PR1 could lead to its ubiquitination and proteasome degradation, so it can also result in functional antagonism of S1PR1.18,19 FTY720 was approved by the FDA in 2010 for the treatment of MS. The main mechanism of action is to induce lymphocyte homing, and the main site of action is S1PR1. S1P is highly expressed in blood and lymph fluid, but the content of S1P in interstitial fluid is low. Therefore, a concentration gradient of S1P between interstitial fluid and the blood-lymphatic system is formed. Due to S1PR1, cells are more likely to move towards the site of high S1P concentration, so this concentration gradient plays an important role in the migration of lymphocytes (including B cells and T cells), NK cells, and dendritic cells. In addition, a high concentration of S1P can induce S1PR1 to internalize and reach cytoplasm, thus resulting in cells losing the ability to migrate to the site of high concentration S1P. FTY720-P is a kind of S1P analogue and can induce the internalization of S1PR1. Thereby FTY720 can induce lymphocyte homing to secondary lymphoid organs and prevent its re-migration, thus achieving the effect of immunosuppression. In recent years, with the gradual deepening of research on the SphK/S1P/S1PRs signal axis and FTY720, a number of studies have shown that the S1PRs modulator FTY720 has significant protective effects in autoimmune myocarditis, uveal retinitis, systemic lupus erythematosus, atherosclerosis, and other models.20–23
FTY720 and DM
DM is one of the most common metabolic diseases in humans and is characterized by a reduction in the mass of functional islet β cells and insulin resistance. Previous studies have shown that the function and survival of islet β cells and insulin sensitivity play important roles in DM, both of which are regulated by the S1P signaling axis.8,24
He et al reviewed the positive role of S1P signaling pathway in T2DM from the perspective of “ominous octet”: (1) S1P can promote the proliferation of islet β cells and reduce their apoptosis; (2) S1P indirectly improve Insulin resistance in muscle tissue through adiponectin; (3) It inhibits hepatic glucose output by activating the Akt pathway; (4) S1P can protect the liver and pancreas from lipotoxic damage through fighting cytotoxic substances produced during oxidative stress, reducing JNK phosphorylation and inhibiting the C/EBP homologous protein signaling pathway; (5) It promotes the secretion of glucagon-like peptide 1(GLP-1) and produce the same biological effects as GLP-1; (6) S1P can inhibit the secretion of glucagon by islet alpha cells; (7) It improves the imbalance of glucose processing in the kidney; (8) S1P promotes the release and transport of neurotransmitters to target organs such as the liver, kidneys, and muscles to increase glucose utilization.25 As the important roles of S1P and related molecules in the pathogenesis of DM have been increasingly known, its related drug FTY720 in the field of DM has been gradually explored.
Zhao et al reported that oral administration of FTY720 in db/db mice can normalize hyperglycemia by increasing the viability and regeneration of islet β cells. The main mechanism is that FTY720 up-regulates the expression of PDX-1 and cyclinD3 in islets and down-regulates the expression of P57KIP2 to stimulate the proliferation of islet β cells through the S1PR-PI3K signaling pathway.26 Similarly, Moon et al also confirmed that early intervention of FTY720 in db/db mice can protect the islet β cells by up-regulating the expression of Bcl-2 and BcL-xL to inhibit apoptosis and increase the survival rate of islet β cells, thereby preventing the development of T2DM.27 In addition, FTY720 may protect pancreatic tissue in SDT rats by depleting circulating lymphocytes, ultimately inhibiting the development of T2DM.28 A recent study investigated the effects of FTY720 on glucose homeostasis and diabetic complications in spontaneous diabetes translates into non-human primates(NHP, an animal model of T2DM). The study showed that in the NHP model which has T2DM combined with cardiac and renal dysfunction, FTY720 sustainably reduced blood glucose and restored islet β cells function, accompanied by improvements in cardiac function and reduction in proteinuria. This may be due to FTY720 significantly reducing CD4+ T and CD8+ T lymphocytes and increasing dendritic cells, thereby preventing islet β cells from being destroyed by autoimmune cells and improving glucose homeostasis.29
In addition to protecting islet β cells function, FTY720 also improved glucose tolerance and insulin sensitivity in obese and insulin-resistant rodent models. In diet-induced obesity mice, FTY720 treatment can prevent weight gain, improve insulin sensitivity, and reduce lymphocytes and macrophages in adipose tissue.30 In addition, ceramide accumulates in the skeletal muscles of people and rodents who have obesity and insulin resistance, which aggravates insulin resistance by impairing the Akt signaling pathway. High-fat-fed mice treated with FTY720 had significantly reduced ceramide, diacylglycerol and triacylglycerol content in muscle. Therefore, insulin resistance and glucose homeostasis had been improved.31
