Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
The Value of Inflammatory Biomarkers in Differentiating Asthma–COPD Overlap from COPD
Authors Li M, Yang T , He R , Li A, Dang W, Liu X, Chen M
Received 27 July 2020
Accepted for publication 23 September 2020
Published 20 November 2020 Volume 2020:15 Pages 3025—3037
DOI https://doi.org/10.2147/COPD.S273422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Meng Li,* Tian Yang,* Ruiqing He, Anqi Li, Wenhui Dang, Xinyu Liu, Mingwei Chen
Department of Respiratory and Critical Care Medicine of the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingwei Chen
Department of Respiratory and Critical Care Medicine of the First Affiliated Hospital of Xi’an Jiaotong University, 277 West Yanta Road, Xi’an, Shaanxi 710061, People’s Republic of China
Tel/Fax +86-29-85323850
Email [email protected]
Purpose: To evaluate the accuracy of inflammatory biomarkers in differentiating patients with asthma–COPD overlap (ACO) from those with COPD alone.
Methods: Clinical data of 134 patients with COPD and 48 patients with ACO admitted to the First Affiliated Hospital of Xi’an Jiaotong University from January 2016 to June 2019 were retrospectively analyzed. Receiver operating characteristic (ROC) curve analysis was performed to determine the best cut-off values of fractional exhaled nitric oxide (FeNO), blood eosinophil counts (EOS), and neutrophil to lymphocyte ratio (NLR) for differentiating between ACO and COPD alone. Spearman correlation analysis was conducted to evaluate the relationships between these inflammatory biomarkers and the forced expiratory volume in one second/prediction (FEV1%pred).
Results: FeNO and EOS in the ACO patients were significantly higher than those in the COPD patients (FeNO: median 37.50 vs 24.50 ppb, P < 0.001; EOS: median 0.20 vs 0.10 × 109/L, P = 0.004). FeNO was positively correlated with FEV1%pred (r = 0.314, P = 0.030), while NLR was negatively correlated with FEV1%pred (r = − 0.372, P = 0.009) in patients with ACO. In addition, a positive correlation between FeNO and EOS was also found in ACO, especially in patients without history of inhaled corticosteroids (ICS) use (r = 0.682, P < 0.001). The optimal cut-off value of FeNO was 31.5 ppb (AUC = 0.758, 95% CI = 0.631– 0.886) in patients with smoking history, with 70.0% sensitivity and 89.9% specificity for differentiating ACO from COPD. In patients without history of ICS use, the best cut-off value of FeNO was 39.5 ppb (AUC = 0.740, 95% CI = 0.610– 0.870), with 58.3% sensitivity and 84.9% specificity. Among patients without history of ICS use and smoking, 27.5 ppb was optimal cut-off level for FeNO (AUC = 0.744, 95% CI = 0.579– 0.908) to diagnose ACO, with 81.8% sensitivity and 60.7% specificity, and the sensitivity was improved to 91.7% when FeNO was combined with EOS.
Conclusion: The inflammatory biomarkers FeNO and EOS can be used as indicators for differentiating between ACO and COPD alone.
Keywords: fractional exhaled nitric oxide, blood eosinophil counts, neutrophil to lymphocyte ratio, chronic obstructive pulmonary disease, asthma–COPD overlap
Introduction
Asthma–COPD overlap (ACO) is characterized by persistent airflow limitation with several features usually associated with both asthma and COPD.1 It has been reported that ACO has a prevalence of 15–20% among patients with COPD,2,3 the incidence increases in an age-dependent manner, with a prevalence of about 23% in patients aged 50–59 years but 52% in those over 70 years.4 The last Global Initiative for Chronic Obstructive Lung Disease (GOLD) update suggests that the patient should be treated accordingly when asthma is suspected.5 However, sometimes this is hard to be performed since several smokers have reversibility and increased sputum eosinophils making the recognition of the asthma component in a COPD patient difficult.5 By now, there are no unified standards to differentiate ACO from COPD alone. The stepwise approach for the diagnosis of ACO, proposed jointly by Global Initiative for Asthma (GINA) and GOLD, are mainly based on symptoms but lack objective indicators such as imaging characteristics and inflammatory biomarkers.1 The diagnostic procedure based on this criteria is also complicated for clinical application, especially to outpatients. Therefore, it is of great practical significance to find new objective indicators for recognizing the asthma component in COPD and diagnosing ACO.
