Back to Journals » Journal of Inflammation Research » Volume 16
Therapeutic Effects of HLA-G5 Overexpressing hAMSCs on aGVHD After Allo-HSCT: Involving in the Gut Microbiota at the Intestinal Barrier
Authors Bu X, Pan W, Wang J , Liu L, Yin Z, Jin H , Liu Q, Zheng L, Sun H, Gao Y, Ping B
Received 1 June 2023
Accepted for publication 11 August 2023
Published 24 August 2023 Volume 2023:16 Pages 3669—3685
DOI https://doi.org/10.2147/JIR.S420747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Xiaoyin Bu,1,2,* Weifeng Pan,1,* Junhui Wang,1 Liping Liu,1 Zhao Yin,1 Hua Jin,1 Qifa Liu,1 Lei Zheng,3 Haitao Sun,4 Ya Gao,3 Baohong Ping1,2
1Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 2Department of Hematology, Huiqiao Medical Center, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 3Department of Laboratory Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 4Department of Laboratory Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Baohong Ping, Department of Hematology, Huiqiao Medical Center, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China, Tel +86 15625042109, Fax +86 2062781875, Email [email protected] Ya Gao, Department of Laboratory Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China, Email [email protected]
Background: Acute graft-versus-host disease (aGVHD) initiated by intestinal barrier dysfunction and gut microbiota dysbiosis, remains one of the main obstacles for patients undergoing allogenic hematopoietic stem cell transplantation (allo-HSCT) to achieve good prognosis. Studies have suggested that mesenchymal stem cells (MSCs) can suppress immune responses and reduce inflammation, and human leukocyte antigen-G5 (HLA-G5) plays an important role in the immunomodulatory effects of MSCs, but very little is known about the potential mechanisms in aGVHD. Thus, we explored the effect of HLA-G5 on the immunosuppressive properties of human amnion MSCs (hAMSCs) and demonstrated its mechanism related to the gut microbiota at the intestinal barrier in aGVHD.
Methods: Patients undergoing allo-HSCT were enrolled to detect the levels of plasma-soluble HLA-G (sHLA-G) and regulatory T cells (Tregs). Humanized aGVHD mouse models were established and treated with hAMSCs or HLA-G5 overexpressing hAMSCs (ov-HLA-G5-hAMSCs) to explore the mechanism of HLA-G5 mediated immunosuppressive properties of hAMSCs and the effect of ov-HLA-G5-hAMSCs on the gut microbiota at the intestinal barrier in aGVHD.
Results: The plasma levels of sHLA-G on day +30 after allo-HSCT in aGVHD patients were lower than those in patients without aGVHD, and the sHLA-G levels were positively correlated with Tregs percentages. ov-HLA-G5-hAMSCs had the potential to inhibit the expansion of CD3+CD4+ T and CD3+CD8+ T cells and promote Tregs differentiation, suppress proinflammatory cytokine secretion but promote anti-inflammatory cytokines release. Besides, ov-HLA-G5-hAMSCs also could reverse the intestinal barrier dysfunction and gut microbiota dysbiosis in aGVHD.
Conclusion: We demonstrated that HLA-G might work with Tregs to create a regulatory network together to reduce the occurrence of aGVHD. HLA-G5 mediated hAMSCs to exert higher immunosuppressive properties in vivo and reverse the immune imbalance caused by T lymphocytes and cytokines. Furthermore, HLA-G5 overexpressing hAMSCs could restore gut microbiota and intestinal barriers, thereby ameliorating aGVHD.
Keywords: acute graft-versus-host disease, amniotic mesenchymal stem cells, human leukocyte antigen-G5, gut microbiota, intestinal barrier
Introduction
Allogenic hematopoietic stem cell transplantation (allo-HSCT) is a promising cellular therapy to cure various hematological malignancies, non-hematological malignancies and some genetic and metabolic diseases.1 However, acute graft-versus-host disease (aGVHD), in which donor-derived T cells recognize recipient tissues as heterologous, resulting in high morbidity and mortality, remains the main cause of short-term death after allo-HSCT and the major obstacle to allo-HSCT.2 Systemic corticosteroids are still the standard first-line therapy for aGVHD, whereas 35~50% of patients are refractory to steroid therapy.3 There was no uniformly adopted or approved second-line therapy for patients with steroid-refractory aGVHD (SR-aGVHD), whose 2-year overall survival is only 25%.4,5 It is thus the impetus to develop more effective strategies for this devastating disease while maintaining graft-versus-leukemia (GVL) effects to avoid a potential increase in recurrence-related mortality. The gastrointestinal tract, harboring a dense and diverse microbial community, is the primary severely affected target organ by aGVHD. Under physiological conditions, the crosstalk between the intestinal barrier, immunity, gut microbiota and its metabolites is in a steady state, maintaining intestinal homeostasis and protecting against pathogens.6 During allo-HSCT, antibiotics and conditioning regimen including radiotherapy and chemotherapy result in the brokenness of intestinal homeostasis including intestinal barrier damage and gut microbiota dysbiosis. Antigen presenting cells recognize gut microbiota components, which induce T cell immune imbalance and inflammatory cascade to exacerbate tissue damage and promote aGVHD development.7 Thus, it is the imputes to maintain the homeostasis in the intestines to reduce aGVHD.
Mesenchymal stem cells (MSCs) are adult progenitor cells found in bone marrow, amniotic membrane, umbilical cord, adipose tissue and other postnatal tissues and organs.8 Nowadays, stem cell therapy with MSCs has aroused great interest as a treatment strategy for immunomodulatory and immune-mediated disorders, including GVHD, systemic lupus erythematosus and arthritis.9 Human amniotic mesenchymal stem cells (hAMSCs) are a type of novel MSCs derived from post-partum placenta, a medical waste, which could be obtained easily, massively and safely without invasive procedures and ethical or moral disputes compared with bone marrow mesenchymal stem cells (BM-MSCs), making them an ideal source of MSCs for GVHD therapy.10 We have demonstrated that hAMSCs are yet a favorable therapeutic strategy beneficial to aGVHD,11 and the gut microbiota could be altered after hAMSCs treatment,12 but the mechanism remains unclear.