Furthermore, it is been reported that FTY720 plays a role in protecting the pancreatic in several animal models of T1DM. The non-obese diabetic(NOD) mouse is a well-established mouse model of spontaneous T1DM and is similar to features of patients with T1DM. Yang et al tested the ability of FTY720 to prevent autoimmune diabetes in NOD mice. The results show that FTY720 prevents the onset of T1DM by inducing lymphocytopenia, preventing the infiltration of islet inflammatory cells, and the destruction of β cells caused by pro-inflammatory cytokines.32 Similarly, Penaranda et al consistently treated NOD mice with FTY720, where they found that FTY720 can “lock” lymphocytes in the pancreatic lymph nodes and pancreas, and control the exit and entry of tertiary lymphoid organs into adjacent pancreatic tissue, thus preventing islet destruction and development of T1DM.33 The LEW.1AR1-iddm(IDDM) rat is a T1DM animal model without lymphocytopenia. Jorns et al found that FTY720 prevents the progression of diabetes in IDDM rats by promoting the retention of activated immune cells in lymph nodes, thereby avoiding islet invasion and the destruction of islet β cells by proinflammatory cytokines.34
Based on these data, FTY720 has been shown to prevent DM by promoting islet β cells proliferation, inhibiting apoptosis, and reducing insulin resistance. However, many studies have found that animal models with DM treated with FTY720 have significant improvements in its complications, but it has no significant effect on lowering blood glucose.12,14,35,36 This may be due to these differences in the model building, the dosage and method of medication. Hence, in the future, the role of FTY720 in diabetes control needs to be further evaluated in large-scale and long-term studies.
FTY720 and diabetic heart diseases
Diabetic heart diseases includes various cardiovascular diseases secondary to diabetes, such as coronary artery disease and cardiomyopathy. In addition to coronary atherosclerosis, the microvascular circulation of the heart is also worthy of attention. Coronary flow reserve(CFR) is a measure of coronary vascular function and an important functional parameter to understand the pathophysiology of coronary microcirculation. Low CFR is an independent risk factor for cardiovascular events, and CFR impairment is prevalent in diabetic patients and animal models.37 Therefore, the study of drugs to improve coronary microcirculation has important clinical value. Xu et al confirmed that the CFR of T1DM rats increased after continuous oral administration of FTY720 (1.25mg/kg/day) for 9 weeks. This suggests that FTY720 can improve coronary microcirculation disorders.37 Furthermore, FTY720 treatment can reduce the expression of transforming growth factor(TGF-β) and collagen in heart tissue, and the expression of vascular inflammatory factors and endothelial adhesion molecules such as vascular cell adhesion molecule-1(VCAM-1), intercellular adhension molecule-1(ICAM-1) and interleukin-6(IL-6), which have indirect effects on the recovery of CFR.37
Cardiac microangiopathy is characterized by increased apoptosis of vascular endothelial cells, increased permeability, and pathological angiogenesis. Previous studies have shown that S1PR1 and S1PR3 are expressed on endothelial cells and contribute to vascular stability. Activation of S1PR1 promotes angiogenesis, while stimulation of S1PR3 leads to impaired barrier function.38 One study found that increased cardiac microvascular permeability and pathological angiogenesis in diabetic rats were accompanied by downregulation of S1PR1 and translocation of S1PR3 from the nucleus to the membrane. For this reason, FTY720 can protect the cardiac microvessels by up-regulating S1PR1 and stimulating the translocation of S1PR3 from the cell membrane to the nucleus.12 In addition, the study also found that FTY720 reduced the expression of protein kinase C β II by regulating S1PR1/3, thereby improving the occurrence of diabetic heart microangiopathy.12
Persistent hyperglycemia impairs the coronary microcirculatory response to ischemia and removes the protection provided by ischemic preconditioning(IPC). IPC is a powerful endogenous protective phenomenon. Before the subsequent long-term ischemic injury, brief intermittent ischemia and reperfusion circulation make the myocardium more resistant to the harmful injury induced by ischaemic reperfusion(I/R) within a short time.39 The cellular and molecular mechanisms of DM lead to an imbalance between the phosphorylation and dephosphorylation states of lipid and protein kinases, which can regulate mitochondrial permeability transition pores(mPTP), thereby weakening the heart protective effects of IPC. It is reported that diabetic rats were pretreated with FTY720 at twenty minutes before ischemia, the infarction size, and the expression levels of lactic dehydrogenase(LDH), creatine kinase-MB(CK-MB), myeloperoxidase(MPO), tumor necrosis factor α(TNF-α) and glycogen synthase kinase 3β(GSK-3β) of their heart were significantly reduced. Thus, the protective effect of IPC on the heart of diabetic rats was significantly restored by alleviating myocardial damage induced by I/R, lowering inflammation, and regulating the opening of mPTP.40
In addition, myocardial fibrosis is one of the main features of diabetic cardiomyopathy and contributes to increased ventricular stiffness, which leads to systolic dysfunction of the failing diabetic heart. Previous studies have found increased T cells infiltration in diabetic heart muscle.41 T lymphocytes can activate prefibrotic cells and participate in the continuation of fibrosis by secreting pro-inflammatory cytokines. Mature T cells express higher S1PR1 on their surfaces, allowing them to sense increased S1P concentration gradients in blood and lymph. Mature T cells express higher S1P1 receptors on their surfaces, allowing them to sense increased S1P concentration gradients in blood and lymph. Since FTY720 binds to S1PR1 with high affinity, it can lead to ligand-induced receptor internalization and degradation, resulting in functional antagonism. Abdullah et al confirmed that after treatment with FTY720 in diabetic mice, the depletion of CD4+ T cells and CD8+ T cells in blood and the increase of CD3+ T cells in myocardial interstitium were reduced by down-regulation of S1PR1 on the surface of mature T lymphocytes. This illustrates that FTY720 has a protective effect on the heart and can prevent heart remodeling in diabetic mice.36 Notably, FTY720 provides benefits in diabetic cardiomyopathy by down-regulating S1PR1, while it has beneficial effects in diabetic cardiac microangiopathy by up-regulating S1PR1. It may be that in different cell types, S1PR1 is associated differently with specific molecules in lipid rafts(a cellular niche in which G-protein-coupled receptor-specific signaling and internalization/recycling tasks occur), therefore there is a difference in the effects of S1PR1 on T cells and cardiac microvascular endothelial cells.