Fractional exhaled nitric oxide (FeNO), blood eosinophil counts (EOS), and neutrophil to lymphocyte ratio (NLR), as indicators of airway or systemic inflammation, have been used to improve the accuracy in diagnosing asthma, guide asthma interventions, monitor the response to inhaled corticosteroids (ICS) treatment, evaluate eosinophilic airway inflammation, and assess the risk of acute exacerbation of COPD (AECOPD).6–12 FeNO and EOS were suggested by the GINA/GOLD joint document and Spanish ACOS Diagnostic Consensus 2017 to be used as inflammatory biomarkers to differentiate ACO from COPD.1,13 Neutrophils and NLR, as indicators of circulating immune complexes, were found remarkably higher in patients with airflow limitation (including COPD and ACO) than those in the healthy population,14 which suggested that NLR may be used as a biomarker to distinguish and diagnose different types of obstructive diseases.
Although some relevant studies have proved that FeNO and EOS are useful indicators to a certain extent in distinguishing ACO from COPD,15–20 the value of these inflammatory biomarkers in ACO diagnosis remains contradictory and inconclusive. In addition, most studies included patients with a history of smoking or ICS use,15–20 which might affect the expressions of the inflammatory biomarkers.21–24 What’s more, there is no research on the NLR in distinguishing between COPD and ACO. To address these problems, the present retrospective study was performed to further evaluate the accuracy of FeNO, EOS, and NLR for the clinical diagnosis of ACO after eliminating the influence of confounding factors.
Patients and Methods
Patients and Ethics Statement
The present study recruited 134 patients with COPD alone and 48 patients with ACO. All subjects with COPD were defined as a post-bronchodilator with a forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC) less than 0.70 with at least one of the following appropriate symptoms: cough, expectoration, wheezing and significant exposures to noxious stimuli (tobacco, occupation, indoor and outdoor air pollution).5 Asthma should be excluded from all patients with COPD alone. The definition of asthma according to GINA diagnostic criteria,1 patients fulfilled a history of various respiratory symptoms, such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, and determination of variable expiratory flow limitations (increased in FEV1≥200 mL and ≥12% from baseline after the use of a bronchodilator or 4 weeks after the anti-inflammatory treatment, outside respiratory infections).
ACO patients were confirmed according to the universally accepted definition of the GINA/GOLD joint document.1 The characteristics that suggest the diagnosis of asthma and COPD (including the following general categories: age at onset, pattern of symptoms, lung function, patient or family history, time course, and chest X-ray) were listed in Supplementary Table 1.1 Patients who satisfied the 3 or more characteristics of asthma or COPD can be diagnosed accordingly. If the number of characteristics of asthma and COPD are similar, ACO can be diagnosed. In addition, the following conditions must be also satisfied for ACO patients in this study: (1) age ≥ 40; (2) a history of chronic cough, phlegm and exertion dyspnea; (3) evidence of persistent airflow limitation (FEV1/FVC < 0.7 after bronchodilator); (4) a past history of asthma or strong evidence of reversible airflow limitation (increase in FEV1≥400mL and ≥15% from baseline after inhaling bronchodilator). Exclusion criteria were as follows: (1) diagnosis of bronchiectasis, interstitial lung disease, lung cancer, or tuberculosis; (2) suffering from other diseases affecting levels of inflammatory markers, such as severe autoimmune diseases, hematologic disease and metabolic diseases; (3) having received oral or intravenous glucocorticoid therapy in the preceding 4 weeks; (4) history of severe liver and kidney dysfunction or malignant tumor; (5) incomplete clinical data of patients. These diagnostic features were evaluated and extracted from the Electronic Medical Record System (EMRS). The study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, and written informed consents from patients were waived because it was a non-interventional retrospective study. We confirmed that the data of patients was maintained with confidentiality.
Methods
Pulmonary Function Test
Pulmonary function and bronchial dilation tests were performed using the pulmonary function equipment (MSD+APS, Germany). The tests were performed by professional medical technicians and repeated twice to obtain the best results.
FeNO Measurement
FeNO level were measured by using the Sunvou device (Sunvou Medical Electronics Co. LTD, Wuxi, CHN) with the method recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) Committee.25 The patient sat or stood straight, placed the filter in their mouth, and exhaled at a steady rate of 50 mL/s for 6–10 seconds immediately after deep inhalation, during which aeration and breath-holding were prohibited. For the test, patients had to satisfy the following conditions: no strenuous exercise, smoking and feeding one hour before the test; no broccoli, lettuce, celery and smoked or pickled foods three hours before the test; no history of respiratory infection or antibiotic use within one week before the test; no history of oral or intravenous glucocorticoids use within four weeks before the test. The results of FeNO were represented as parts per billion (ppb). FeNO level was classified as follows: normal, <25 ppb, non-eosinophilic airway inflammation; intermediate, 25–50 ppb, mixed airway inflammation; and high, >50 ppb, eosinophilic airway inflammation.
EOS and NLR Measurement
EOS and NLR were determined from peripheral blood samples. EOS levels were reported as ×109/L. Other clinical data were extracted from the EMRS. All of the pulmonary function test, FeNO, and blood test were performed on the same day.