Human leukocyte antigen-G (HLA-G), a functional non-classical HLA class Ib molecule, plays a critical role in inducing immune tolerance. HLA-G is reported to exert immunomodulatory properties and induce peripheral tolerance by directly interacting with its connate inhibitory receptors expressing on T cells, dendritic cells, mononuclear cells and other immune cells.13,14 Thus, due to its ability to inhibit innate and adaptive immune responses and its tolerogenic effects, HLA-G has become attractive to the fields of reproduction, infections, autoimmune diseases and organ transplantation.15–18 HLA-G5, due to its unique structure, is a key molecular subtype in the HLA-G family that plays an immunosuppressive role.19,20 Although studies have shown that the immunomodulatory functions of MSCs are mainly mediated by immunosuppressive molecules secreted by MSCs, the immunoregulatory mechanisms are still not fully elucidated.21,22 It was found that MSCs not only express HLA-G proteins but also secrete soluble HLA-G5, which mediates and participates in a variety of immunomodulatory effects of MSC in vitro.23–26 However, whether hAMSCs alleviate aGVHD through HLA-G5 and their mechanisms concerning gut microbiota in the intestinal barrier are rarely reported in allo-HSCT.
In this study, we first observed the expression and the role of HLA-G in aGVHD patients after allo-HSCT. We then established humanized aGVHD mouse models to explore the effect of HLA-G5 on the immunosuppressive properties of hAMSCs and the alterations of gut microbiota to demonstrate its mechanism in aGVHD post-HSCT.
Materials and Methods
Patients and Study Design
Thirteen patients who underwent allo-HSCT at the Department of Hematology, Nanfang Hospital of Southern Medical University from May 2020 to December 2020 were consented and enrolled in the study. The included patients were aged between 14 and 62. Five patients received a reduced intensity conditioning and eight received a myeloablative conditioning. All patients were given standard immunosuppressive prophylaxis with cyclosporine, methotrexate and mycophenolate mofetil with or without ATG depending on the type of donor and the source of cells. Among them, seven patients suffered aGVHD (herein aGVHD group) within days +100 after transplantation, while six patients did not (herein non-aGVHD group). The detailed clinical characteristics of patients were presented in Table S1. Additionally, 6 healthy volunteers were included as control (control group). Ethylenediaminetetraacetate plasma samples were collected from patients before transplantation, on days +15 and +30 after transplantation, as well as from healthy volunteers. Within 24 hours after collection, the plasmas were obtained by a 20 min 3000 rpm centrifugation at 4°C, and stored at −20°C until required.
Cell Isolation, Culture and Validation of hAMSCs
hAMSCs were isolated, cultured and validated as previously described.11 Briefly, the human amniotic membrane was collected from postnatal placenta of healthy pregnant women with consent, and hAMSCs were extracted from the amniotic membrane by tissue block culture. After incubating hAMSCs with antibodies against CD105 (eBioscience, Clone: SN6), CD73 (BD Pharmingen, Clone: AD2), CD90 (BD Pharmingen, Clone: 5E10), CD34 (BD Pharmingen, Clone: 581), CD45 (BD Pharmingen, Clone: HI30), CD11b (BioLegend, Clone: ICRF44), and HLA-DR (BD Pharmingen, Clone: G46-6), the surface markers of hAMSCs were identified using flow cytometry analysis (BD FACS Canto II). hAMSCs adipogenic and osteogenic differentiation potential were assessed after cultured in adipogenic and osteogenic differentiation media (BGscience, Chian), respectively. hAMSCs from 2 to 6 passages were used for the experiments.
Lentiviral Transduction
Lenti-B2M-HLA-G5-2AG lentiviruses were constructed and packaged by Guangzhou Shanze Biotechnology Co., Ltd. hAMSCs were seeded in 10cm petri dishes in advance, and passaged at 1:3 when they reached 85–90% confluence. Refresh culture medium with α-MEM (Gibco, USA) plus 10% fetal bovine serum (FBS, Gibco, USA), 1% Penicillin–Streptomycin (100U/mL, Thermo Fisher), 1% L-Glutamine (200mM Thermo Fisher, USA), 1% non-essential amino acids (100mM Thermo Fisher, USA) and 1% sodium pyruvate (100mM, Thermo Fisher, USA). Add the corresponding virus into each petri dish based on the multiplicity of infection of 20. Extract virus-containing culture medium at 12 h and replace it with fresh culture medium. Then continue to cultivate at 37°C for 48 hours and detect the expression of green fluorescent protein by fluorescence microscopy and flow cytometry. Stably transduced cell lines were used for the following experiments.
Western Blotting
Proteins were extracted from hAMSCs and HLA-G5 overexpressing hAMSCs (ov-HLA-G5-hAMSCs) using a cell lysis buffer (Keygen, China). The total protein concentrations were measured with the Pierce BCA Protein Assay Kit (Thermo Fisher, USA) according to the manufacturer’s recommendations. The samples were then boiled at 100°C for 10 min. Proteins (10 μg) were electrophoresed and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in 5% bovine serum albumin, the membranes were then incubated with primary antibodies against HLA-G5 (1:1000, Abcam, UK) or β-tubulin (1:5000, Affinity, China) overnight at 4°C. The membranes were washed with tris-buffered saline with Tween (TBST), then incubated with the corresponding secondary antibody goat anti-mouse IgG (H+l)-HRP (1:5000, Fdbio science, China) for 1 h at room temperature, and then washed again with TBST. Signals were visualized using an ECL detection kit (Fedbio Science, China) and protein bands were quantified using GelView 6000 ProII (Guangzhou Biolight Biotechnology Co., Ltd., China). Images were analyzed using ImageJ.