FTY720 and DR
DR, one of the common microvascular complications of DM, is the leading cause of blindness in the working-age population worldwide. It is characterized by structural changes in retinal endothelial vessels and the breakdown of the blood-retina barrier(BRB). The manifestations of early DR are endothelial and pericellular apoptosis, vascular leakage, and leukocyte adhesion. Then, as the disease progresses, microaneurysms, retinal vein occlusion, diabetic macular edema(DME), and proliferative diabetic retinopathy(PDR) that threaten vision are likely to occur.42,43 At present, the clinical treatment of DR mainly includes metabolic disorder control, laser photocoagulation, vitrectomy, glucocorticoid therapy, and anti-vascular endothelial growth factor(VEGF) therapy, but the above treatment methods have their own limitations and adverse reactions respectively.44–46 Therefore, it is important to further develop new therapeutic approaches for DR.
More evidence confirm that low-grade inflammation plays a key role in the development and progression of DR.47 Under normal physiological conditions, neurotrophic factors and inflammatory mediators in the retina maintain a dynamic balance, but the occurrence of DM breaks the balance, causing chronic inflammation of retinal endothelial cells and nerve cells. Various inflammatory mediators such as TNF-α, interleukin-1β(IL-1β), and IL-6 were elevated in vitreous and retina of patients with DR.48,49 The combination of these pro-inflammatory factors with VEGF ultimately leads to increased vascular permeability and/or pathological angiogenesis. These clinical manifestations are DME and PDR.50 TNF-α is an effective mediator of retinal vascular white matter block, which can mediate the breakdown of BRB and the formation of retinal new blood vessels induced by ischemia.51 It also mediates cell death or apoptosis of retinal neurons and vascular endothelial cells in DR, which contributes to the breakdown of BRB.52 IL-1β can increase endothelial permeability in DR through perithelial cells apoptosis induced by nuclear transcription factor κB(NF-κB)activation.53 IL-6 can inhibit the formation and development of retinal vascular endothelial cells, promote endothelial cell apoptosis, destroy the normal function of blood vessel wall, and affect retinal vascular permeability.54 Therefore, inhibiting the expression of these inflammatory factors is beneficial to DR. Fan et al found that after the treatment of FTY720, the expressions of TNF-α, IL-6, and IL-1β in diabetic rats’ retina were significantly reduced, and the vascular permeability was significantly reduced and the destruction of BRB was significantly improved.35 In addition, the activation of NF-κB controls the expression of many genes involved in the inflammatory response and plays an important role in the pathogenesis of DR. The research shows that FTY720 therapy exerts anti-inflammatory effects by inhibiting the activation of NF-κB in the retina.35 Moreover, in the early stage of DR, increased expression of adhesion molecules leads to increased adhesion of white blood cells in retinal blood vessels, forming a local inflammatory environment and white blood cell stasis. Then endothelial cell damage and death are caused, thus leading to the destruction of the blood-retina barrier.55 Among them, it is focused on ICAM-1 and VCAM-1. It was found that FTY720 can down-regulate the expression of ICAM-1 and VCAM-1 in the retina of diabetic rats, thereby inhibiting the adhesion of retinal blood vessel white blood cells, reducing BRB destruction and secondary inflammatory cell leakage, and delaying the progression of DR.35
Previous studies have shown that the activation of melanocortin receptors 1(MCR1) and melanocortin receptors 5(MCR5) reduces pro-inflammatory cytokines and chemokines, improves the levels of manganese superoxide dismutase and glutathione peroxidase, and protects the integrity of photoreceptors, which has a protective effect on DR.56 Similarly, Rossi et al showed that the activation of MCR1 and MCR5 can regulate retinal occlude proteins, VGEF level, and change macrophage polarization, thereby reducing the damage of DR.57 Based on these findings, FTY720, as an agonist for MCR1 and MCR5, has been proven to have a protective effect on DR through binding to MCRs by Gesualdo et al.13 In addition, DR is characterized by BRB destruction. Both the outer barrier and the inner barrier of BRB were damaged, but the inner barrier was dominant. Vascular endothelial cells and their close connections are the functional basis of the inner barrier. S1PRs on the surface of endothelial cells, especially S1PR1 and S1PR3, are major mediators that help maintain the integrity of the inner barrier. FTY720 regulates and maintains the inner barrier by acting on S1PR1/S1PR3 directly, thereby reducing retinal microvascular permeability and maintaining the integrity of BRB.35 Therefore, FTY720 has great application prospects in the treatment of DR, and a large number of studies should be carried out to explore its efficacy and long-term side effects.
