Back to Journals » Journal of Inflammation Research » Volume 17
The Use of the Neoglycolipid-Based Oligosaccharide Microarray System in the Diagnosis of Endometriosis – Preliminary Study
Authors Wojtyla C, Tołwiński I , Laudański P
Received 11 September 2023
Accepted for publication 1 January 2024
Published 9 February 2024 Volume 2024:17 Pages 899—908
DOI https://doi.org/10.2147/JIR.S439709
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Cezary Wojtyla,1,2 Ignacy Tołwiński,3 Piotr Laudański1,2,4
1Women’s Health Research Institute, Calisia University, Kalisz, Poland; 2OVIklinika Infertility Center, Warsaw, Poland; 3Warsaw Southern Hospital, Warsaw, Poland; 4Department of Obstetrics, Gynecology and Gynecological Oncology, Medical University of Warsaw, Warsaw, Poland
Correspondence: Cezary Wojtyla, Women’s Health Research Institute, Calisia University, Kalisz, Poland, Email [email protected]
Objective: Endometriosis presents diagnostic challenges, and there is a need for developing novel biomarkers with satisfactory specificity and sensitivity. Glycomics, exploring glycosylation changes in glycoproteins, offers potential solutions. The aim of this study was to analyze the carbohydrate-binding properties of IgG and IgM antibodies in the plasma and peritoneal fluid samples and to identify any differences in the presence and the specificities of anti-carbohydrate antibodies in the endometriosis patient and the controls.
Methods: Multicenter study was conducted in Poland between 2018 and 2019. Plasma and peritoneal fluid samples were collected from women undergoing laparoscopic surgery. Endometriosis patients (n=8) and controls (n=8), matched for cycle phase and disease stage, were selected. The neoglycolipid-based oligosaccharide microarray system was used to investigate IgG and IgM antibody binding properties to glycan-related probes in biological materials.
Results: In peritoneal fluid samples, IgM binding to the following probes was significantly higher in endometriosis: GSC-915-4 (new), LNFP-I, NeuAcα-(6’)LNnO (F1), B-like decaosylceramide, log10(GM1-penta), and log10(GSC-915-5). In a control group higher IgG binding to log10(Orsay-5-AO) was observed. In plasma samples, endometriosis showed higher IgG binding to log10(NeuAcα-(6’)LNnO (F1)) and lower IgG binding to Gal2GlcNAc(1-3)-AO. After Benjamin–Hochberg correction, differences were not significant. Effect sizes highlighted some glycan probes in both plasma and peritoneal fluid. Strong correlations were observed among binding to certain glycan probes.
Conclusion: This preliminary study suggests glycomics’ potential contribution to endometriosis diagnosis and understanding of its pathophysiology. Neoglycolipid-based microarrays hold promise for non-invasive endometriosis diagnostic tools. Further investigations with larger cohorts are warranted to validate these findings and explore potential correlations with antibody levels in plasma and peritoneal fluid. Glycomics emerges as a valuable diagnostic asset in endometriosis research.
Keywords: endometriosis, glycomics, diagnosis, microarray
Introduction
Endometriosis is a gynecological condition, which is defined as endometrium-like lesions outside of the uterus.1 Its diagnosis still remains a challenge, and there is a demand for developing new biomarkers with satisfactory specificity and sensitivity. Glycomics can be a source of such biomarkers. Almost all proteins associated with cell membranes and human extracellular proteins are glycosylated, and in many diseases there may be changes in glycoprotein connections.2 Oligosaccharide microarrays revolutionized researches on carbohydrate–protein interactions. One of these technologies is microarray based on neoglycolipid technology. It is based on connection of oligosaccharides with aminophospholipids to produce novel glycolipids.3 We hypothesized that plasma (P) and peritoneal fluid (PF) in endometriosis contain unique glycan profiles. To evaluate this hypothesis we created a feasibility study, which at the same time would be able to assess the usefulness of the neoglycolipid-based oligosaccharide microarray system in the analysis of P and PF in endometriosis. The aim of our study was to analyze the carbohydrate-binding properties of IgG and IgM antibodies in the P and PF samples and to identify any differences in the presence and the specificities of anti-carbohydrate antibodies in the endometriosis patient group and the control group.