Murine aGVHD Model and Treatment
NPG mice (eight to ten weeks old and 25 to 30 g) were purchased from Beijing Vitalstar Biotechnology Co., Ltd. and housed in a SPF environment at a cycle of 12:12 light/dark. Humanized aGVHD mouse models induced by irradiation and human peripheral blood mononuclear cells (PBMCs) injection were constructed using an established protocol in our group.11 Briefly, NPG mice were randomly divided into four groups, namely the Control group, aGVHD group, hAMSCs group and HLA-G5 group, and there were 14 mice in each group apart from 4 mice in the Control group. Mice in aGVHD, hAMSCs and HLA-G5 groups were irradiated followed by 3 × 106 PBMCs injection by tail vein after 3–4 h. On the third day after transplantation, mice in each group were given a tail vein infusion of 5 × 105 hAMSCs (hAMSCs group), 5 × 105 HLA-G5 overexpressing hAMSCs (HLA-G5 group) or 500 μL PBS (aGVHD group and Control group). After the treatment, 10 mice were randomly picked out from each group to observe mouse body weight, aGVHD symptoms and survival every two days to plot body weight curve, aGVHD clinical scoring curve and survival curve until death of mice or termination of the study at day 28. Based on aGVHD clinical scoring system adapted from Cooke’s description,27 each mouse was graded from 0 to 12. Meanwhile, 4 mice in the Control group, the remaining 4 aGVHD mice suffered from severe aGVHD (weight loss of 25%, severe hunched posture, severe ruffled fur, severe skin lesions, less or no mobility, or hematochezia) and the mice in the two treatment groups (hAMSCs group and HLA-G5 group) on the sixth day after transplantation were euthanized to obtain aGVHD target organs and peripheral blood. Each experiment was performed using PBMCs from a single donor. The experiment was repeated three times.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of soluble HLA-G (sHLA-G) in plasmas and HLA-G5 in cell culture and organ tissue supernatants as well as plasmas of mice were measured using the human ELISA kits (CUSABIO, USA; Jingmei, China), and plasma levels of D-Lactate (D-LA) and Diamine oxidase (DAO) in mice were detected using the mouse ELSIA kits (Jingmei, China), respectively, according to the manufacturer’s instructions. Measure the absorbance at 450 nm using a microplate reader (Multiskan MK3, Thermo Fisher Scientific).
Histological and Immunohistochemical Staining
Collect mouse target organ tissues including livers, spleens, lungs and intestines at the time of necropsy, fix them in 10% buffered formalin, and embed them in paraffin. Cut the tissues into 5-μm-thick slices and stain then with hematoxylin and eosin (H&E). Based on the degree of tissue destruction and lymphocyte infiltration, histopathological scores of 0, 1, 2, 3 and 4 were performed on each organ, respectively, corresponding to normal, mild, moderate, severe, and extremely severe.28 For immunohistochemistry, 2 μm tissue sections were incubated with anti-human CD45 antibody or ZO-1 antibody (Abcam, England) for 1 hour at 37°C, then rinsed with PBS for 5 times, and incubated with the secondary antibody goat anti-rabbit IgG antibody-HRP (Genetech, USA) at 37°C for 30 min. Develop with DAB for 2 min after washing with PBS. The positive area was analyzed by ImageJ.
Flow Cytometric Analysis
Mouse anti-human CD3-PE-Cyanine7 (BioLegend, Clone: UCHT1), CD4-FITC (BioLegend, Clone: RPA-T4), CD8-PE (BioLegend, Clone: RPA-T8), CD25-APC (BioLegend, Clone: BC96), Foxp3-PE (eBioscience, Clone: 236A/E7), IgG1-PE (eBioscience, Clone: P3.6.2.8.1), IgG1-FITC (eBioscience, Clone: P3.6.2.8.1), IgG2b-PE-Cyanine7 (BD Pharmingen, Clone: A95-1) and IgG2a-APC (eBiocience, Clone: eBR2a) antibodies were used for flow cytometry analysis. Patients’ blood samples as well as peripheral blood and single-cell suspension of target organs of mice at the time of necropsy were analyzed by flow cytometry. Briefly, red blood cells were treated with Lysing Buffer (BD Pharm LyseTM) at 4°C for 15 min according to the protocol. Intracellular Foxp3 staining was performed according to the protocol (Fixation/Permeabilization Solution Kit; eBioscience). All samples were incubated with antibodies or isotype matched control IgG for 30 min in the dark. Then, flow cytometric analyses were performed on the Flow Cytometer (BD FACS Canto II) and further analyzed using FlowJo 10.0.
Cytokine Examination
The single-cell suspension of target organs and peripheral blood collected at the time of necropsy were centrifuged and the supernatants were collected and stored at −80°C until required. The levels of cytokines in the tissue supernatant and peripheral blood were measured using Cytometric Bead Array (CBA) Human Th1/Th2/Th17 cytokine kit (BD Pharmingen, USA) according to the manufacturer’s protocol, and analyzed by flow cytometry.
16S rRNA Sequencing
The feces in all groups were collected before sacrifice for further analysis. Fecal genomic DNA extraction and 16S rRNA sequencing were conducted by Magigene Technology Co., Ltd. (Guangzhou, China) and analyzed the data using Magichand platform (http://cloud.magigene.com/).
Quantitative Real-Time PCR (RT-qPCR)
The intestinal tissues were lysed with Trizol Reagent (TaKaRa, China) to extract total RNA. Reverse-transcribed cDNA using total RNA as a template. The data was normalized to the expression of β-actin. The primer sequences were as follows: ZO-1-F, 5’-ACCACCAACCCGAGAAGAC-3’, and ZO-1-R, 5’-CAGGAGTCA TGGACGCACA-3’; Occludin-F, 5’-TTGAAAGTCCACCTCCTTACAGA-3’, and Occludin-R, 5’-CCGGATAAAAAGAGTACGCTGG-3’; β-actin-F, 5’-GGCTGTATTCCCCTCCATCG-3’, and β-actin-R, 5’-CCAGTTGGTAACAATGCCATGT-3’. The mRNA expression levels were measured using the 2−ΔΔCt method.
Statistical Analysis
Statistical analyses and presentation were performed using GraphPad Prism 8.0. The data were presented as the means ± standard deviation (SD). Comparisons between two means were evaluated using the independent samples t-test. Multiple comparisons were performed using one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison tests. Receiver operating characteristic (ROC) analysis and area under curve (AUC) were performed to define the optimal threshold value of sHLA-G to predict the sensitivity and specificity of aGVHD. Survival curves were generated with Kaplan–Meier method. Statistical significance was defined as p < 0.05.