FTY720 and DN
As the number of people with DM has skyrocketed, studies have shown that about 40% of patients with DM are more likely to have DN.58 DN is the main cause of end-stage renal failure. The clinical manifestation of DN is progressive renal failure. And the pathological features were glomerular hypertrophy, basement membrane thickening, extracellular matrix protein deposition, podocyte process reduction, and renal tubule interstitial fibrosis.59,60 Previous studies have shown that the S1PR1 signaling pathway may reduce the severity of acute and chronic kidney disease by reducing inflammation and fibrosis, and protecting blood vessels, while the S1PR2 signaling pathway may exacerbate these diseases by accelerating fibrosis and inflammation.61 Moreover, FTY720 can activate S1PR1 to protect kidneys by reducing inflammation, leukocyte infiltration, and vascular permeability caused by acute renal ischemia-reperfusion injury.62,63 Therefore, FTY720 is presumed to have a renal protective effect in DN. The research results of Award et al prove this hypothesis.64 After activating S1PR1, FTY720 controlled glomerular permeability and podocyte function by maintaining the expression of podocyte specific proteins, TNF-α and VEGF. Besides, significant reductions in tubule damage and vacuolation were observed, suggesting that FTY720 also had a protective effect on proximal tubule cells. Progressive nephropathy due to DN is caused by glomerular and tubular interstitial injury, so the broad action of FTY720 may be more effective than current treatments for DN.
FTY720 and erectile dysfunction(ED)
ED is one of the peripheral vascular complications of DM. Previous studies have shown that up to 52% of DM patients may be associated with ED.65 Penile vascular endothelial cells are damaged and apoptotic due to oxidative stress, which affects the activities of endothelial nitric oxide synthase(eNOS), resulting in decreased nitric oxide(NO) level and endothelial diastolic function, thus affecting erectile function. The study of Cui et al on FTY720 treatment in T1DM rat models showed that phosphorylated FTY720 binds to S1PR3 on the surface of endothelial cells to activate the Akt-eNOS signaling pathway and further promote the release of NO. Subsequently, NO can activate the accumulation of soluble guanidine cyclase and cGMP, resulting in smooth muscle relaxation and penile erection, thereby improving erectile function.66 In addition, fibrosis is known to contribute to the development of ED. The study also confirmed that FTY720 can reduce the lower body fibrosis by inhibiting Smad and non-Smad pathways, and then reduce the apoptosis of corporal smooth muscle cells, which is a potential new treatment for diabetic ED.