Materials and Methods
Sample Collection
A multicenter, cross-sectional study was conducted between 2018 and 2019 in eight centers in Poland. Biological material (plasma and peritoneal fluid) was obtained from women undergoing laparoscopic surgery for the following reasons: ovarian cyst, pelvic pain, and/or infertility. The exclusion criteria were as follows: age under 18 and over 45 years old; irregular menstruation (<25 or >35 days), any form of hormonal therapy during the last three months before surgery, pelvic inflammatory disease, uterine fibroids, polycystic ovary syndrome, any autoimmune diseases, malignant or suspected malignant diseases, and any previous history of surgical treatment. The details of the study have recently been published.4 The cycle phase was calculated from the last menstrual period and average length of the menstrual cycle.
Women from the endometriosis group were diagnosed through laparoscopic findings, and each case was histopathologically confirmed. As controls, we recruited patients without visible endometriosis during laparoscopy. In this study we analysed P and PF samples collected from 16 patients (8 from the endometriosis group and 8 from controls). In order to maintain homogeneity of studied groups, we selected only samples taken in the proliferative phase of the cycle and from women with stage III/IV endometriosis according to the revised American Fertility Society classification.5 All the women belonged to the white European ethnic group. Women completed a World Endometriosis Research Foundation (WERF) clinical questionnaire and signed consent form to participate in the study. The Bioethics Committee operating at the Medical University of Warsaw approved the collection of the material in accordance with opinion no. KB 223/2017, issued on December 12, 2017. All patients gave their informed consent to participate in the study.
Diagnostic laparoscopy was performed in all patients by trained gynecologists. Peritoneal fluid was collected through aspiration using a Veress needle under direct visualization immediately upon introduction of the laparoscope in order to avoid contamination with blood. The procedure was meticulously performed in line with the Endometriosis Phenome and Biobanking Harmonization Project standard operating procedures.6 The collected PF was centrifuged at 1000 × g for 10 min at 4 °C. The supernatant was transferred to a fresh 10 mL tube (Sarstedt) and divided into 500 µL tubes. Blood samples were collected before laparoscopy (before anesthesia) in ethylenediaminetetraacetic acid (EDTA) 10 mL tubes (Sarstedt). We used tubes of the same type for blood and PF. The time lapse between sample collection (both P and PF) and processing was <45 min. Blood samples were centrifuged at 2500 × g for 10 min at 4 °C. Then, the P samples were split into 500 μL aliquots. Both materials were stored at −80 °C until further use. Afterward, single tubes of PF and P were selected for shipment on dry ice to the Glycosciences Laboratory (Imperial College, London, UK) to conduct the neoglycolipid-based oligosaccharide microarray system as a custom service.3 The Bioethics Committee operating at the Calisia University approved the method of analysis of the previously collected material using the abovementioned technology (opinion 04/2021, issued on December 20, 2021).
Microarray Analyses
Samples (P and PF) were analyzed using two sequence-defined glycan array sets:
- CIG array (CIG Set 1,2,3) which stands for CLL and I/i Glycan array set containing 122 lipid-linked oligosaccharide probes
- BGG array (BGG set 1) which stands for Blood Group and Galactose-terminating sequence array set containing 46 glycan probes
The two array sets have 168 glycan probes printed; among these 137 probes have unique sequences (31 probes were common to both array sets). Included were sequences of A, B, O(H) blood group antigens, Lewis types (Lewis a, b, x, y), alpha-GalNAc/Gal terminating sequences (such as Forssmann glycolipid and B-like sequences), and antigens associated with their backbones such as the I- and i-antigens terminated in Gal or GlcNAc. A limited number of sialylated sequences and beta-glucan related sequences were also included. The probe lists of the two array sets are in Table S1.
Analysis steps were performed essentially as described in the paper by Liu et al,7 except that the blocking and incubation steps of the microarray binding analyses were performed in a cold room at 4 °C. This is mainly because certain autoantibodies to glycan antigens bind optimally below body temperature, and some dissociate at 20 °C and higher temperatures.
In brief, microarray slides were blocked for 1 h using the blocker/diluent solution (1% BSA + 1% casein in HBS). The sample supernatants were diluted to 1/20 in the blocker/diluent solution and overlaid onto the arrays for 1.5 h. Then the detecting solution composed of anti-human IgG-cAF488 + anti-human IgM mu chain-AF647 (2 µg/mL) was overlaid onto the arrays for 1 h.