Results
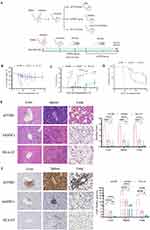
Soluble HLA-G Expression in aGVHD After Allo-HSCT
As shown in Figure 1A, the sHLA-G levels were independent of the patients’ sex, age and disease status including disease diagnosis and curative effect evaluation pre-HSCT. There was no obvious difference in sHLA-G levels among healthy volunteers (control group), aGVHD patients and non-aGVHD patients before transplantation (Figure 1B). On day +15 after transplantation, the sHLA-G plasma level between aGVHD group and non-aGVHD group was not significantly different (Figure 1C). However, the level of sHLA-G on day +30 in aGVHD group was lower than that in non-aGVHD group (p < 0.05) (Figure 1D). To evaluate whether sHLA-G could serve as an early biomarker for predicting aGVHD, ROC analysis was used to define the optimal thresholds for sensitivity and specificity of sHLA-G levels obtained pre-HSCT, +15 and +30 days post-HSCT (Figure 1E–G). The sHLA-G cut-off levels of pre-HSCT (Figure 1E) and day +15 (Figure 1F) were not related to aGVHD, while sHLA-G cut-off level of day +30 (cut off 4.275 ng/mL, sensitivity 85.71%, specificity 83.33%, AUC 0.8571; p = 0.0321) was associated with aGVHD post-HSCT (Figure 1G). As sHLA-G level was increased on day +30 post-HSCT in patients without aGVHD compared to aGVHD patients, we wondered whether T cells were influenced by sHLA-G secretion. As shown in Figure 1H, the sHLA-G level was positively correlated with the percentage of CD4+CD25+Foxp3+T regulatory cells (Tregs) on day +30. These results suggested the induction of transplantation tolerance in patients without aGVHD, as validated by high levels of both sHLA-G and Tregs in the periphery.
Identification of hAMSCs
hAMSCs were purified from human amniotic membrane and characterized via morphological properties and differentiation to adipocytes and osteocytes. They displayed adherent growth characteristics of primary culture cells and exhibited the expected spindle or polygon shape in morphology (Figure 2A and B). Intracytoplasmic lipid vacuoles and droplet accumulation and a high degree of mineral deposits verified the great potential for adipocytic and osteoblastic differentiation of hAMSCs (Figure 2C and D). Furthermore, hAMSCs expressed typical MSCs phenotypic markers, as previously defined by the International Society for Cellular Therapy (ISCT),29 being strongly positive for CD90, CD105 and CD73, but negative for CD45, CD34, HLA-DR and CD11b (Figure 2E), confirming that the isolated cells retained the characteristics of MSCs.
hAMSCs That Overexpressing HLA-G5 Alleviated aGVHD
The authors confirmed that patients without aGVHD had higher sHLA-G levels post-HSCT compared to aGVHD patients so as to implicate that sHLA-G molecules could be a participant in the process of aGVHD prevention. HLA-G5, as a significant anti-inflammatory and immunosuppressive molecule in HLA-G family, could mediate hAMSCs to play a crucial role in immunomodulatory function in vitro. The authors also declared that hAMSCs could mitigate aGVHD by regulating T cell immune balance in vivo.11 Therefore, the author established hAMSCs overexpressing HLA-G5 to verify whether they have an effective therapy for aGVHD in vivo. A schematic of the animal experimental design is shown in Figure 3A, and examination of the expression of HLA-G5 protein in hAMSCs and lentivirus transfected hAMSCs through Western Blot and ELISA was shown in Figure S1. As expected, hAMSCs and ov-HLA-G5-hAMSCs administration significantly attenuated the aGVHD severity, as demonstrated by less body weight loss (Figure 3B), lower aGVHD clinical score (Figure 3C) and better survival (Figure 3D) at all the time points observed. Although the body weight loss showed a comparable tendency between hAMSCs group and HLA-G5 group, ov-HLA-G5-hAMSCs-treated aGVHD mice displayed lower aGVHD clinical score and better survival compared with hAMSCs-treated mice (Figure 3B–D). Less leukocyte infiltration accompanied by milder pathological tissue damage in the target organs in ov-HLA-G5-hAMSCs-treated aGVHD mice was also remarkable according to H&E staining of the histology score (Figure 3E). These results were consistent with those of immunohistochemistry experiments (Figure 3F). Taken together, these findings showed that ov-HLA-G5-hAMSCs treatment were effective in ameliorating aGVHD.
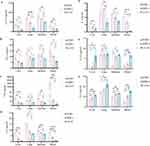
HLA-G5 Mediated hAMSCs to Exert Higher Immunomodulatory Properties in vivo
To explore the in vivo immunosuppressive effect of HLA-G5 mediated hAMSCs treatment, mice were immunophenotyped after transplantation. We first examined the level of HLA-G5 secretion in the peripheral blood and target organs among the groups. The secretion of HLA-G5 in the supernatants of the peripheral blood and the target organs in HLA-G5 group was higher than that in the aGVHD group and hAMSCs group (Figure S2). As shown in Figures 4A and B and Figures S3 and 4, the percentages of CD3+CD4+ T cells (Figure 4A) and CD3+CD8+ T cells (Figure 4B) in peripheral blood and target organs were increased in hAMSCs group compared with those in aGVHD group. Furthermore, there was a dramatical decrease in the percentages of CD3+CD4+ T cells and CD3+CD8+ T cells in peripheral blood and target organs except for CD3+CD4+ T cells in the intestines of the mice treated with ov-HLA-G5-hAMSCs compared with those treated with hAMSCs (Figures 4A and B and Figures S3 and 4). Of note, the percentage of CD3+CD4+T cells in HLA-G5 group was also decreased in the intestines compared to hAMSCs group, although the difference was not statistically significant. Additionally, hAMSCs infusion significantly increased the percentage of Tregs derived from donor CD4+T cells in the target organs and blood in aGVHD mice except for Tregs in the livers (Figure 4C and Figure S5). In the meantime, overexpression of HLA-G5 in hAMSCs significantly enhanced their promotion effect on Tregs (Figure 4C and Figure S5). Thus, the enhancement of the efficacy of hAMSCs on aGVHD by HLA-G5 is through inhibiting CD3+CD4+ and CD3+CD8+ T cells response and promoting Tregs differentiation. Next, we detected the levels of various cytokines in vivo (Figure 5). Compared to aGVHD group, the levels of proinflammatory cytokines IL-17A (Figure 5A), IFN-γ (Figure 5B), TNF (Figure 5C), IL-6 (Figure 5D) and IL-2 (Figure 5E) in the target organs and blood in HLA-G5 group were decreased significantly except for IFN-γ in the intestine. We also observed that the levels of these proinflammatory cytokines in HLA-G5 group were lower than those in the hAMSCs group, in addition to IL-17A in the blood, IFN-γ in the liver and lung, and TNF in the liver. As for anti-inflammatory cytokines, ov-HLA-G5-hAMSCs treatment increased the secretion of IL-10 (Figure 5F) and IL-4 (Figure 5G) in the target organs and blood, apart from IL-10 in the lung. These results demonstrated that HLA-G5 could enhance the immunosuppressive effect of hAMSCs on the secretion of proinflammatory cytokines but promote anti-inflammatory cytokines secretion. Taken together, overexpressing of HLA-G5 in hAMSCs improved their immunomodulatory properties in aGVHD.