FTY720 and diabetes cognitive decline(DCD)
DCD is a common complication of T1DM and T2DM, which is characterized by a loss of learning ability and memory and behavioral impairments. Patients with DM are 1.5 to 2.0 times more likely to develop cognitive decline, cognitive impairment, or dementia than those without DM.67 The onset of DCD is insidious and the pathogenesis is complex. At present, it is believed that it is related to insulin resistance, blood glucose fluctuation, neuroinflammation, oxidative stress, and other factors. S1P has strong neuroprotective effects and is important for normal excitability and synaptic transmission of hippocampal neurons. As a result, the effects of S1PR modulators on cognitive decline in neurodegenerative diseases such as Alzheimer’s disease(AD), Parkinson’s disease(PD), and Huntington’s disease are constantly being explored.68 FTY720 is a structure-like sphingosine drug capable of penetrating the blood-brain barrier.69 Previous studies have shown that the S1PR1 agonist FTY720 can reduce cognitive impairment in AD. One of the neuroprotective mechanisms provided by S1PR1 activation is the regulation of microglial polarization. Microglia are immune cells in the central nervous system that continuously monitor the microenvironment of the central nervous system and maintain nervous system homeostasis. When the brain is damaged, microglia are the first responders.70 After microglia are stimulated by injury, microglia activate and polarize into proinflammatory phenotype M1 and anti-inflammatory phenotype M2, leading to the occurrence of neuroinflammation. Neuroinflammation is the main cause of cognitive decline in diabetic mice.71 A recent study has confirmed that FTY720 induces the polarization of M1 to M2 through the pstat3-jmjd3 axis to improve neuroinflammatory response, further inhibit neuronal apoptosis, improve synaptic plasticity, and provide neuroprotection for diabetic mice.14 In addition, by inhibiting S1PL in the prefrontal cortex and hippocampus of T2DM mice, FTY720 further reduces P53 level and promotes TP53-associated glycolysis and apoptosis regulator expression, thereby increasing anti-inflammatory microglial phenotype and inhibiting apoptosis in the brain of diabetic mice.72
Conclusion
In recent years, the application of FTY720 has gradually become a research hotspot in the biomedical field, and its emergence provides a new target and choice for the treatment of a variety of diseases. Studies in various pathological models have demonstrated that the S1PR modulator FTY720 can protect islet β cells and increase insulin sensitivity, so it has the potential to be developed as a drug for DM. In addition, the review also reveals the promise of FTY720 in improving chronic complications of DM, especially diabetic microvascular complication(Figure 2). However, the clinical application of FTY720 is still limited. First, while many mechanisms have been discovered, much remains to be learned about redundant signaling, and the involvement of normal and pathological immune responses. In addition, due to its broad spectrum effect on S1PR1, S1PR3, S1PR4 and S1PR5, it is easy to cause some serious adverse reactions, such as first dose bradycardia, macular edema, elevated liver enzymes, lymphocytopenia, reduced lung function, hypertension, tumor and so on.15,18,19,73 Therefore, more in-depth studies on the application of FTY720 in DM and its complications are needed, which enable people to have a deeper understanding of its mechanism of action in the disease, thus providing new targets and strategies for the prevention, diagnosis and treatment of related diseases.
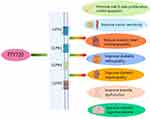 |
Figure 2 FTY720 acts on the above-mentioned organs to improve DM and its chronic complications through S1PR1, 3, 4, 5. |
Abbreviation
AD, Alzheimer’s disease; BRB, blood-retinal barrier; CFR, coronary flow reserve; CK-MB creatine kinase-MB; DCD, diabetes cognitive decline; DM, Diabetes mellitus; DME, diabetic macular edema; DN, diabetic nephropathy; DR, diabetic retinopathy; ED, Erectile dysfunction; EMA, European Medical Agency; eNOS, endothelial nitric oxide synthase; FDA, Food and Drug Administration; FTY720, Fingolimod; FTY720-P, FTY720 phosphate; GLP-1, glucagon-like peptide 1; GSK-3β, glycogen synthase kinase 3β; ICAM-1, intercellular adhension molecule-1; IDDM LEW, 1AR1-iddm; IL-6, interleukin-6; IL-1β, interleukin-1β; IPC, ischemic preconditioning; I/R, ischaemic reperfusion; LDH, lactic dehydrogenase; MCR1, melanocortin receptors 1; MCR5, melanocortin receptors 5; MPO, myeloperoxidase; mPTP, mitochondrial permeability transition pores; NF-κB, nuclear transcription factor κB; MS, multiple sclerosis; NO, nitric oxide; NOD, non-obese diabetic; PD, Parkinson’s disease; PDR, proliferative diabetic retinopathy; S1P, sphingosine-1-phosphate; S1PL, sphingosine-1-phosphate lyase; S1PRs, S1P receptors; SphK1, sphingosine kinases 1; SphK2, sphingosine kinases 2; T1DM, type 1 diabetes mellitus; T2DM type 2 diabetes mellitus; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
