Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Role of PPARγ Gene Polymorphisms, Gut Microbiota in Type 2 Diabetes: Current Progress and Future Prospects
Authors Zhao YK, Zhu XD, Liu R, Yang X, Liang YL, Wang Y
Received 10 July 2023
Accepted for publication 18 October 2023
Published 7 November 2023 Volume 2023:16 Pages 3557—3566
DOI https://doi.org/10.2147/DMSO.S429825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Yi-Kun Zhao,1 Xiang-Dong Zhu,2 Rong Liu,1 Xia Yang,1 Yong-Lin Liang,1 Yan Wang2
1Department of Basic Medical College, Gansu University of Chinese Medicine, Lanzhou City, People’s Republic of China; 2Department of Traditional Chinese Medicine College, Ningxia Medical University, Yinchuan city, People’s Republic of China
Correspondence: Yong-Lin Liang, Gansu University of Chinese Medicine, No. 35, Dingxi East Road, Chengguan District, Lanzhou City, Gansu, 730030, People’s Republic of China, Email [email protected] Yan Wang, Ningxia Medical University, No. 1160 Shengli Street, Xingqing District, Yinchuan City, Ningxia Hui Autonomous Region, 750004, People’s Republic of China, Tel +86-18153952736, Email [email protected]
Abstract: Over the past decade, there has been a significant increase in studies investigating the relationship between the polymorphisms of the Peroxisome Proliferator-Activated Receptor gamma (PPARγ) gene and Type 2 Diabetes (T2D). PPARγ, a critical transcription factor, plays a central role in lipid metabolism, insulin resistance, and inflammatory response. Concurrently, the influence of gut microbiota on the development of T2D has gained increasing attention, especially their role in affecting host metabolism, such as lipid metabolism and the PPARγ signaling pathway. This review provides a comprehensive analysis of recent studies on PPARγ gene polymorphisms and their association with T2D, with a specific emphasis on the implications of gut microbiota and their interaction with PPARγ pathways. We also discuss the potential of manipulating gut microbiota and targeting PPARγ gene polymorphisms in T2D management. By deepening our understanding of these relationships, we aim to pave the way for novel preventative and therapeutic strategies for T2D.
Keywords: PPARγ, T2D, lipid metabolism, insulin resistance, gut microbiota
Introduction
Overview of Type 2 Diabetes (T2D)
In recent years, the global prevalence of Type 2 Diabetes (T2D) has escalated significantly, necessitating innovative preventative and therapeutic strategies. T2D is a chronic metabolic disorder that has evolved into a global public health crisis, affecting an estimated 537 million individuals worldwide in 2021 (International Diabetes Federation, 2021). This surge has coincided with a growing understanding of the genetic underpinnings of T2D, specifically the polymorphisms of the Peroxisome Proliferator-Activated Receptor gamma (PPARγ) gene, which regulates lipid metabolism, insulin resistance, and inflammation.1 Concurrently, burgeoning research focused on the gut microbiota reveals their substantial influence on the onset and progression of T2D, especially through lipid metabolism and PPARγ signaling pathway.2 This review aims to critically analyze recent studies investigating the interplay between PPARγ gene polymorphisms, gut microbiota and their collective impact on T2D. We also explore the potential of harnessing this knowledge for T2D management by manipulating gut microbiota and targeting PPARγ gene polymorphisms. (see Table 1)
 |
Table 1 Mechanistic Connections Between Hyperglycemia, Gut Microbiota, and PPARγ Gene Polymorphisms |
Importance of Studying Gene Polymorphisms in T2D
The rapid escalation of the T2D epidemic worldwide underscores the urgent need for a more comprehensive understanding of its pathophysiology. An important aspect of this understanding comes from studying genetic variations or polymorphisms that are associated with the risk of developing T2D. Polymorphisms are variations in the DNA sequence that are present in at least 1% of the population and they can play a significant role in determining an individual’s susceptibility to certain diseases, including T2D.3