The oligosaccharide probes were all lipid-linked. They were neoglycolipids (NGLs) prepared from reducing oligosaccharides by reductive amination with the amino lipid, 1,2-dihexadecyl-sn-glycero-3-phosphoethanolamine (DHPE), or prepared from reducing oligosaccharides by oxime ligation with an aminooxy (AO) functionalized DHPE.8
The microarray slides were scanned with GenePix 4300A scanner instrument. Fluorescent signals were recorded at PMT 350 with red laser (635 nm) at 100% laser power for IgM binding, and with blue laser (488 nm) at 5% laser power for IgG binding.
Statistical Analysis
Asymmetrical (right-skewed) distributed variables were subjected to logarithmic transformation. Significant differences for individual comparisons were tested using Student’s t-test after verifying assumptions. In cases where there was a lack of homogeneity of variances in subgroups, the Cochrane–Cox test was applied. The Benjamin–Hochberg correction for the hypothesis set was used.
The absolute values of the standardized effect and their 95% confidence intervals were calculated. The study presented results of IgM and IgG levels in peritoneal fluid, for which a minimum of 14 measurements were obtained, and the standardized effects were above 0.5. The χ2 test with a correction for low number of cases was used to assess associations between the results in peritoneal fluid and plasma.
Results
The average age of women diagnosed with endometriosis was 32 years, while in the control group it was 29 years. The mean body mass index (BMI) was 22.4 kg/m2 in both groups of women. Infertility affected 50% of women with endometriosis and over 62% of women in the control group. All women diagnosed with endometriosis had confirmed endometrial cysts, while over 37% of women in the control group underwent surgery due to non-endometrial cysts. One woman in the control group was diagnosed with pelvic pain syndrome, whereas it did not present a problem among women with endometriosis. Clinical characteristics of patients are presented in Table 1.
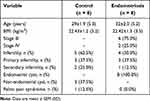 |
Table 1 Clinical Characteristics of Women |
Control and Endometriosis Groups
In PF, significantly higher (p < 0.05) mean binding levels to the following glycan-related probes in CIG array for IgM were observed in endometriosis compared to control group: GSC-915-4 (new), LNFP-I, NeuAcα-(6’)LNnO (F1), log10(B-like decaosylceramide), log10(GM1-penta), and log10(GSC-915-5). Conversely, a significantly higher binding level to probe of log10(Orsay-5-AO) was found in the control group (CIG array for IgG). In BGG array for IgM, a higher mean binding level to B-like decaosylceramide was recorded in the endometriosis group. In P samples, a significantly higher mean binding level to glycan-related probe of log10(NeuAcα-(6’)LNnO (F1)) (CIG array for IgG) and a significantly lower mean binding level to Gal2GlcNAc(1-3)-AO (BGG array for IgG) were observed in endometriosis compared to control group. Table 2 presents binding level to selected glycan-related probes. After applying the Benjamin–Hochberg correction, none of the described comparisons remained statistically significant.
 |
Table 2 Binding Level to Glycan Probes Expressed as Fluorescence Signals (Fluorescence Intensity at 5 fmol/Probe Spot) |
In P, standardized effect sizes above 1.0 were obtained for two glycan probes: Gal2GlcNAc(1-3)-AO in BGG array for IgG and log10(NeuAcα-(6’)LNnO (F1)) in CIG array for IgG. However, no significant correlation was observed between the aforementioned compounds. In PF, standardized effect sizes around 1.0 were obtained for the following glycan probes: log10(Orsay-5-AO) (CIG array for IgG), B-like decaosylceramide (BGG array for IgM), GSC-915-4 (new) (CIG array for IgM), LNFP-I (CIG array for IgM), NeuAcα-(6’)LNnO (F1) (CIG array for IgM), log10(B-like decaosylceramide) (CIG array for IgM), and log10(GM1-penta) (CIG array for IgM). Figure 1 presents absolute values of standardized effects with 95% confidence intervals for comparisons of binding level to glycan-related probes in control and endometriosis groups.