Statistical Analysis
Statistical analysis were performed using SPSS 18.0 (SPSS Inc, Chicago, IL), PASS 11 (NCSS, LLC. Kaysville, Utah, USA) and Microsoft Excel (Microsoft Corp., Redmond, WA). Percentage was used to express the enumeration data. Measurement data were shown as median (interquartile range) or mean ± standard deviation, unless otherwise specified. Chi-square test and t-test were used to compare the distribution of categorical and continuous variables between the two groups, respectively. Mann–Whitney U-test was used to compare the non-normally distributed data between groups. Spearman’s rank correlation coefficient was used to assess the relationship between FeNO and EOS and the correlation between the biomarkers and FEV1%pred. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of inflammatory biomarkers in differentiating ACO from COPD. P<0.05 was considered as statistically significant.
Results
Baseline Characteristics of the Patients
This study enrolled a total of 182 participants, including 134 patients with COPD alone (91 males and 43 females; average age: 67.66±8.63 years) and 48 patients with ACO (26 males and 22 females; average age: 61.13±9.41 years), which met the requirements of sample size calculated before our study (at least 41 patients of ACO and COPD, respectively). The proportion of patients with a history of smoking was lower in the ACO group than in the COPD group (41.7% vs 59.0%, P = 0.044). The ACO patients were comparatively younger (61.13±9.41 years vs 67.66±8.63 years, P < 0.001) and had a lower FEV1/FVC level (50.66±8.22% vs 52.35±10.63%, P = 0.009). Detailed characteristics of the study patients are listed in Table 1.
 |
Table 1 Characteristics of the Study Patients (n=182) |
Levels of FeNO, EOS, and NLR in the Patients
The levels of FeNO and EOS were significantly higher in patients with ACO than in patients with COPD alone [FeNO: median 37.50 (23.00, 60.75) ppb vs 24.50 (16.75, 33.50) ppb, P < 0.001; EOS: median 0.20 (0.07, 0.45)×109/L vs 0.10 (0.04, 0.21)×109/L, P = 0.004; Table 1, Figure 1A and B]. The differences also existed in patients who had never used ICS (FeNO: P < 0.001; EOS: P = 0.005; Figure 1A and B), and patients with a history of smoking (FeNO: P < 0.001; EOS: P = 0.008; Figure 1A and B). In addition, among patients with no history of both ICS use and smoking, only FeNO showed a significant difference between the two groups (P = 0.019; Figure 1A). No significant FeNO and EOS between-group differences were found among patients with a history of ICS use and smoking simultaneously (Figure 1A and B). The level of NLR did not show a difference between COPD and ACO in all conditions (Figure 1C).
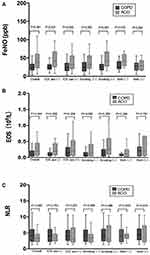 |
Figure 1 Inflammatory biomarkers levels in COPD and ACO. |
Correlation Analysis of FeNO and EOS in ACO Patients
FeNO and EOS were positively correlated in ACO patients (r = 0.497, P < 0.001; Figure 2A), especially in patients that had never used ICS (r = 0.682, P < 0.001; Figure 2B) and patients with a smoking history (r = 0.643, P = 0.002; Figure 2E). Of patients with a history of ICS use and patients without a history of smoking, no correlation of the two markers was found in ACO patients (Figure 2C and D).
 |
Figure 2 Correlation of FeNO with EOS in patients with ACO. |
Correlation Analysis of FeNO, EOS and NLR with FEV1%pred in ACO Patients
Correlation analysis showed that FeNO and NLR were positively and negatively correlated with FEV1%pred in patients with ACO, respectively (FeNO: r = 0.314, P = 0.030; NLR: r = −0.372, P = 0.009; Figure 3A and C). No relationship between EOS and FEV1%pred was found in this study (Figure 3B).
 |
Figure 3 Correlation of inflammatory biomarkers with FEV1%pred in patients with ACO. |
Diagnostic Accuracy of FeNO, EOS, and NLR in Identifying ACO and COPD
ROC curve analysis showed that 39.5 ppb was the best cut-off value of FeNO in identifying ACO and COPD in the entire cohort of patients [AUC = 0.683, 95% confidence interval (CI) = 0.590–0.776], with a sensitivity of 50.0% and a specificity of 83.6% (Table 2, Figure 4A). Among patients without ICS use, the optimal cut-off level was 39.5 ppb (AUC = 0.740, 95% CI = 0.610–0.870), with 58.3% sensitivity and 84.9% specificity (Table 2, Figure 4B). In addition, the optimal cut-off value of FeNO was 31.5 ppb (AUC = 0.758, 95% CI = 0.631–0.886) in patients with a smoking history, and the corresponding sensitivity was 70.0% and the specificity was 89.9% (Table 2, Figure 4D). As for EOS, 0.335×109/L was found to be the best cut-off value for ACO diagnosis in overall patients, with 39.6% sensitivity and 90.3% specificity (AUC = 0.640, 95% CI = 0.542–0.738; Table 2, Figure 4A). In patients with no ICS use and patients with a smoking history, the AUC of EOS increased but was still relatively low (Table 2, Figure 4B and D). It’s worth noting that only FeNO can be used to distinguish the two diseases after excluding the effect of both ICS use and smoking history at the same time, and the best cut off value was 27.5 ppb (AUC = 0.744, 95% CI = 0.579–0.908), with 81.8% sensitivity and 60.7% specificity (Table 2, Figure 4F). However, among patients with ICS treatment, patients with no smoking history, and patients with ICS treatment and smoking history simultaneously, FeNO and EOS showed no diagnostic value in distinguishing ACO from COPD (Table 2, Figure 4C, E and G). In short, both EOS and FeNO had high specificity in distinguishing ACO from COPD, and FeNO showed a higher diagnostic sensitivity in patients without ICS treatment and smoking history. NLR did not show a notable value in differentiating ACO from COPD (Figure 4A–G).