In recent years, genome-wide association studies (GWAS) have identified several genetic loci associated with T2D, leading to insights into the molecular pathways underlying this disease.4 Among the genes identified, the PPARγgene has gained considerable attention due to its central role in lipid metabolism, insulin resistance, and inflammatory response.5 PPARγ polymorphisms, in particular, have been shown to be associated with an increased risk of T2D.6 Understanding these genetic polymorphisms is not only important for identifying individuals at high risk for T2D but also for developing new therapeutic strategies. For instance, studies have shown that individuals with certain PPARγ polymorphisms may respond differently to thiazolidinedione, a class of drugs used to treat T2D.7
Furthermore, elucidating the interaction of these polymorphisms with environmental factors such as gut microbiota, could help us understand the gene-environment interactions that contribute to T2D.23 Given the complexity of T2D pathogenesis, studying gene polymorphisms in T2D can offer new insights into the underlying mechanisms of this disease, and pave the way for personalized prevention and treatment strategies. (see Figure 1)
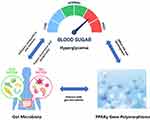 |
Figure 1 Mechanistic Interaction Model among Hyperglycemia, Gut Microbiota, and PPARγ Gene Polymorphisms. |
Brief introduction to the PPARγ Gene
The PPARγ gene is a member of the nuclear hormone receptor superfamily, which acts as a critical transcription factor involved in the regulation of adipogenesis, lipid metabolism, insulin sensitivity, and inflammatory response.24 This gene encodes for two isoforms, PPARγ1 and PPARγ2, which differ at the N-terminal due to alternative promoter usage and splicing.25 PPARγ is predominantly expressed in adipose tissue, where it plays a key role in adipocyte differentiation, promoting the conversion of preadipocytes into mature adipocytes. Its role in promoting lipid storage in adipocytes helps to prevent lipotoxicity in other tissues, which contributes to the maintenance of systemic insulin sensitivity.26
Beyond adipose tissue, PPARγ is also expressed in various tissues including the skeletal muscle, liver, and pancreatic β cells, where it helps regulate lipid metabolism and insulin action. Importantly, it is also expressed in immune cells, where it modulates inflammatory responses.14
Given its diverse roles in metabolism and inflammation, PPARγ is not surprisingly implicated in the pathogenesis of T2D. Polymorphisms in the PPARγ gene can affect its function and have been associated with an increased risk of developing T2D.15 Thus, understanding the role of PPARγ and its polymorphisms can provide critical insights into the pathophysiology of T2D and offer potential therapeutic targets.
Significance of Gut Microbiota in Health and Disease
The gut microbiota, composed of trillions of microorganisms, is an integral part of the human body. These microorganisms, primarily bacteria, play a critical role in maintaining host health by aiding in digestion, synthesizing vitamins, and strengthening the immune system.27 Additionally, they play a vital role in the metabolism of dietary components, including complex carbohydrates, proteins, and lipids, and can significantly influence the host’s metabolic status.28 However, disturbances in the gut microbiota, often referred to as ‘dysbiosis’, have been linked to a variety of diseases, including inflammatory bowel disease, obesity, cardiovascular disease, and T2D.29 Emerging research has shown that the gut microbiota can influence insulin resistance, inflammation, and lipid metabolism – key factors involved in the pathogenesis of T2D.11
Notably, the gut microbiota can also interact with the host’s genetic factors, like the PPARγ signaling pathway, and potentially influence the host’s susceptibility to T2D.30 Therefore, understanding the complex interactions between the gut microbiota, host genes, and metabolic health is essential for the development of novel therapeutic strategies for T2D.
Thus, gut microbiota represents a crucial factor in health and disease, offering a potential target for therapeutic intervention. With the advancements in metagenomic sequencing technologies, the potential to manipulate gut microbiota for disease management, such as T2D, is becoming increasingly feasible.