 |
Figure 1 Absolute values of standardized effects for comparisons of binding level to glycan-related probes in control and endometriosis group in plasma (A) and peritoneal fluid (B) samples. |
Table 3 presents the coefficients of simple correlation between the mentioned compounds. The glycan probes in CIG array set for IgM were significantly and positively correlated, except for log10(B-like decaosylceramide) and log10(GM1-penta), and the correlations between LNFP-I and NeuAcα-(6’)LNnO (F1) as well as log10(GSC-915-5) and NeuAcα-(6’)LNnO (F1) were almost complete.
 |
Table 3 Simple Correlation Coefficients Rxy |
In the plasma of six women without endometriosis, binding to H-Tri-T2-AO (CIG array for IgG) probe was not detected. However, in two women from this group, the mean binding level of H-Tri-T2-AO was above 0 (Table 4). On the other hand, in the endometriosis group, the binding to probe of H-Tri-T2-AO was detected in the plasma samples of all women. A significant association between the presence of binding to this glycan probe in the serum and endometriosis was observed (ϕ=0.77).
 |
Table 4 The Relationship Between Endometriosis and the Presence of H-Tri-T2-AO (IgG CIG) in the Plasma |
Discussion
Glycosylation plays an important role in differentiating protein functions, communication between the cell and its environment, response function of immune system, inflammatory processes, and in the development of tissues and organs. Anti-glycan antibodies are a major component of our immune system. This subpopulation of antibodies can specifically recognize normal or abnormal glycan structures.9 Some recent studies have provided valuable information that has made it possible to exploit glycomics for clinical applications. It is now possible to use glycomics in future diagnosis, profiling of patients, and individualization of medicine.10–13 In our study we touched on identification of the prevalence and the specificities of anti-carbohydrate antibodies in endometriosis. There are not many publications available on this subject as there are challenges of profiling of anti-glycan antibodies due to lack of access to glycans or poor outcomes of traditional assays. Glycan microarrays provide a powerful, high-throughput tool to profile antibody populations and identify specific subsets with clinical or biological relevance.14 Hence, we used microarrays based on neoglycolipid technology to identify any differences of anti-carbohydrate antibodies in P and PF samples collected from endometriosis patients.
In our study, the binding profiles of the P and PF samples from the same individual were almost identical for both IgG and IgM binding. The signal intensities with the P samples were higher than with the corresponding PF samples, but our study confirms that PF samples are also suitable for microarray analyses.
Considering the analysis of IgM and IgG detection, we observed good binding signals with all the P and PF samples tested for both IgG and IgM binding under the assay conditions used. As the binding was detected with anti-IgG and anti-IgM from different commercial sources carrying different fluorescent labels, and the images were also recorded using different lasers with different laser power, the binding scores of IgG and IgM should not be compared directly. It is, nevertheless, possible to compare the IgG and IgM binding patterns and overall intensities. In general, with all the samples tested (P or PF), a broader range of glycan probes showed binding with the IgM detection system compared to the IgG detection system. Endometriosis shows some features of malignant neoplasms like aggressive migration and invasion. In addition, endometriosis like cancer cells is part of the host’s own tissue. Thus, in both cases the organism's attempt to eliminate them must be based on innate immune responses. In cancers natural IgM antibodies are crucial for anti-cancer defense and immunological functions.15–17 A similar mechanism may exist in endometriosis, which would explain our observations indicating the advantage of the IgM detection system compared to the IgG detection system.
Data show that anti-glycan antibodies are possible diagnostic options in other fields of medicine. For instance specific anti-carbohydrate antibodies are associated with islet immunity and progression to type 1 diabetes.17 In gynecological oncology, glycomics, as a method of diagnosis, has a potential of replacing the current clinically used biomarker as they found a discriminating anti-glycan antibody panel, which possibly could diagnose ovarian cancers with higher sensitivity and specificity than CA125.18 However more recent study has shown that glycans at this point may not replace the currently used ovarian cancer biomarker CA125 but certainly may serve as a complementary marker and predictor in the diagnosis of this disease.19 Furthermore, studies on oral squamous cell carcinoma patients after serum N-glycome characterization and anti-carbohydrate antibody profiling shows that two IgM antibodies were elevated accompanied with the decreased levels of nine IgG antibodies in patient serum, which matches our results about changes in profiles of IgM and IgG.20 IgM anti-glycan antibodies as a possible method of diagnosis were also found in multiple sclerosis.21 Although it is still highly speculative that endometriosis is a risk factor for or a consequence of autoimmune diseases, the most recent systematic review and meta-analysis on the relationship between endometriosis and autoimmune disease showed a possible connection between endometriosis and multiple sclerosis.22 On the other hand our most recent study found no differences in levels of autoantibodies between endometriosis and controls.4
While in our present study anti-glycan antibodies have been analyzed for the first time in endometriosis, variation in n-glycome concentration has been previously reported.2 It indicates that glycomics may be a promising source of data on this condition. Our study highlights a possible contribution of glycomics to diagnosis of endometriosis as we performed a wide anti-glycan antibody screening of the P and PF using a high-throughput tool, widely applicable to desired glycan sequences, based on neoglycolipid technology.