 |
Table 2 Diagnostic Accuracy of Inflammatory Biomarkers |
 |
Figure 4 ROC curve for inflammatory markers for differentiating ACO from COPD. |
Diagnostic Accuracy of FeNO and EOS in Combination
Given the low sensitivity of either FeNO or EOS in distinguishing ACO from COPD, further analysis was conducted to explore the diagnostic value of the two inflammatory biomarkers in combination. The multi-index combined ROC curve showed that the AUC of the combined indexes was larger than that of EOS alone (Figure 4). In addition, the value of the combined indexes was also further calculated, in which ACO was confirmed as long as either index was above the cut-off value. As shown in Table 2 and Figure 4A, combination of FeNO≥39.5 ppb and EOS≥0.335×109/L had 69.8% sensitivity and 75.5% specificity in overall patients (AUC = 0.678, 95% CI = 0.580–0.775). Among patients not having used ICS, 75.7% sensitivity and 80.2% specificity were achieved under the combination of FeNO≥39.5ppb and EOS≥0.285×109/L (AUC = 0.740, 95% CI = 0.610–0.869; Table 2, Figure 4B). 83.5% sensitivity and 66.0% specificity were identified under the combination of FeNO≥31.5ppb and EOS≥0.360×109/L among patients with a smoking history (AUC = 0.742, 95% CI = 0.600–0.885; Table 2, Figure 4D). What’s more, in patients without a history of both ICS use and smoking, 91.7% sensitivity and 43.3% specificity were achieved (AUC = 0.727, 95% CI = 0.560–0.894; Table 2, Figure 4F) when the two biomarkers combined. These results confirmed that the combination of FeNO and EOS can improve the sensitivity of ACO diagnosis, but reduce the specificity to some extent.
Discussion
Up to now, the clinical management of ACO has remained difficult due to the lack of precise definition and unified diagnostic criteria. As multiple phenotypes have been verified in ACO,26,27 there is an increased awareness of the importance and clinical significance in the diagnosis, treatment, prognosis, and recurrence of ACO by monitoring the level of inflammation. Compared to the induced sputum method recommended in previous guidelines,28 inflammatory biomarkers FeNO, EOS and NLR are more rapid, convenient, reproducible, and less painful. However, all of them are affected by factors such as therapeutic drugs and smoking.21–24 Therefore, the present study was conducted to further evaluate the accuracy of FeNO, EOS, and NLR in ACO diagnosis after excluding the influence of ICS and smoking use.
Congruent with some previous studies,15–20 our results confirmed the higher levels of FeNO and EOS in ACO patients than in COPD patients, especially in those without ICS use or with a smoking history. A possible reason for the results may be that chronic airway inflammation in asthma and COPD is mainly characterized by eosinophils and neutrophils, respectively,1,5 and ACO shares the airway inflammation characteristics of both COPD and asthma.1 In addition, FeNO has been used as an alternative indicator for eosinophilic airway inflammation and is a useful tool for diagnosing and monitoring asthma.6,7 What’s more, steroid responsiveness, airway inflammation, and airway remodeling that occur in asthma and COPD are associated with cigarette smoking.24 Patients with asthma who smoke have larger numbers of neutrophils and eosinophils.24,29,30 Therefore, the FeNO and EOS levels were higher in the ACO patients, and increased FeNO and EOS levels in ACO patients also manifest that ACO has an eosinophil airway inflammatory response similar to asthma. Unlike previous studies,29,31 we found that the FeNO level increased in patients with a smoking history. It may be due to the existence of some other factors that affect the level of FeNO, such as a nitrate rich diet before examination and contamination of nasal exhaled nitric oxide (nNO). In addition, Rouhos et al23 demonstrated that smoking seems to attenuate the increase in FeNO in atopic but not in nonatopic asthmatics. This may be due to the reason that some ACO patients in this study showed characteristics of nonatopic asthmatics. It’s worth noting that although 41.0% (55/134) of COPD and 58.3% (28/48) of ACO patients in this study had no smoking history, all of them were chronically exposed to high levels of fine particulate air pollution or biomass for cooking or heating, which is the main causes of COPD and ACO. Consistent with previous studies,3,15,32 the ACO patients in our study were younger than those with COPD, and they had poorer lung functions. Several researches confirmed that ACO patients tend to have more severe symptoms, faster deterioration and higher mortality rates compared to asthma and COPD alone,33–35 and most patients seek medical treatment earlier for these above reasons, which may increase the early detection of the disease.