 |
Figure 4 Overexpression of HLA-G5 in hAMSCs inhibited CD3+CD4+ T cells and CD3+CD8+ T cells expansion while promoted Tregs expansion in aGVHD mice. Notes: Mice in aGVHD that suffered severe aGVHD and mice in hAMSCs and HLA-G5 groups on day 6 after transplantation were sacrificed to harvest target organs and peripheral blood to analyze frequencies of T cell subsets. (A–C) The percentages of CD3+CD4+ T cells (A), CD3+CD8+ T cells (B) and Tregs (C). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns, no significance, based on the One-way ANOVA and Tukey’s post hoc test. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, based on the Student’s t-test. n = 4 per group. The data were representative of three independent experiments. See also Figures S3–5. |
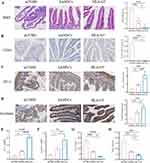
Intestinal Barrier Dysfunction and Gut Microbiota Disorder Were Reversed Upon Treatment in aGVHD Mice
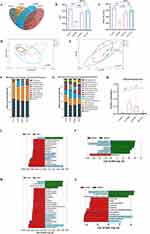
We next evaluated the impact of hAMSCs or ov-HLA-G5-hAMSCs on the intestinal barrier. As shown in Figure 6A and B, aGVHD mice displayed tissue damage and infiltration of leukocytes, while no or less such defects were observed in the intestine of the hAMSCs group or HLA-G5 group. Additionally, the protein and mRNA expression levels of intestinal tight junction (TJ) proteins ZO-1 and Occludin were lower in the aGVHD group, whereas hAMSCs or ov-HLA-G5-hAMSCs treatment increase their expression levels, and the HLA-G5 group showed the most obvious increase (Figure 6C–F). As indices of intestinal permeability, the plasma levels of D-LA and DAO in the hAMSCs or HLA-G5 group were obviously higher than those in the aGVHD group, with the HLA-G5 group showing the most significant increase (Figure 6G and H). Thus, hAMSCs treatment could reduce intestinal tissue damage and promote the recovery of the intestinal tight junction proteins and permeability, and such effect in HLA-G5 group was better. We further investigated the alternation of the gut microbiota at the intestinal barrier through 16S rRNA sequencing. The α-diversity of the gut microbiota was measured by operational taxonomic units (OUT) and Chao1 index (Figure 7A–C). A total of 1055 OTUs were observed (Figure 7A), and the OUT (Figure 7B) and Chao1 index (Figure 7C) showed that the α-diversity of the gut microbiota in the aGVHD group was lower than that in the Control group, and both of these were higher in the hAMSCs group and HLA-G5 group compared with aGVHD group. In terms of β-diversity of gut microbiota, the principal coordinate analysis (PCoA) based on Bray–Curtis distance and ANOSIM displayed that the four groups, namely, Control group, aGVHD group, hAMSCs group and HLA-G5 group were dramatically different from each other (Figure 7D). And the result from principal component analysis (PCA) was consistent with the PCoA result (Figure 7E). The above results indicated the diversity of gut microbiota was lost when aGVHD occurred, and it can be increased after hAMSCs or ov-HLA-G5-hAMSCs treatment. Next, we explored the alternation of the gut microbiota composition. The bacteria with the highest abundance in all groups belonged to the phylum Firmicutes and Bacteroidetes (Figure 7F). At the family level, Muribaculaceae, Lactobacillaceae and Ruminococcaceae were the most predominant microbiota in the four groups (Figure 7G). As shown in Figure 7H, compared with Control group, the relative abundance of Akkermansiaceae was increased in aGVHD group, whereas decreased after ov-HLA-G5-hAMSCs administration. Furthermore, we performed Line Discriminant Analysis (LDA) Effect Size (LEfSe) analysis to find out the differences among groups. At the genus level, the Control group harbored the beneficial bacteria such as Lachnospiraceae_NK4A136_group, Roseburia and Ruminiclostridium, and the genus Akkermansia expansion was one of the key characterizations causing gut microbiota dysbiosis in the aGVHD group (Figure 7I). After hAMSCs or ov-HLA-G5-hAMSCs treatment, the beneficial bacteria mainly Ruminiclostridium, Lachnospiraceae_NK4A136_group and Roseburia displayed a relative enrichment (Figure 7J and K), which was similar to the composition of Control group and might be involved in the intestinal barrier repair. Compared with the hAMSCs group, the HLA-G5 group had a higher abundance of Bifidobacterium and Lachnospiraceae_NK4A136_group, and lower abundance of Ruminococcaceae_UCG_014 (Figure 7L). Thus, the diversity and composition of the gut microbiota can be normalized beneficial to the body organism through hAMSCs or ov-HLA-G5-hAMSCs administration. Collectively, these results suggested that hAMSCs or ov-HLA-G5-hAMSCs might modulate the intestinal homeostasis, involved in the repair of intestinal barrier dysfunction and regulation of gut microbiota disorder.