The Role of PPARγ Gene in T2D
Biological Function of the PPARγ Gene
Role in Lipid Metabolism
The PPARγ gene is intimately involved in the regulation of lipid metabolism, largely through its expression in adipose tissue and liver, which are critical sites of lipid storage and processing.26
In adipose tissue, PPARγ promotes the differentiation of pre-adipocytes into mature adipocytes, a process that allows the effective storage of lipids.25 It also regulates the transcription of numerous genes involved in lipid uptake, storage, and mobilization. For instance, PPARγ stimulates the expression of genes such as lipoprotein lipase (LPL) and fatty acid transport protein (FATP), facilitating the entry and esterification of fatty acids within adipocytes.31
In the liver, PPARγ influences lipid homeostasis by modulating the expression of genes involved in fatty acid oxidation, triglyceride synthesis, and lipoprotein assembly. PPARγ activation results in increased lipid uptake and decreased lipogenesis, thus potentially attenuating hepatic steatosis, a condition often seen in T2D patients.32 It’s worth noting that dysregulation of these metabolic processes due to genetic variations in the PPARγ gene may be implicated in the development of insulin resistance and T2D.15
Impact on Insulin Resistance
Insulin resistance, a characteristic feature of T2D, is also intricately associated with PPARγ activity. As a critical modulator of adipocyte differentiation and function, PPARγ influences systemic insulin sensitivity.33 PPARγ promotes the transcription of a suite of insulin-sensitizing adipokines, including adiponectin and leptin, which enhance insulin signaling in peripheral tissues. Dysregulation of these adipokines has been implicated in the pathogenesis of insulin resistance and T2D.34
Furthermore, PPARγ also regulates lipid storage in adipocytes, which is crucial for preventing ectopic fat accumulation in insulin-responsive tissues like liver and muscle. The buildup of lipids in these non-adipose tissues can trigger lipotoxicity and insulin resistance.35 Therefore, the capacity of PPARγ to direct fatty acids towards adipocytes is an essential component of systemic insulin sensitivity.
The PPARγ gene variants have been linked to altered insulin sensitivity and the risk of developing T2D, further emphasizing the importance of PPARγ in the regulation of insulin action.15
Involvement in the Inflammatory Response
Beyond its role in lipid metabolism and insulin sensitivity, PPARγ is increasingly recognized for its involvement in the regulation of inflammatory responses, which are also significant in the pathogenesis of T2D.36 PPARγ exerts anti-inflammatory effects by negatively regulating the expression of pro-inflammatory genes. This is achieved through transrepression mechanisms where PPARγ physically interacts with pro-inflammatory transcription factors, such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), thus preventing their binding to DNA and subsequent transcriptional activation.14
In the context of T2D, inflammation in adipose tissue is associated with insulin resistance, and PPARγ can alleviate this through its anti-inflammatory action. Moreover, PPARγ activation in macrophages, a significant source of inflammation in adipose tissue, promotes a switch towards an anti-inflammatory M2 phenotype, further contributing to insulin sensitivity.16
Polymorphisms in the PPARγ gene may also influence its anti-inflammatory function and, consequently, susceptibility to T2D, but more research is required to confirm these associations.17
Description of PPARγ Gene Polymorphisms
Genetic polymorphisms are variations in the DNA sequence that exist within a population. They can significantly affect gene function and consequently influence an individual’s susceptibility to various diseases, including T2D. Over the past decade, numerous polymorphisms in the PPARγ gene have been identified and studied for their potential role in the pathogenesis of T2D.15
One of the most extensively studied polymorphisms in the PPARγ gene is Pro12Ala, which is a single nucleotide polymorphism (SNP) that results in the substitution of the amino acid proline by alanine at codon 12 of the PPARγ2 protein. The frequency of this SNP varies across populations, but it has consistently been associated with a reduced risk of T2D in several large-scale meta-analyses.37 This protective effect is believed to be due to increased insulin sensitivity conferred by the Ala12 variant.38 In contrast, some other polymorphisms like the C1431T SNP have been associated with an increased risk of T2D, although the evidence is less robust and more heterogeneous across studies.39 Further investigation into the PPARγ gene polymorphisms and their functional consequences can lead to a better understanding of T2D pathogenesis and potentially pave the way for personalized medicine strategies in its management.
Relationship Between PPARγ Gene Polymorphisms and T2D
Summary of Recent Studies
Numerous studies have examined the association between PPARγ gene polymorphisms and T2D, reflecting the significance of this gene in glucose homeostasis and lipid metabolism.40 As previously mentioned, the Pro12Ala polymorphism has been extensively studied, with a meta-analysis of 57 studies involving nearly 23,000 participants finding that individuals carrying the Ala allele had a 20% reduced risk of developing T2D.15 Furthermore, a study by Deeb et al found that this polymorphism was associated with improved insulin sensitivity.37
Other polymorphisms, such as the C1431T, have also been investigated. A recent study found an increased risk of T2D in carriers of the T allele, suggesting a detrimental effect on insulin resistance.39 However, the results for this polymorphism are inconsistent across different populations and more research is needed.