Several studies revealed a weak-to-moderate correlation between eosinophils in sputum and FeNO in patients with asthma,36,37 while Takayama et al18 claimed that the correlation between FeNO and EOS was insignificant in patients with either ACO or COPD. This might be because FeNO and EOS are involved in two different inflammatory pathways, namely, the interleukin-4 (IL-4) and IL-13-mediated pathway38 and the IL-5-mediated pathway.39 Different from these studies, our results showed a moderate correlation between FeNO and EOS in patients with ACO, especially in patient without ICS use and patients with a smoking history. Prado et al40 confirm that neurokinins and nitric oxide (NO) are involved in iNOS-negative eosinophil and iNOS-positive eosinophil recruitment, respectively. NO may promote eosinophil and mononuclear cell response in the distal airways. Gao et al37 identified a significant positive correlation between FeNO and sputum eosinophils, and Hartjes et al41 detected a tight association of higher eosinophils levels in blood with higher eosinophils levels in sputum. These findings, together with ours, suggested that FeNO and EOS may interact in a certain inflammatory pathway, and combination of the two biomarkers may increase the diagnostic sensitivity for the ACO. Whereas, further analysis revealed that the association between FeNO and EOS disappeared in patients with the experience of ICS treatment. This may be attributed to the reduced expression of inflammatory biomarkers when using ICS.21,22
Shi et al42 confirmed that FeNO was negatively associated with FEV1%pred in COPD and ACO. However, Liu et al43 demonstrated a higher proportion of patients with GOLD III–IV and more exacerbations in patients with low FeNO levels. Vedel-krogh et al10 found that FEV1%pred was slightly lower in individuals with a high EOS level in patients with COPD, while higher FEV1%pred was found in patients with a high EOS level in the ECLIPSE study.44 Furutate et al12 found inversely correlation between NLR and FEV1 in COPD patients. Up to now, the correlation among EOS, NLR and FEV1%pred in ACO patients has not been systematically explored. Similar to the previous researches,43,45 our study revealed that FeNO was positively correlated with FEV1%pred in patients with ACO. Although previous studies have found that increased neuronal nitric oxide synthase (nNOS) in patients with severe COPD and promote the production of FeNO,46 these patients with exacerbation and poor lung function may use more ICS to relieve dyspnea symptoms, which reduced the concentrations of FeNO. So its clinical relevance still remains controversial and needs to be confirmed in other studies. In addition, our study also found a negative relation between NLR and FEV1%pred in patients with ACO. AECOPD is generally thought to be significantly associated with infection,47 previous studies have manifested that infectious AECOPD is characterized by an increased level of NLR, which may be caused by pathogens inducing a stronger inflammatory response mediated by neutrophils rather than lymphocyte.48,49 Neutrophils have been shown to influence the pulmonary ventilation function by participating in the inflammatory response and remodeling in the airway.50 Therefore, the level of NLR were gradually increased along with the aggravation of airflow limitation in patients with ACO. In this study, no correlation between EOS and FEV1%pred was found in patients with ACO, which is attributed to the large proportion of patients with a history of ICS use in the included samples. ICS can reduce the FEV1 decline in patients with higher blood eosinophils counts at baseline.51
Although many previous studies have demonstrated the value of inflammatory markers in ACO diagnosis,15–19 the results remain highly controversial.20 Kobayashi et al15 found that 156.2/mm3 was the best diagnostic cut-off level of EOS for ACO diagnosis, with the sensitivity and specificity being 49.5% and 83.8%, respectively. Deng et al19 reported that the optimal cut-off level of FeNO was 29.0 ppb, with 80.0% sensitivity and 73.0% specificity. Takayama et al18 demonstrated 21.0 ppb and 250 cells/mL as the optimal diagnostic cut-off levels of FeNO and EOS for differentiating ACO from COPD in overall patients, but among patients naive to ICS, the cut-off value of FeNO was 25.0 ppb with 60.6% sensitivity and 87.7% specificity. However, Goto et al20 pointed out that FeNO alone was insufficient to discriminate ACO from COPD. Notably, the above studies had certain limitations. First, the diagnosis of ACO was not performed based on the accepted method, and some diseases that may affect the expression of inflammatory markers were not excluded. Second, some articles did not mention whether FeNO, lung function and blood test were completed on the same day. Third, patients with a history of ICS