Discussion
Allogeneic hematopoietic stem cell transplantation has the potential to cure malignant hematological disorders. Graft-versus-host disease is the most prevalent complication and the leading cause of mortality and morbidity following allo-HSCT. HLA-G, as a functional non-classical MHC class Ib molecule, has 7 different protein isoforms, among which HLA-G1 to HLA-G4 are membrane-bound proteins and HLA-G5 to HLA-G7 are soluble proteins.30 It has been proven that HLA-G5 has immunomodulatory properties and could induce the maintenance of tolerance, indicating its potential in the prevention and treatment of aGVHD.13 Previous studies have confirmed that a decrease in sHLA-G levels is correlated with moderate-to-severe aGVHD. Le Maux et al31 demonstrated that decreased sHLA-G levels were positively attached to aGVHD, involving in the aGVHD development. Nomura et al32 suggested that elevated sHLA-G participated in the pathophysiology and prevention of aGVHD. In a study involving 32 patients who underwent allo-HSCT, Kordelas et al33 indicated that increased sHLA-G level was associated with less severe aGVHD and was positively associated with the proportion of Tregs in patients. It was confirmed by Liu et al34 that HLA-G5 rather than HLA-G6 or HLA-G7 level might be served as a predictor of the development and severity of aGVHD. However, they nearly did not clarify the relationship between sHLA-G and T-cell immunity in patients, which may contribute to the aGVHD development. Herein, we found significantly decreased levels of sHLA-G on day +30 post-HSCT in patients with aGVHD compared with those without aGVHD. We also indicated that the level of sHLA-G on day +30 has the certain ability to predict the occurrence of aGVHD. Furthermore, the sHLA-G level was positively associated with Tregs percentage. Therefore, we speculated that elevated levels of sHLA-G might induce transplantation tolerance through Tregs expansion and might be served as a good predictive marker for aGVHD occurrence. However, it is worth noting that the number of patients enrolled in the study is relatively small, which is also a limitation of this study. Further verification was required in a larger patient cohort.
Mesenchymal stem cells possess the capacity to modulate immune or inflammation response, regulate the tissue microenvironment, repair tissue damage and support hematopoiesis, which have been widely used in regenerative medicine and autoimmune diseases treatment.35 We have previously shown that MSCs derived from human amnion could exhibit immunosuppressive ability in aGVHD by regulating the balance of Tregs and T effector cells,11 however, the corresponding mechanism by which hAMSCs participate in alleviating aGVHD has not yet been fully clarified. Accumulating evidence suggests that paracrine secretion is an effective pathway for MSCs to exert an immunomodulatory perspective to mitigate immune diseases such as aGVHD.36,37 MSCs have the ability to secrete soluble suppressive molecules such as HLA-G, which is known to have anti-proliferative effect on naïve T cells.36 It was shown that HLA-G played a critical role in inhibiting the allogeneic proliferative T cell response, CD4+ T cell activation directly and CD8+ T cells maturation.16,38 Selmani et al25 showed that HLA-G5 secreted by BM-MSCs was required to suppress allogeneic T-cell proliferation and then to promote Tregs expansion in vitro. Nasef et al23 also draw the same conclusion that BM-MSCs harbored HLA-G protein, which had immunosuppressive effect on lymphocytes proliferation. These studies all indicated that HLA-G5 expressed and secreted by MSCs contributed to the immunosuppressive perspective of MSCs in vitro. Therefore, we explored whether hAMSCs that overexpressing HLA-G5 had the same effect in a humanized aGVHD mouse model. Our results showed the positive therapeutic effects of overexpressing HLA-G5 in hAMSCs treated in aGVHD mice with decreased weight loss, reducing aGVHD clinical scores, improved survival and attenuated tissue damage. Yang et al39 indicated that human umbilical cord blood-derived MSCs overexpressing HLA-G could reduce aGVHD symptoms and prolong survival. However, this study did not investigate the effect of HLA-G/HLA-G5 on T lymphocytes and cytokines in target organs and peripheral blood of aGVHD mice. aGVHD is an immune-mediated process, driven by mature donor T lymphocytes, leading to cytokine cascade and the proliferation of activated T cells and their homing to target tissues, ultimately resulting in tissue damage.40,41 Donor CD8+ T cells alone are sufficient to induce aGVHD when MHC class I only is mismatched, and CD4+ T cells can cause GVHD by cognate interaction or can destruct tissues without homologous interactions by releasing pro-inflammatory cytokines such as TNF-α.4 In our research, when different T cell subsets were analyzed, we found that the percentages of CD3+CD4+ T cells and CD3+CD8+ T cells were markedly reduced in target organs in hAMSCs group and HLA-G5 group, which were more pronounced in aGVHD mice treated with hAMSCs overexpressing HLA-G5. Additionally, while arresting naïve T cells functionally differentiated into effector type, augment HLA-G5 expression in hAMSCs also retained the CD4+CD25+Foxp3+Tregs population. It is now widely recognized that activated T cells result in massive release of pro-inflammatory mediators, thereby amplifying the immune response and lead to tissue damage.4,41 HLA-G protein could enhance the expression of Th2 anti-inflammatory cytokines and decrease Th1 pro-inflammatory cytokines.38 Here, we found that the secretion of pro-inflammatory cytokines TNF-α, IFN-γ, IL-6 and IL-2 were decreased and anti-inflammatory cytokines IL-10 and IL-4 were increased in target organs and peripheral blood after hAMSCs treatment, and overexpression of HLA-G5 in hAMSCs significantly enhanced their immunosuppressive effect on pro-inflammatory cytokines but promotion effect on anti-inflammatory cytokines. Hence, we interpreted that HLA-G5 mediated hAMSCs-reduced activation of pathogenic T cells and hindered their migration to target organs while increasing Tregs generation, decreasing pro-inflammatory cytokines but increasing anti-inflammatory cytokines secretion, leading to lower inflammatory response and milder tissue damage, thus ameliorating aGVHD. Although studies have shown that hAMSCs exert immunomodulatory effects through their secretion factors, the potential mechanisms are not fully understood. Further in-depth research was needed to elucidate.