Analysis of the Results
The identified association between PPARγ gene polymorphisms and T2D suggests that these polymorphisms could alter the function of the PPARγ protein, thereby influencing an individual’s susceptibility to T2D.15 For example, the Pro12Ala polymorphism may enhance insulin sensitivity, reducing the risk of T2D. Conversely, the C1431T polymorphism may impair insulin action, increasing T2D risk.39
These findings highlight the role of genetic factors in the pathogenesis of T2D and underscore the potential of genetic screening and personalized treatment strategies in managing the disease. However, it’s important to acknowledge the complexity of T2D and the likely interaction of multiple genes, environmental factors, and lifestyle factors in its development.
The Influence of Gut Microbiota on T2D
Overview of Gut Microbiota
The gut microbiota is a complex and dynamic community of trillions of microorganisms living in our gastrointestinal tract, comprising bacteria, viruses, fungi, and other microscopic life forms.27 This microbiota coexists with us in a mutualistic relationship - it assists in various physiological functions including the extraction of energy and nutrients from food, regulation of immune responses, and protection against harmful pathogens.41
In recent years, scientific evidence has indicated that the gut microbiota plays a pivotal role in metabolic health, including the regulation of glucose metabolism and insulin sensitivity, which are critical in the pathophysiology of T2D.29,42 Disturbances in the gut microbiota, a condition referred to as dysbiosis, have been associated with increased inflammation, altered gut barrier function, and metabolic endotoxemia, all of which may contribute to insulin resistance and the development of T2D.42
Furthermore, specific changes in the composition and functionality of the gut microbiota have been observed in T2D patients compared to healthy individuals, suggesting a possible link between the gut microbiota and T2D.11
Interactions Between Gut Microbiota and Host Metabolism
Effect on Lipid Metabolism
The gut microbiota plays a substantial role in modulating lipid metabolism. Some bacterial species can ferment dietary fibers into short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate.43 These SCFAs are absorbed into the bloodstream and contribute to host energy metabolism. In addition, butyrate specifically is a major energy source for colonocytes and has been found to reduce cholesterol levels.44
Furthermore, gut microbiota has been found to affect lipid absorption by altering the expression of genes involved in intestinal lipid metabolism. For instance, gut microbiota depletion in mice led to an increase in fasting-induced adipose factor (FIAF), an inhibitor of lipoprotein lipase (LPL) and thus reduced lipid storage in adipose tissue.45
Connection to the PPARγ Signaling Pathway
There’s an intricate relationship between gut microbiota and the PPARγ signaling pathway. Some SCFAs, particularly butyrate and propionate, act as ligands for PPARγ, influencing its transcriptional activity.46 Moreover, several gut bacteria can produce conjugated linoleic acid (CLA), which is a potent activator of PPARγ.47 This activation is essential for maintaining adipose tissue homeostasis and insulin sensitivity, thereby reducing the risk of developing T2D.
On the other hand, alterations in gut microbiota composition can disrupt PPARγ signaling, leading to metabolic dysfunctions and potentially contributing to the pathogenesis of T2D.12
Recent Research on Gut Microbiota and T2D
The past decade has seen an explosion of research into the role of the gut microbiota in health and disease, and the onset and progression of T2D is no exception. Several studies have identified specific gut microbiota compositions that correlate with T2D, suggesting that dysbiosis could contribute to disease pathogenesis.11
For example, it has been observed that individuals with T2D have a lower abundance of butyrate-producing bacteria and an overgrowth of opportunistic pathogens compared to healthy controls.13 Butyrate, produced by the fermentation of dietary fiber by gut bacteria, plays a key role in maintaining gut health and regulating host metabolism, as previously discussed. The lack of these beneficial bacteria could potentially lead to metabolic dysfunctions, insulin resistance, and eventually T2D.
Moreover, a study by Karlsson et al18 demonstrated that the gut microbiota in individuals with T2D was characterized by a decrease in the abundance of some universal butyrate-producing bacteria and an increase in various opportunistic pathogens. These changes in microbiota composition were associated with an increase in markers of inflammation and metabolic dysfunctions.