Acknowledgments
The co-authors thank the Natural Science Foundation of Hebei Province of China (H2020105) and the Central Guiding Local Science and Technology Development Funding Project (216Z7706G) for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Yang XD, Yang YY. Ferroptosis as a novel therapeutic target for diabetes and its complications. Front Endocrinol. 2022;13:853822. doi:10.3389/fendo.2022.853822
3. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi:10.1038/s41581-020-0278-5
4. Entezari M, Hashemi D, Taheriazam A, et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563. doi:10.1016/j.biopha.2021.112563
5. Susan Van D, Beulens JWJ, Yvonne T, Van Der S, Grobbee DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(1_suppl):s3–s8. doi:10.1097/01.hjr.0000368191.86614.5a
6. Yang M, Chen J, Chen L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front Endocrinol. 2022;13:1027686. doi:10.3389/fendo.2022.1027686
7. Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetes-associated pathologies (Review). IntJ Mol Med. 2017;39(2):243–252. doi:10.3892/ijmm.2017.2855
8. Jessup F, Bonder CS, Pitson CM, Toby S, Coates H. The sphingolipid rheostat: a potential target for improving pancreatic islet survival and function. EMIDDT. 2011;11(4):262–272. doi:10.2174/187153011797881201
9. Yazdi A, Ghasemi‐Kasman M, Javan M. Possible regenerative effects of fingolimod (FTY720) in multiple sclerosis disease: an overview on remyelination process. J Neurosci Res. 2020;98(3):524–536. doi:10.1002/jnr.24509
10. White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget. 2016;7(17):23106–23127. doi:10.18632/oncotarget.7145
11. Zhang L, Wang H. FTY720 in CNS injuries: molecular mechanisms and therapeutic potential. Brain Res Bull. 2020;164:75–82. doi:10.1016/j.brainresbull.2020.08.013
12. Yin Z, Fan L, Wei L, et al. FTY720 protects cardiac microvessels of diabetes: a critical role of S1P1/3 in diabetic heart disease. PLoS One. 2012;7(8):e42900. doi:10.1371/journal.pone.0042900
13. Gesualdo C, Balta C, Platania CBM, et al. Fingolimod and diabetic retinopathy: a drug repurposing study. Front Pharmacol. 2021;12:718902. doi:10.3389/fphar.2021.718902
14. Sood A, Fernandes V, Preeti K, Khot M, Khatri DK, Singh SB. Fingolimod alleviates cognitive deficit in type 2 diabetes by promoting microglial M2 polarization via the pSTAT3-jmjd3 axis. Mol Neurobiol. 2023;60(2):901–922. doi:10.1007/s12035-022-03120-x
15. Bravo GÁ, Cedeño RR, Casadevall MP, Ramió-Torrentà L. Sphingosine-1-Phosphate (S1P) and S1P signaling pathway modulators, from current insights to future perspectives. Cells. 2022;11(13):2058. doi:10.3390/cells11132058
16. Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–195. doi:10.1124/pr.107.07113
17. Huwiler A, Zangemeister-Wittke U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol Ther. 2018;185:34–49. doi:10.1016/j.pharmthera.2017.11.001
18. Cartier A, Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 2019;366(6463):eaar5551. doi:10.1126/science.aar5551
19. Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170. doi:10.1016/j.phrs.2019.02.009
20. Boldizsar F, Tarjanyi O, Olasz K, et al. FTY720 (Gilenya) treatment prevents spontaneous autoimmune myocarditis and dilated cardiomyopathy in transgenic HLA-DQ8-BALB/c mice. Cardiovasc Pathol. 2016;25(5):353–361. doi:10.1016/j.carpath.2016.05.003
21. Commodaro AG, Peron JPS, Lopes CT, et al. Evaluation of experimental autoimmune uveitis in mice treated with FTY720. Invest Ophthalmol Vis Sci. 2010;51(5):2568. doi:10.1167/iovs.09-4769
22. Okazaki H, Hirata D, Kamimura T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol. 2002;29(4):707–716.
23. Keul P, Tölle M, Lucke S, et al. The Sphingosine-1-Phosphate Analogue FTY720 reduces atherosclerosis in apolipoprotein E–deficient mice. Arteriosclerosis Thrombosis Vasc Biol. 2007;27(3):607–613. doi:10.1161/01.ATV.0000254679.42583.88
24. Fayyaz S, Japtok L, Kleuser B. Divergent role of Sphingosine 1-phosphate on insulin resistance. Cell Physiol Biochem. 2014;34(1):134–147. doi:10.1159/000362990