Intestinal homeostasis, a dynamic equilibrium state formed by the interplay of intestinal barrier, immunity and gut microbiota and their metabolites, prevents pathogen invasion and maintains intestinal health, which could be disrupted after allo-HSCT, leading to aGVHD occurrence and development.6 Under normal conditions, the intestinal barrier prevents gut microbiota invasion into deeper host tissues, and meanwhile, gut microbiota at the intestinal barrier maintains intestinal barrier integrity and shapes the mucosal immune system.7,42 Understanding the interaction between the intestinal barrier and gut microbiota is essential to maintain the intestinal homeostasis and may promote the advancement of aGVHD treatment.42 Our recent research has found that hAMSCs may improve aGVHD by regulating the interaction between gut microbiota and intestinal immunity,12 and therefore, we sought to explore whether overexpressing HLA-G5 in hAMSCs has the same effect on the gut microbiota. Here, we found that the hAMSCs had the capability to repair intestinal barrier damage, as manifested by the increased expressions of ZO-1 and Occludin as well as decreased plasma levels of D-LA and DAO, where these indicators represent the intestinal barrier function and intestinal permeability. We observed a loss of microbial diversity in aGVHD mice compared with control group, which could be improved after hAMSCs or ov-HLA-G5-hAMSCs treatment. In terms of microbial composition, in our research, we found that aGVHD mice had higher abundance of genus Akkermansia affiliated to the family Verrucomicrobiaceae compared with normal NPG mice, and ov-HLA-G5-hAMSCs administration could reduce the Akkermansia expansion induced by aGVHD. Akkermansia muciniphila is a commensal bacterium with the ability to degrade mucus, which can raise the possibility of mucus degradation. 16S rRNA sequencing of mouse stool samples by Shono et al43 showed that expansion of Akkermansia muciniphila induced by imipenem-cilastatin could cause the loss of the colonic mucus layer and compromised intestinal barrier function, thus contributing to murine GVHD, which was similar with our results. Yuan et al44 also drew the same conclusion that Akkermansiaceae promoted the decomposition of mucus layer. In contrast, Ilett et al45 observed that aGVHD patients harbored a low abundance of Akkermansia muciniphila using shotgun metagenomic sequencing. Zhu et al46 detected a low abundance of genus Akkermansia in the normal mice and not detected in the MSCs treatment group, but a high abundance in MSCs and endothelial progenitor cell combination infusion group. Given the above, it appears that Akkermansia is bound by intestinal homeostasis, but more details regarding the causations are needed. Whether Akkermansia expansion could exacerbate GVHD in humans remains an open question. In addition, hAMSCs or ov-HLA-G5-hAMSCs injection could increase the relative abundance of beneficial bacteria such as Ruminiclostridium, Lachnospiraceae_NK4A136_group and Roseburia, which all can produce short-chain fatty acids,47–49 one of the gut microbiota metabolites protecting intestinal barrier integrity.42 Therefore, under the function of hAMSCs or ov-HLA-G5-hAMSCs, the intestinal barrier dysfunction and gut microbiota disorder could be reshaped and then the intestinal homeostasis was restored.
Conclusion
In conclusion, we demonstrated that HLA-G might induce transplantation tolerance with Tregs to reduce the occurrence of aGVHD. Animal experiments showed that HLA-G5 overexpressing hAMSCs had greater suppression of immune responses induced by effector T cells and pro-inflammatory cytokines, of which process involved the reconstruction of gut microbiota and intestinal barrier, resulting in better therapeutic effect on aGVHD.
Ethics Approval and Consent to Participate
All experiments were performed in accordance with the institutional guidelines of Nanfang Hospital of Southern Medical University. The use of animals in this study was approved by Southern Medical University Institutional Animal Care and Use Committee (No. L2019132). Informed consent was obtained from all subjects and/or their legal guardian(s) under the ethical committee of the Nanfang Hospital of Southern Medical University.
Acknowledgments
This work was supported by Natural Nature Science Foundation of China (81870145, 82200234), Science and Technology Planning Project of Guangzhou (201904010481) and President Foundation of Nanfang Hospital, Southern Medical University (2022B008).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi:10.1016/S0140-6736(09)60237-3
2. Staffas A, Burgos da Silva M, van den Brink MRM. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129(8):927–933. doi:10.1182/blood-2016-09-691394
3. Malard F, Huang X, Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. 2020;34(5):1229–1240. doi:10.1038/s41375-020-0804-2
4. Zeiser R, Blazar BR. Acute graft-versus-host disease – biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–2179. doi:10.1056/NEJMra1609337
5. Hill GR, Koyama M. Cytokines and costimulation in acute graft-versus-host disease. Blood. 2020;136(4):418–428. doi:10.1182/blood.2019000952
6. Lin D, Hu B, Li P, et al. Roles of the intestinal microbiota and microbial metabolites in acute GVHD. Exp Hematol Oncol. 2021;10(1):49. doi:10.1186/s40164-021-00240-3
7. Chen Y, Zhao Y, Cheng Q, Wu D, Liu H. The role of intestinal microbiota in acute graft-versus-host disease. J Immunol Res. 2015;2015:145859. doi:10.1155/2015/145859
8. Bulati M, Miceli V, Gallo A, et al. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-γ produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front Immunol. 2020;11:54. doi:10.3389/fimmu.2020.00054
9. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi:10.1038/ni.3002
10. Liu Q, Huang Q, Wu H, et al. Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci. 2021;22(2):970. doi:10.3390/ijms22020970
11. Gao Y, Li W, Bu X, et al. Human amniotic mesenchymal stem cells inhibit aGVHD by regulating balance of treg and T effector cells. J Inflamm Res. 2021;14:3985–3999. doi:10.2147/JIR.S323054
12. Bu X, Wang J, Yin Z, et al. Human amniotic mesenchymal stem cells alleviate aGVHD after allo-HSCT by regulating interactions between gut microbiota and intestinal immunity. Stem Cell Rev Rep. 2023. doi:10.1007/s12015-023-10522-4
13. Bu X, Zhong J, Li W, et al. Immunomodulating functions of human leukocyte antigen-G and its role in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2021;100(6):1391–1400. doi:10.1007/s00277-021-04486-z
14. Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: at the Interface of Maternal-Fetal Tolerance. Trends Immunol. 2017;38(4):272–286. doi:10.1016/j.it.2017.01.009
15. González A, Rebmann V, LeMaoult J, et al. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49(3):63–84. doi:10.3109/10408363.2012.677947
16. Zaborek-łyczba M, Łyczba J, Mertowska P, et al. The HLA-G immune checkpoint plays a pivotal role in the regulation of immune response in autoimmune diseases. Int J Mol Sci. 2021;22(24). doi:10.3390/ijms222413348
17. Lin A, Yan W. Perspective of HLA-G Induced Immunosuppression in SARS-CoV-2 Infection. Front Immunol. 2021;12:788769. doi:10.3389/fimmu.2021.788769
18. Lila N, Amrein C, Guillemain R, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation. 2002;105(16):1949–1954. doi:10.1161/01.cir.0000015075.89984.46
19. Curigliano G, Criscitiello C, Gelao L, Goldhirsch A. Molecular Pathways: human Leukocyte Antigen G (HLA-G). Clin Cancer Res. 2013;19(20):5564–5571. doi:10.1158/1078-0432.CCR-12-3697
20. HoWangYin K, Loustau M, Wu J, et al. Multimeric structures of HLA-G isoforms function through differential binding to LILRB receptors. Cell Mol Life Sci. 2012;69(23):4041–4049. doi:10.1007/s00018-012-1069-3
21. Zoehler B, Fracaro L, Senegaglia AC, Bicalho MDG. Infusion of mesenchymal stem cells to treat graft versus host disease: the role of HLA-G and the impact of its polymorphisms. Stem Cell Rev Rep. 2020;16(3):459–471. doi:10.1007/s12015-020-09960-1
22. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi:10.1038/s41581-018-0023-5
23. Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237.