While this association between the gut microbiota and T2D has been well established, the exact mechanism of how gut bacteria influence the development of T2D remains to be fully understood. Future research could focus on identifying the specific microbial taxa that contribute to or protect against T2D, thereby informing novel therapeutic strategies targeting the gut microbiota.
In light of the findings presented in this study, it is worth mentioning and discussing the effects of pioglitazone and other thiazolidinediones (TZDs) on gut microbiota and glucolipid metabolism, as observed in similar studies. For instance, a recent study by Wang et al19 demonstrated that pioglitazone treatment could alleviate high-fat diet-induced insulin resistance and obesity by modulating the gut microbiota and bile acid metabolism in mice. The authors reported that pioglitazone administration increased the abundance of beneficial bacteria, such as Akkermansia and Lactobacillus, and reduced the abundance of harmful bacteria, including Desulfovibrio and Allobaculum. Moreover, pioglitazone treatment was associated with a decrease in secondary bile acid levels, which may contribute to improved insulin sensitivity and lipid metabolism. These findings, together with the results of the present study, suggest that the combination of metformin and pioglitazone may exert synergistic or additive effects in improving glucolipid metabolism and insulin sensitivity by modulating gut microbiota composition and metabolic activities. The observed alterations in gut microbiota and their metabolites, following the combined therapy, support the potential of this approach as an effective therapeutic strategy for type 2 diabetes management. Further research is warranted to elucidate the precise molecular mechanisms underlying the interactions between metformin, pioglitazone, gut microbiota, and their metabolites in the context of diabetes and obesity. Additionally, comprehensive investigations into the clinical implications and long-term safety of combined metformin and pioglitazone therapy are necessary to establish its efficacy in the management of type 2 diabetes.
Interplay Between Gut Microbiota and PPARγ Pathways in T2D
Influence of Gut Microbiota on PPARγ Gene Polymorphisms
Research has revealed that gut microbiota composition can influence host genetic expression, including genes like PPARγ. The PPARγ gene is highly polymorphic and different polymorphisms may be influenced variably by gut microbiota. For instance, certain gut bacteria species produce short-chain fatty acids (SCFAs) such as butyrate, which have been demonstrated to upregulate the expression of PPARγ.8 On the other hand, dysbiosis or imbalanced gut microbiota might be involved in downregulating PPARγ, thereby affecting insulin sensitivity and lipid metabolism.
Implications of Gut Microbiota-PPARγ Interaction on the Pathogenesis of T2D
The crosstalk between gut microbiota and the PPARγ pathways has significant implications for the pathogenesis of T2D. Altered gut microbiota may influence PPARγ signaling pathways, affecting lipid metabolism, insulin sensitivity, and inflammatory responses. Furthermore, variations in the PPARγ gene may also affect its interaction with gut microbiota, potentially contributing to T2D risk.9 For instance, PPARγ polymorphisms that lead to reduced gene expression could render individuals more susceptible to the metabolic disruptions induced by gut dysbiosis.
Summary of Recent Studies and Their Findings
Recent studies have shed light on the interplay between gut microbiota and PPARγ pathways. For example, research has revealed a complex interaction between the gut microbiota, PPARγ gene polymorphisms, and metabolic health outcomes, pointing to the potential utility of microbiota manipulation in managing T2D.10 Further exploration of this relationship may pave the way for personalized medicine approaches for T2D prevention and treatment.
Potential Therapeutic Strategies for T2D
Manipulating Gut Microbiota as a Therapeutic Strategy
Given the significant role gut microbiota play in metabolism and the pathogenesis of T2D, altering the gut microbiota composition could serve as a potential therapeutic strategy. For instance, the administration of probiotics or prebiotics could help regulate the gut microbiota composition and promote a healthier metabolic profile.20 Similarly, fecal microbiota transplantation (FMT) is another method of reshaping the gut microbiota, which has shown promise in preliminary studies for the management of T2D.21
Targeting PPARγ Gene Polymorphisms in T2D Management
The understanding of PPARγ gene polymorphisms and their implications in T2D offers another therapeutic target. Potential strategies may include developing drugs that specifically target certain polymorphisms of PPARγ, enhancing the gene’s beneficial metabolic effects and mitigating its adverse effects.48 However, such strategies require further research and clinical trials to confirm their efficacy and safety.