use and smoking were not excluded, and the diagnostic value of NLR in the two diseases has not been studied. In the present study, the definition in the GINA/GOLD joint document was used in ACO diagnosis, and all of the pulmonary function, blood and FeNO tests were performed on the same day, what’s more, some diseases that may affect the expression of inflammatory markers and the effects of ICS treatment and smoking history were excluded when evaluating the diagnostic value of inflammatory markers. Our results clearly showed that either FeNO or EOS has a high specificity in distinguishing ACO from COPD among patients without ICS use or patients with a smoking history alone, and the sensitivity for diagnosis can be improved to 91.7% when the two indexes are a combination of patients without a history of ICS use and smoking. However, there is no value in them in differentiating between ACO and COPD in patients with ICS and a smoking history. Since both ICS and smoking can affect FeNO and EOS levels,22–24 and the diagnostic value of them may be weakened simultaneously. The cut-off value of FeNO for ACO diagnosis in this study were higher than those in previous studies, there are several potential reasons, as follows: first, this study was conducted in Asians, a meta-analysis showed that the FeNO value of Asians appears to be higher than Caucasians;52 second, Huang et al53 found that FeNO measured by Sunvou device (used in this study) showed a higher value compared to FeNO measured by NIOX VERO (used in several previous studies15,16,18,20); third, people exposed to higher levels of air pollutant, especially PM2.5, had a higher FeNO level.54 Shaanxi Province was found to be the main exogenous source of total particulate matter in northwest China, and the PM2.5 concentrations of the provincial capital Xi’an mainly originates from local emissions.55,56 Therefore, we consider it appropriate to further investigate the optimal cut-off value of FeNO and EOS for ACO diagnosis based on ethnic and regional groups.
In this study, we identified and assessed the values of inflammatory biomarkers in ACO diagnosis. Compared with previous studies, our research has some advantages. First, the diagnostic accuracy of FeNO, EOS and NLR in ACO was evaluated with the exclusion of confounding factors, including ICS use and smoking, and some diseases that may affect the expression of inflammatory markers were excluded in our study. Second, the diagnostic values of NLR and combination of FeNO and EOS were investigated, and the ACO patients in our study were screened according to the widely accepted criteria defined in the GINA/GOLD joint document, and all patients with ACO must meet the 3 main criteria of GesEPOC 2017 and a consensus definition of ACOS from a round table discussion, which guaranteed the accuracy of ACO diagnosis. Third, the inflammatory biomarkers were performed on the same day to guarantee the accuracy of the study, and we analyzed the differential value of NLR between the two diseases for the first time. Of course, our study may have some limitations. Selection bias might exist as the data were from the same hospital. Smokers have not been classified as current and ex-smoker groups for further analysis. The sample size was not large enough to support some results in the stratified analysis. Therefore, the results of this study need to be confirmed with data from multiple centers, with a larger sample size, and probably by prospective research.
Conclusion
Our results demonstrate that the inflammatory biomarkers FeNO and EOS can be used to support the diagnosis of ACO, especially in patients without a history of ICS use and smoking. However, there was no diagnostic value of them in patients with a history of smoking and ICS use.
Acknowledgment
Thanks to all the patients and researchers who participated in this study.
Funding
Financial support was provided by National Key R&D Program of China (2018YFC1315101).
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Global Initiative for Asthma, Global Initiative for Chronic Obstructive Lung Disease, Diagnosis of Disease of Chronic Airflow Limitation. Asthma, COPD, and asthma–COPD overlap syndrome (ACOS); 2015. Available from: http://www.ginasthma.org/.
2. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21(1):74–79. doi:10.1097/mcp.0000000000000118
3. Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi:10.1016/j.rmed.2013.03.007
4. Zeki AA, Schivo M, Chan A, et al. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011;2011:861926. doi:10.1155/2011/861926
5. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease; 2020. Available from:https://goldcopd.org/.
6. Arnold RJ, Massanari M, Lee TA, et al. A review of the utility and cost effectiveness of monitoring fractional exhaled nitric oxide (FeNO) in asthma management. Manag Care. 2018;27:34–41.