25. He Q, Bo J, Shen R, et al. S1P signaling pathways in pathogenesis of type 2 diabetes. J Diabetes Res. 2021;2021:1–12. doi:10.1155/2021/1341750
26. Zhao Z, Choi J, Zhao C, Ma ZA. FTY720 normalizes hyperglycemia by stimulating β-cell in vivo regeneration in db/db mice through regulation of cyclin D3 and p57KIP2. J Biol Chem. 2012;287(8):5562–5573. doi:10.1074/jbc.M111.305359
27. Moon H, Chon J, Joo J, et al. FTY720 preserved islet β -cell mass by inhibiting apoptosis and increasing survival of β -cells in db/db mice: FTY720 preserves islet β -cell in mice. Diabetes Metab Res Rev. 2013;29(1):19–24. doi:10.1002/dmrr.2341
28. Kobayashi K, Sasase T, Ishii Y, et al. The sphingosine‐1‐phosphate receptor modulator, FTY720, prevents the incidence of diabetes in Spontaneously Diabetic Torii rats. Clin Exp Pharma Physio. 2021;48(6):869–876. doi:10.1111/1440-1681.13405
29. Wang Y, Wang X, An A, et al. Immunomodulator FTY720 improves glucose homeostasis and diabetic complications by rejuvenation of β ‐cell function in nonhuman primate model of diabetes. Fund Clin Pharm. 2022;36(4):699–711. doi:10.1111/fcp.12760
30. Kendall MR, Hupfeld CJ. FTY720, a sphingosine‐1‐phosphate receptor modulator, reverses high‐fat diet–induced weight gain, insulin resistance and adipose tissue inflammation in C57BL/6 mice. Diabetes Obesity Metab. 2008;10(9):802–805. doi:10.1111/j.1463-1326.2008.00910.x
31. Bruce CR, Risis S, Babb JR, et al. The sphingosine-1-phosphate analog FTY720 reduces muscle ceramide content and improves glucose tolerance in high fat-fed male mice. Endocrinology. 2013;154(1):65–76. doi:10.1210/en.2012-1847
32. Yang Z, Chen M, Fialkow LB, et al. The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice☆. Clin Immunol. 2003;107(1):30–35. doi:10.1016/S1521-6616(02)00054-2
33. Penaranda C, Tang Q, Ruddle NH, Bluestone JA. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes. 2010;59(6):1461–1468. doi:10.2337/db09-1129
34. Jörns A, Rath KJ, Terbish T, et al. Diabetes prevention by immunomodulatory FTY720 Treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology. 2010;151(8):3555–3565. doi:10.1210/en.2010-0202
35. Fan L, Yan H. FTY720 attenuates retinal inflammation and protects blood–retinal barrier in diabetic rats. Invest Ophthalmol Vis Sci. 2016;57(3):1254. doi:10.1167/iovs.15-18658
36. Abdullah CS, Li Z, Wang X, Jin ZQ. Depletion of T lymphocytes ameliorates cardiac fibrosis in streptozotocin-induced diabetic cardiomyopathy. Int Immunopharmacol. 2016;39:251–264. doi:10.1016/j.intimp.2016.07.027
37. Xu H, Jin Y, Ni H, Hu S, Zhang Q. Sphingosine-1-phosphate receptor agonist, FTY720, restores coronary flow reserve in diabetic rats. Circ J. 2014;78(12):2979–2986. doi:10.1253/circj.CJ-14-0521
38. English D, Welch Z, Kovala AT, et al. Sphingosine 1‐phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB j. 2000;14(14):2255–2265. doi:10.1096/fj.00-0134com
39. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi:10.1161/01.CIR.74.5.1124
40. Rana A, Sharma S. Mechanism of sphingosine-1-phosphate induced cardioprotection against I/R injury in diabetic rat heart: possible involvement of glycogen synthase kinase 3 β and mitochondrial permeability transition pore. Clin Exp Pharmacol Physiol. 2016;43(2):166–173. doi:10.1111/1440-1681.12516
41. Becher PM, Lindner D, Fröhlich M, Savvatis K, Westermann D, Tschöpe C. Assessment of cardiac inflammation and remodeling during the development of streptozotocin-induced diabetic cardiomyopathy in vivo: a time course analysis. IntJ Mol Med. 2013;32(1):158–164. doi:10.3892/ijmm.2013.1368
42. Ivanova E, Kovacs-Oller T, Sagdullaev BT. Vascular pericyte impairment and connexin43 gap junction deficit contribute to vasomotor decline in diabetic retinopathy. J Neurosci. 2017;37(32):7580–7594. doi:10.1523/JNEUROSCI.0187-17.2017
43. Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology. 2017;24(4):229–241. doi:10.1016/j.pathophys.2017.07.001
44. Simó R, Simó-Servat O, Bogdanov P, Hernández C. Neurovascular unit: a new target for treating early stages of diabetic retinopathy. Pharmaceutics. 2021;13(8):1320. doi:10.3390/pharmaceutics13081320
45. Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of Corticosteroids for Diabetic Macular Edema. Invest Ophthalmol Vis Sci. 2018;59(1):1. doi:10.1167/iovs.17-22259
46. Stewart MW. Treatment of diabetic retinopathy: recent advances and unresolved challenges. World J Diabetes. 2016;7(16):333. doi:10.4239/wjd.v7.i16.333
47. Tang L, Xu GT, Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023;18(5):976. doi:10.4103/1673-5374.355743