Exploration of Preventative Measures Based on PPARγ Gene Polymorphisms and Gut Microbiota
Understanding the interaction between PPARγ gene polymorphisms and gut microbiota could also lead to preventative measures. These could include personalized nutrition plans, lifestyle modifications, and early intervention strategies that take into account an individual’s genetic makeup and gut microbiota composition. Such personalized approaches could have the potential to reduce the risk of T2D development in susceptible individuals.22
Role of the Microbiota in Modulating PPARγ Activity and Its Potential Impact on the Development and Progression of T2DM
Microbiota can influence PPARγ activity both directly and indirectly. Some studies have shown that certain bacterial metabolites, such as short-chain fatty acids (SCFAs), can act as ligands for PPARγ, thereby modulating its activity.49 SCFAs such as butyrate, propionate, and acetate are produced through the fermentation of dietary fibers by gut bacteria.50 Additionally, research has indicated that the gut microbiota composition can change in response to different PPARγ agonists, suggesting a potential feedback loop between PPARγ activity and the gut microbiome.51
Furthermore, PPARγ polymorphisms have been associated with alterations in gut microbiota composition.51 For instance, individuals carrying specific PPARγ risk alleles have been found to harbor distinct gut microbiota profiles as compared to non-carriers, suggesting that PPARγ genetic variants might influence not only metabolism but also the gut microbiome.
In conclusion, the tri-axis association between PPARγ, microbiota, and T2DM is a complex and intricate relationship that warrants further investigation. Understanding the interplay between these factors will provide valuable insights into the pathophysiology of T2DM and may pave the way for novel therapeutic approaches.
Conclusion
Recap of the Significance of PPARγ Gene Polymorphisms and Gut Microbiota in T2D
This review underscores the increased research interest in the association between PPARγ gene polymorphisms and T2D. PPARγ, a pivotal transcription factor in T2D pathogenesis, is implicated in lipid metabolism, insulin resistance, and inflammation. Alongside, investigations into gut microbiota’s impact on T2D, particularly on host metabolism and PPARγ signaling, are gaining momentum. The review identifies a correlation between PPARγ gene polymorphisms and heightened T2D risk, implying a genetic predisposition. Moreover, gut microbiota dysbiosis, typified by gut microbial community imbalance, can affect lipid metabolism and disrupt PPARγ signaling, thus influencing T2D development and progression.
Implications of These Relationships for Future Research
The relationships between PPARγ gene polymorphisms, gut microbiota, and T2D hold significant implications for future research. Firstly, further investigation is warranted to elucidate the underlying molecular mechanisms through which PPARγ gene polymorphisms influence T2D susceptibility. This may involve exploring the specific genetic variants within the PPARγ gene and their functional consequences on PPARγ activity, as well as the interplay between these variants and environmental factors.
Additionally, more research is needed to comprehensively understand how gut microbiota composition and function affect T2D development. Studying the dynamic interactions between the gut microbiota, host metabolism, and the PPARγ signaling pathway will provide valuable insights into the complex mechanisms underlying T2D pathogenesis.
Moreover, considering the potential bidirectional relationship between PPARγ and gut microbiota, future investigations should explore whether modulating gut microbiota can influence PPARγ activity and function, potentially providing novel therapeutic strategies for T2D management.
Potential for New Preventative and Therapeutic Strategies for T2D
The knowledge gained from studying PPARγ gene polymorphisms and the influence of gut microbiota on T2D opens up possibilities for new preventative and therapeutic strategies. Firstly, identifying individuals with PPARγ gene polymorphisms associated with increased T2D risk could enable targeted interventions and personalized approaches for disease prevention. Early detection and intervention in high-risk individuals may mitigate the progression of T2D.
Furthermore, interventions targeting gut microbiota composition and function hold promise as potential therapeutic avenues. Modulating the gut microbiota through approaches such as probiotics, prebiotics, dietary interventions, or fecal microbiota transplantation may help restore microbial balance and improve T2D outcomes. Additionally, developing strategies that enhance PPARγ activity or address PPARγ dysregulation could provide novel therapeutic options for T2D management.
Directions for Future Research
To advance our understanding of the relationships between PPARγ gene polymorphisms, gut microbiota, and T2D, several areas require further investigation. Firstly, more comprehensive studies are needed to determine the causal relationship between PPARγ gene polymorphisms and T2D. Longitudinal studies, functional analyses, and large-scale genetic association studies are necessary to establish the causality and clarify the mechanistic links between PPARγ variants and T2D susceptibility.