This is a preliminary, proof-of-concept study, therefore the statistical significance of the differences observed requires investigation with larger sample cohorts. Also a correlation with plasma and peritoneal fluid antibodies levels should be considered. Besides this, the findings from the microarray analyses of this small number of samples are promising. We found higher binding levels to some of the analyzed glycan-related probes in the patient group compared to the control group. A wide range of recent studies that emphasized glycomics contribution to diagnosis of diseases in the fields of autoimmunological disturbances and oncology also match our results which may be summarized that glycans are highly diverse structures with a specific interaction mediating a wealth of important biological and physiological processes as the titers of anti-glycan antibodies are closely associated with various immunological responses, hence our conclusions put glycomics studies as a highly probable diagnostic asset in endometriosis.
Conclusion
This is the first study that analyzed carbohydrate binding properties in the P and PF samples in endometriosis. Our study highlights the possible contribution of glycomics to diagnosis of endometriosis and better understanding of its pathophysiology. It also confirms that neoglycolipid-based oligosaccharide microarray system is a promising technology for the development of a non-invasive endometriosis diagnostic tool.
Abbreviations
AO, aminooxy; BMI, body mass index; DHPE, 1,2-dihexadecyl-sn-glycero-3-phosphoethanolamine; EDTA, ethylenediaminetetraacetic acid; P, plasma; PF, peritoneal fluid; NGLs, neoglycolipids.
Institutional Review Board Statement
The Bioethics Committee operating at the Medical University of Warsaw approved the collection of the material in accordance with opinion No. KB 223/2017, issued on December 12, 2017. The Bioethics Committee operating at the Calisia University approved the method of analysis of the previously collected material using neoglycolipid-based oligosaccharide microarray system (opinion 04/2021, issued on December 20, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank our collaborators from the MZ project for their participation in the recruitment of selected samples for this project. We would also like to thank the Glycosciences Laboratory team for conducting glycomics analysis.
Author Contributions
Cezary Wojtyła was responsible for study conception, study design, acquisition, analysis, interpretation, and visualization of the data. He acquired financial support for the project leading to this publication, and he was responsible for managing and coordinating the planning and implementation of research activities, selecting patients and material for this project, and analysis. He wrote and edited the manuscript. Ignacy Tołwiński was responsible for study conception, analysis, and interpretation of the data, and he was also responsible for data visualization and writing the manuscript. Piotr Laudański was responsible for study conception and study design. He acquired financial support for the project leading to this publication. He was responsible for organizing the collection of biological material, editing the manuscript, and critical revision. All authors have agreed on the journal to which the article will be submitted. All authors have reviewed and agreed on all versions of the article. All authors have agreed to take responsibility and be accountable for the contents of the article.