48. Chernykh V, Varvarinsky E, Smirnov E, Chernykh D, Trunov A. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol. 2015;63(1):33. doi:10.4103/0301-4738.151464
49. Boss JD, Singh PK, Pandya HK, et al. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(12):5594. doi:10.1167/iovs.17-21973
50. Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J of Cellular Biochem. 2016;117(11):2443–2453. doi:10.1002/jcb.25575
51. Vinores SA, Xiao WH, Shen J, Campochiaro PA. TNF-α is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J Neuroimmunol. 2007;182(1–2):73–79. doi:10.1016/j.jneuroim.2006.09.015
52. Huang H, Gandhi JK, Zhong X, et al. TNFα Is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52(3):1336. doi:10.1167/iovs.10-5768
53. Yun JH. Interleukin-1β induces pericyte apoptosis via the NF-κB pathway in diabetic retinopathy. Biochem Biophys Res Commun. 2021;546:46–53. doi:10.1016/j.bbrc.2021.01.108
54. Yao Y, Li R, Du J, Long L, Li X, Luo N. Interleukin-6 and diabetic retinopathy: a systematic review and meta-analysis. Curr Eye Res. 2019;44(5):564–574. doi:10.1080/02713683.2019.1570274
55. Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semi Ophthalmol. 1999;14(4):233–239. doi:10.3109/08820539909069542
56. Maisto R, Gesualdo C, Trotta MC, et al. Melanocortin receptor agonists MCR 1-5 protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture. J Cell Mol Med. 2017;21(5):968–974. doi:10.1111/jcmm.13036
57. Rossi S, Maisto R, Gesualdo C, et al. Activation of melanocortin receptors MC 1 and MC 5 attenuates retinal damage in experimental diabetic retinopathy. Mediators Inflammation. 2016;2016:1–13. doi:10.1155/2016/7368389
58. Danta CC, Boa AN, Bhandari S, Sathyapalan T, Xu SZ. Recent advances in drug discovery for diabetic kidney disease. Expert Opin Drug Discov. 2021;16(4):447–461. doi:10.1080/17460441.2021.1832077
59. Koch A, Pfeilschifter J, Huwiler A. Sphingosine 1-phosphate in renal diseases. Cell Physiol Biochem. 2013;31(6):745–760. doi:10.1159/000350093
60. Deng Y, Lan T, Huang J, Huang H. Sphingosine Kinase-1/sphingosine 1-phosphate pathway in diabetic nephropathy. Chin Med J. 2014;127(16):3004–3010.
61. Kurano M, Tsukamoto K, Shimizu T, Hara M, Yatomi Y. Apolipoprotein M/sphingosine 1-phosphate protects against diabetic nephropathy. Transl Res. 2023;258:16–34. doi:10.1016/j.trsl.2023.02.004
62. Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290(6):F1516–F1524. doi:10.1152/ajprenal.00311.2005
63. Bajwa A, Jo SK, Ye H, et al. Activation of Sphingosine-1-Phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21(6):955–965. doi:10.1681/ASN.2009060662
64. Awad AS, Rouse MD, Khutsishvili K, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011;79(10):1090–1098. doi:10.1038/ki.2010.544
65. Esposito K, Maiorino MI, Bellastella G. Diabetes and sexual dysfunction: current perspectives. Diab Metab Synd Obes. 2014;95. doi:10.2147/DMSO.S36455
66. Cui K, Ruan Y, Wang T, et al. FTY720 supplementation partially improves erectile dysfunction in rats with streptozotocin-induced type 1 diabetes through inhibition of endothelial dysfunction and corporal fibrosis. J Sex Med. 2017;14(3):323–335. doi:10.1016/j.jsxm.2017.01.006
67. Ennis GE, Saelzler U, Umpierrez GE, Moffat SD. Prediabetes and working memory in older adults. Brain Neurosci Adv. 2020;4:239821282096172. doi:10.1177/2398212820961725
68. Miguez A, García-Díaz Barriga G, Brito V, et al. Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75 NTR up-regulation and astrocyte-mediated inflammation. Hum Mol Genet. 2015;24(17):4958–4970. doi:10.1093/hmg/ddv218
69. Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs. 2016;30(2):135–147. doi:10.1007/s40263-015-0297-0
70. Bilbo S, Stevens B. Microglia: the brain’s first responders. Cerebrum. 2017;2017:14–17.
71. Rom S, Zuluaga-Ramirez V, Gajghate S, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both Diabetes Mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol. 2019;56(3):1883–1896. doi:10.1007/s12035-018-1195-5
72. Sood A, Fernandes V, Preeti K, Khatri DK, Singh SB. Sphingosine 1 phosphate lyase inhibition rescues cognition in diabetic mice by promoting anti-inflammatory microglia. Behav Brain Res. 2023;446:114415. doi:10.1016/j.bbr.2023.114415
73. Lucaciu A, Brunkhorst R, Pfeilschifter J, Pfeilschifter W, Subburayalu J. The S1P–S1PR axis in neurological disorders—insights into current and future therapeutic perspectives. Cells. 2020;9(6):1515. doi:10.3390/cells9061515
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