24. Deschaseaux F, Gaillard J, Langonné A, et al. Regulation and function of immunosuppressive molecule human leukocyte antigen G5 in human bone tissue. FASEB J. 2013;27(8):2977–2987. doi:10.1096/fj.13-227264
25. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T Lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem Cells. 2008;26(1):212–222. doi:10.1634/stemcells.2007-0554
26. Soontararak S, Chow L, Johnson V, et al. Mesenchymal Stem Cells (MSC) derived from Induced Pluripotent Stem Cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7(6):456–467. doi:10.1002/sctm.17-0305
27. Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239.
28. Yañez R, Lamana ML, García-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24(11):2582–2591.
29. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317.
30. Martín-Villa JM, Vaquero-Yuste C, Molina-Alejandre M, et al. HLA-G: too much or too little? Role in cancer and autoimmune disease. Front Immunol. 2022:13. doi:10.3389/fimmu.2022.796054
31. Le Maux A, Noël G, Birebent B, et al. Soluble human leucocyte antigen-G molecules in peripheral blood haematopoietic stem cell transplantation: a specific role to prevent acute graft-versus-host disease and a link with regulatory T cells. Clin Exp Immunol. 2008;152(1):50–56. doi:10.1111/j.1365-2249.2008.03598.x
32. Nomura S, Ito T, Katayama Y, et al. Effects of recombinant thrombomodulin therapy and soluble human leukocyte antigen-G levels during hematopoietic stem cell transplantation. Transpl Immunol. 2019;53:28–33. doi:10.1016/j.trim.2018.12.001
33. Kordelas L, Da Silva Nardi F, Wagner B, et al. Elevated soluble human leukocyte antigen G levels in patients after allogeneic stem cell transplantation are associated with less severe acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2018;53(9):1149–1156. doi:10.1038/s41409-018-0145-1
34. Liu H, Chen Y, Xuan L, et al. Soluble human leukocyte antigen G molecule expression in allogeneic hematopoietic stem cell transplantation: good predictor of acute graft-versus-host disease. Acta Haematol. 2013;130(3):160–168. doi:10.1159/000350488
35. Zhao K, Liu Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol. 2016;9(1):46. doi:10.1186/s13045-016-0276-z
36. Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28(8):1446–1455. doi:10.1002/stem.459
37. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi:10.1146/annurev-pathol-011110-130230
38. Liu S, Bos NA, Verschuuren EAM, van Baarle D, Westra J. Biological Characteristics of HLA-G and its role in solid organ transplantation. Front Immunol. 2022;13:902093. doi:10.3389/fimmu.2022.902093
39. Yang S, Wei Y, Sun R, et al. Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy. 2020;22(12):718–733. doi:10.1016/j.jcyt.2020.07.008
40. Zhang L, Chu J, Yu J, Wei W. Cellular and molecular mechanisms in graft-versus-host disease. J Leukoc Biol. 2016;99(2):279–287. doi:10.1189/jlb.4RU0615-254RR
41. Magenau J, Runaas L, Reddy P. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol. 2016;173(2):190–205. doi:10.1111/bjh.13959
42. Kayama H, Okumura R, Takeda K. Interaction between the microbiota, Epithelia, and immune cells in the intestine. Annu Rev Immunol. 2020;38:23–48. doi:10.1146/annurev-immunol-070119-115104
43. Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339r–371r. doi:10.1126/scitranslmed.aaf2311
44. Yuan M, Lin L, Cao H, et al. Intestinal microbiota participates in the protective effect of HO-1/BMMSCs on liver transplantation with steatotic liver grafts in rats. Front Microbiol. 2022;13:905567. doi:10.3389/fmicb.2022.905567
45. Ilett EE, Jørgensen M, Noguera-Julian M, et al. Associations of the gut microbiome and clinical factors with acute GVHD in allogeneic HSCT recipients. Blood Adv. 2020;4(22):5797–5809. doi:10.1182/bloodadvances.2020002677
46. Zhu S, Li H, Lv C, et al. Combination of mesenchymal stem cell and endothelial progenitor cell infusion accelerates injured intestinal repair by regulating gut microbiota after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(2):151–152. doi:10.1016/j.jtct.2020.10.013
47. Yuan Y, Liu S, Ding X, et al. Early intestinal microbiota changes in aged and adult mice with sepsis. Front Cell Infect Microbiol. 2022;12:1061444. doi:10.3389/fcimb.2022.1061444
48. Lee SH, Park H, Kang CD, et al. High dose intramuscular vitamin D3 supplementation impacts the gut microbiota of patients with clostridioides difficile infection. Front Cell Infect Microbiol. 2022;12:904987. doi:10.3389/fcimb.2022.904987
49. Cao Y, Liu H, Teng Y, et al. Gut microbiota mediates the anti-colitis effects of polysaccharides derived from Rhopilema esculentum Kishinouye in mice. Food Funct. 2023;14(4):1989–2007. doi:10.1039/d2fo02712g
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.