Funding
This study was supported by grants from National Science Centre (Narodowe Centrum Nauki - MINIATURA 5, grant no. 2021/05/X/NZ1/00646), Polish Ministry of Health (grant no. 6/6/ 4/1/NPZ/2017/1210/1352) and MSCA-RISE-2020 project TRENDO (grant no. 101008193).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tomassetti C, Johnson NP, Petrozza J, et al.; International Working Group of AAGL, ESGE, ESHRE and WES. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021(4):hoab029. doi:10.1093/hropen/hoab029
2. Berkes E, Mužinić A, Rigo JJ, Tinneberg HR, Oehmke F. The analysis of the human plasma N-glycome in endometriosis patients. Eur J Obstet Gynecol Reprod Biol. 2013;171:107–115. doi:10.1016/j.ejogrb.2013.08.008
3. Li Z, Feizi T. The neoglycolipid (NGL) technology-based microarrays and future prospects. FEBS Lett. 2018;592:3976–3991. doi:10.1002/1873-3468.13217
4. Laudański P, Rogalska G, Warzecha D, et al. Autoantibody screening of plasma and peritoneal fluid of patients with endometriosis. Hum Reprod. 2023;38:629–643. doi:10.1093/humrep/dead011
5. Canis M, Donnez JG, Guzick DS, et al. Revised American society for reproductive medicine classification of endometriosis. Fertil Steril. 1997;67:817–821. doi:10.1016/S0015-0282(97)81391-X
6. Rahmioglu N, Fassbender A, Vitonis AF, et al.; WERF EPHect Working Group. World endometriosis research foundation endometriosis phenome and biobanking harmonization project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril. 102;2014:1233–1243. doi:10.1016/j.fertnstert.2014.07.1208
7. Liu Y, Childs RA, Palma AS, et al. Neoglycolipid-based oligosaccharide microarray system: preparation of NGLs and their noncovalent immobilization on nitrocellulose-coated glass slides for microarray analyses. Methods Mol Biol. 2012;808:117–136.
8. Liu Y, Feizi T, Campanero-Rhodes MA, et al. Neoglycolipid probes prepared via oxime ligation for microarray analysis of oligosaccharide-protein interactions. Chem Biol. 2007;14:847–859. doi:10.1016/j.chembiol.2007.06.009
9. Sterner E, Flanagan N, Gildersleeve JC. Perspectives on anti-glycan antibodies gleaned from development of a community resource database. ACS Chem Biol. 2016;11:1773–1783. doi:10.1021/acschembio.6b00244
10. Luetscher RND, McKitrick TR, Gao C, et al. Unique repertoire of anti-carbohydrate antibodies in individual human serum. Sci Rep. 2020;10:15436. doi:10.1038/s41598-020-71967-y
11. Muthana SM, Gildersleeve JC. Factors affecting anti-glycan IgG and IgM repertoires in human serum. Sci Rep. 2016;6:19509. doi:10.1038/srep19509
12. Durbin SV, Wright WS, Gildersleeve JC. Development of a multiplex glycan microarray assay and comparative analysis of human serum anti-glycan IgA, IgG, and IgM repertoires. ACS Omega. 2018;3:16882–16891. doi:10.1021/acsomega.8b02238
13. Greenbaum H, Galper BL, Decter DH, Eisenberg VH. Endometriosis and autoimmunity: can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun Rev. 2021;20:102795. doi:10.1016/j.autrev.2021.102795
14. Muthana SM, Gildersleeve JC. Glycan microarrays: powerful tools for biomarker discovery. Cancer Biomark. 2014;14:29–41. doi:10.3233/CBM-130383
15. Vollmers HP, Brändlein S. Natural antibodies and cancer. N Biotechnol. 2009;25:294–298. doi:10.1016/j.nbt.2009.03.016
16. Vollmers HP, Brändlein S. Natural human immunoglobulins in cancer immunotherapy. Immunotherapy. 2009;1:241–248. doi:10.2217/1750743X.1.2.241
17. Rodrigues JG, Balmaña M, Macedo JA, et al. Glycosylation in cancer: selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018;333:46–57. doi:10.1016/j.cellimm.2018.03.007
18. Wanyama FM, Blanchard V. Glycomic-Based Biomarkers for Ovarian Cancer: advances and Challenges. Diagnostics. 2021;11:643. doi:10.3390/diagnostics11040643
19. Pochechueva T, Chinarev A, Schoetzau A, et al. Blood plasma-derived anti-glycan antibodies to sialylated and sulfated glycans identify ovarian cancer patients. PLoS One. 2016;11:e0164230. doi:10.1371/journal.pone.0164230
20. Paul S, Boschetti G, Rinaudo-Gaujous M, et al. Association of anti-glycan antibodies and inflammatory bowel disease course. J Crohns Colitis. 2015;9:445–451. doi:10.1093/ecco-jcc/jjv063
21. Dotan N, Altstock RT, Schwarz M, Dukler A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus. 2006;15:442–450. doi:10.1191/0961203306lu2331oa
22. Shigesi N, Kvaskoff M, Kirtley S, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:486–503. doi:10.1093/humupd/dmz014
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