7. Petsky HL, Kew KM, Turner C, et al. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016;9:Cd011440. doi:10.1002/14651858.CD011440.pub2
8. Petsky HL, Li A, Chang AB. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2017;8:Cd005603. doi:10.1002/14651858.CD005603.pub3
9. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
10. Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. the copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
11. Kerkhof M, Sonnappa S, Postma DS, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J. 2017;50. doi:10.1183/13993003.00761-2017
12. Furutate R, Ishii T, Motegi T, et al. The neutrophil to lymphocyte ratio is related to disease severity and exacerbation in patients with chronic obstructive pulmonary disease. Intern Med. 2016;55(3):223–229. doi:10.2169/internalmedicine.55.5772
13. Plaza V, Alvarez F, Calle M, et al. Consensus on the asthma-COPD overlap syndrome (ACOS) between the Spanish COPD guidelines (GesEPOC) and the Spanish guidelines on the management of asthma (GEMA). Arch Bronconeumol. 2017;53(8):443–449. doi:10.1016/j.arbres.2017.04.002
14. Pilaczynska-Cemel M, Golda R, Dabrowska A, et al. Analysis of the level of selected parameters of inflammation, circulating immune complexes, and related indicators (neutrophil/lymphocyte, platelet/ lymphocyte, CRP/CIC) in patients with obstructive diseases. Cent Eur J Immunol. 2019;44(3):292–298. doi:10.5114/ceji.2019.87498
15. Kobayashi S, Hanagama M, Yamanda S, et al. Inflammatory biomarkers in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:2117–2123. doi:10.2147/copd.S113647
16. Chen FJ, Huang XY, Liu YL, et al. Importance of fractional exhaled nitric oxide in the differentiation of asthma–COPD overlap syndrome, asthma, and COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2385–2390. doi:10.2147/copd.S115378
17. Guo Y, Hong C, Liu Y, et al. Diagnostic value of fractional exhaled nitric oxide for asthma-chronic obstructive pulmonary disease overlap syndrome. Medicine (Baltimore). 2018;97(23):e10857. doi:10.1097/md.0000000000010857
18. Takayama Y, Ohnishi H, Ogasawara F, et al. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. Int J Chron Obstruct Pulmon Dis. 2018;13:2525–2532. doi:10.2147/copd.S167600
19. Deng DD, Zhou AY, Shuang QC, et al. The value of fractionated exhaled nitric oxide in the diagnosis of asthma-chronic obstructive pulmonary disease overlap syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2017;40:98–101. doi:10.3760/cma.j.issn.1001-0939.2017.02.004
20. Goto T, Camargo CA
21. Gao SJ, Ge YP, Zhang CJ. Correlation between fractional exhaled nitric oxide levels and efficacy of inhaled corticosteroids in children with bronchial asthma. Am J Ther. 2018;25(6):e617–e625. doi:10.1097/mjt.0000000000000423
22. Demarche SF, Schleich FN, Henket MA, et al. Effectiveness of inhaled corticosteroids in real life on clinical outcomes, sputum cells and systemic inflammation in asthmatics: a retrospective cohort study in a secondary care centre. BMJ Open. 2017;7(11):e018186. doi:10.1136/bmjopen-2017-018186
23. Rouhos A, Ekroos H, Karjalainen J, et al. Smoking attenuates increase in exhaled nitric oxide in atopic but not in nonatopic young adults with asthma. Int Arch Allergy Immunol. 2010;152(3):226–232. doi:10.1159/000283029
24. Chalmers GW, MacLeod KJ, Thomson L, et al. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120(6):1917–1922. doi:10.1378/chest.120.6.1917
25. ATS/ERS. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi:10.1164/rccm.200406-710ST.
26. Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis. 2014;6(Suppl 1):S146–151. doi:10.3978/j.issn.2072-1439.2014.03.04
27. Toledo-Pons N, van Boven JFM, Roman-Rodriguez M, et al. ACO: time to move from the description of different phenotypes to the treatable traits. PLoS One. 2019;14(1):e0210915. doi:10.1371/journal.pone.0210915
28. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi:10.1016/s0300-2896(14)70070-5
29. Jacinto T, Malinovschi A, Janson C, et al. Differential effect of cigarette smoke exposure on exhaled nitric oxide and blood eosinophils in healthy and asthmatic individuals. J Breath Res. 2017;11(3):036006. doi:10.1088/1752-7163/aa746b
30. Al-Sawalha NA, Migdadi AM, Alzoubi KH, et al. Effect of waterpipe tobacco smoking on airway inflammation in murine model of asthma. Inhal Toxicol. 2017;29(2):46–52. doi:10.1080/08958378.2017.1280105
31. Shimoda T, Obase Y, Kishikawa R, et al. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma. Allergy Asthma Proc. 2016;37(4):50–58. doi:10.2500/aap.2016.37.3944
32. Alshabanat A, Zafari Z, Albanyan O, et al. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One. 2015;10(9):e0136065. doi:10.1371/journal.pone.0136065
33. Andersen H, Lampela P, Nevanlinna A, et al. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342–346. doi:10.1111/crj.12013
34. Kurashima K, Takaku Y, Ohta C, et al. COPD assessment test and severity of airflow limitation in patients with asthma, COPD, and asthma–COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:479–487. doi:10.2147/copd.S97343
35. Sorino C, Pedone C, Scichilone N. Fifteen-year mortality of patients with asthma-COPD overlap syndrome. Eur J Intern Med. 2016;34:72–77. doi:10.1016/j.ejim.2016.06.020
36. Wang W, Huang KW, Wu BM, et al. Correlation of eosinophil counts in induced sputum and fractional concentration of exhaled nitric oxide and lung functions in patients with mild to moderate asthma. Chin Med J (Engl). 2012;125:3157–3160.
37. Gao J, Wu F. Association between fractional exhaled nitric oxide, sputum induction and peripheral blood eosinophil in uncontrolled asthma. Allergy Asthma Clin Immunol. 2018;14:21. doi:10.1186/s13223-018-0248-7
38. Alving K, Malinovschi A. Basic aspects of exhaled nitric oxide. Eur Respir Monogr. 2011;49:1–31.
39. Stirling RG, van Rensen EL, Barnes PJ, et al. Interleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164(8):1403–1409. doi:10.1164/ajrccm.164.8.2010002
40. Prado CM, Leick-Maldonado EA, Arata V, et al. Neurokinins and inflammatory cell iNOS expression in guinea pigs with chronic allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L741–748. doi:10.1152/ajplung.00208.2004
41. Hartjes FJ, Vonk JM, Faiz A, et al. Predictive value of eosinophils and neutrophils on clinical effects of ICS in COPD. Respirology. 2018;23(11):1023–1031. doi:10.1111/resp.13312
42. Shi F, Qiu C, Yu J, et al. Comparison of fractional exhaled nitric oxide in elderly patients with asthma-chronic obstructive pulmonary disease overlap and other airway inflammatory diseases. Iran J Allergy Asthma Immunol. 2018;17:232–239.
43. Liu X, Zhang H, Wang Y, et al. Fractional exhaled nitric oxide is associated with the severity of stable COPD. Copd. 2020;17(2):121–127. doi:10.1080/15412555.2019.1704231
44. Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
45. Ye M, Li Q, Xiao L, et al. Serum magnesium and fractional exhaled nitric oxide in relation to the severity in asthma-chronic obstructive pulmonary disease overlap. Biol Trace Elem Res. 2020. doi:10.1007/s12011-020-02314-5
46. Brindicci C, Kharitonov SA, Ito M, et al. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(1):21–30. doi:10.1164/rccm.200904-0493OC
47. Kuwal A, Joshi V, Dutt N, et al. A prospective study of bacteriological etiology in hospitalized acute exacerbation of COPD patients: relationship with lung function and respiratory failure. Turk Thorac J. 2018;19(1):19–27. doi:10.5152/TurkThoracJ.2017.17035
48. Tanriverdi H, Ornek T, Erboy F, et al. Comparison of diagnostic values of procalcitonin, C-reactive protein and blood neutrophil/lymphocyte ratio levels in predicting bacterial infection in hospitalized patients with acute exacerbations of COPD. Wien Klin Wochenschr. 2015;127(19–20):756–763. doi:10.1007/s00508-014-0690-6
49. van de Geijn GM, Denker S, Meuleman-van Waning V, et al. Evaluation of new laboratory tests to discriminate bacterial from nonbacterial chronic obstructive pulmonary disease exacerbations. Int J Lab Hematol. 2016;38(6):616–628. doi:10.1111/ijlh.12550
50. Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis. 2014;5(5):212–227. doi:10.1177/2040622314532862
51. Low EV, Hughes SM, Zaffarullah S, et al. ICS use may modify FEV1 decline in α1-antitrypsin deficiency patients with relatively high blood eosinophils. Respiration. 2018;95(2):114–121. doi:10.1159/000481867
52. Blake TL, Chang AB, Chatfield MD, et al. Does ethnicity influence fractional exhaled nitric oxide in healthy individuals? A systematic review. Chest. 2017;152(1):40–50. doi:10.1016/j.chest.2017.02.007
53. Huang T, Liu B, Yang D, et al. Fractional exhaled nitric oxide measurement: comparison between the Sunvou-CA2122 analyzer and the NIOX VERO analyzer. J Asthma. 2019:1–8. doi:10.1080/02770903.2019.1658206.
54. He L, Li Z, Teng Y, et al. Associations of personal exposure to air pollutants with airway mechanics in children with asthma. Environ Int. 2020;138:105647. doi:10.1016/j.envint.2020.105647
55. Yang X, Xiao H, Wu Q, et al. Numerical study of air pollution over a typical basin topography: source appointment of fine particulate matter during one severe haze in the megacity Xi’an. Sci Total Environ. 2020;708:135213. doi:10.1016/j.scitotenv.2019.135213
56. Yu H, Feng J, Su X, et al. A seriously air pollution area affected by anthropogenic in the central China: temporal-spatial distribution and potential sources. Environ Geochem Health. 2020;42(10):3199–3211. doi:10.1007/s10653-020-00558-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