Additionally, investigations into the gut microbiota’s role in mediating the effects of PPARγ gene polymorphisms on T2D development should be expanded. Understanding the specific mechanisms by which gut microbiota dysbiosis influences PPARγ signaling and host metabolism will be crucial for developing targeted therapeutic interventions.
Lastly, clinical trials exploring the efficacy and safety of interventions targeting gut microbiota and PPARγ pathways in the management of T2D are warranted. These trials should assess the long-term effects, optimal interventions, and potential side effects of modulating gut microbiota and targeting PPARγ to ensure the development of evidence-based and clinically effective strategies.
In conclusion, the interplay between PPARγ gene polymorphisms, gut microbiota, and T2D represents a promising avenue for future research. By deepening our understanding of these relationships, we can pave the way for innovative preventative and therapeutic strategies that may revolutionize T2D management and improve patient outcomes.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Research on clinical application basic and transformation development of Shenqitangluo Pill in the treatment of type 2 diabetes based on quantity-effect relationship (2022BEG02034) and research on clinical application and transformation development of Dahuangtangluo Pill in the treatment of type 2 diabetes based on quantity-effect relationship (2021CYZC-03).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and Development of PPAR Modulators in Health and Disease: an Update of Clinical Evidence. Int J Mol Sci. 2019;20(20):5055. doi:10.3390/ijms20205055
2. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi:10.1038/nature05414
3. Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008;322(5903):881–888. doi:10.1126/science.1156409
4. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–1513. doi:10.1038/s41588-018-0241-6
5. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi:10.1016/S0140-6736(13)62154-6
6. Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JP. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2010;171(6):645–655. doi:10.1093/aje/kwp450
7. Kang ES, Park SY, Kim HJ, et al. The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with type 2 diabetes. Diabetes Care. 2005;28(5):1139–1144. doi:10.2337/diacare.28.5.1139
8. Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi:10.1007/s00125-007-0791-0
9. Moya-Pérez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS One. 2015;10(7):e0126976. doi:10.1371/journal.pone.0126976
10. Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85–92. doi:10.1016/j.gene.2013.11.081
11. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi:10.1038/nature11450
12. Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127(4):1202–1214. doi:10.1172/JCI88894
13. Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi:10.1371/journal.pone.0009085
14. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi:10.1038/34178
15. Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26(1):76–80. doi:10.1038/79216
16. Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4(11):619–626. doi:10.1038/ncpendmet0976
17. Tikhanovich I, Zhao J, Olson J, et al. Protein arginine methyltransferase 1 modulates innate immune responses through regulation of peroxisome proliferator-activated receptor γ-dependent macrophage differentiation. J Biol Chem. 2017;292(17):6882–6894. doi:10.1074/jbc.M117.778761
18. Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi:10.1038/nature12198
19. Wang D, Liu J, Zhong L, et al. Potential benefits of metformin and pioglitazone combination therapy via gut microbiota and metabolites in high-fat diet-fed mice. Front Pharmacol. 2022;11(13):1004617. doi:10.3389/fphar.2022.1004617
20. Kootte RS, Vrieze A, Holleman F, et al. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(2):112–120. doi:10.1111/j.1463-1326.2011.01483.x
21. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.e7. doi:10.1053/j.gastro.2012.06.031
22. Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev. 2007;3(1):33–39. doi:10.2174/157339907779802067
23. Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40–54. doi:10.1038/s41577-019-0198-4
24. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. doi:10.1016/j.cmet.2014.08.005
25. Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi:10.1146/annurev.biochem.77.061307.091829
26. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi:10.1038/nm1025
27. Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533. doi:10.1371/journal.pbio.1002533
28. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267.
29. Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33(6):954–964.
30. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24(1):41–50.
31. Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135(2):798–800. doi:10.1210/endo.135.2.8033830
32. Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40(8):1426–1433. doi:10.1016/S0022-2275(20)33384-8
33. Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–37734. doi:10.1074/jbc.R100034200
34. Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276(44):41245–41254.
35. Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118–21. doi:10.2337/diabetes.50.2007.S118
36. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi:10.1016/j.cmet.2012.04.001
37. Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20(3):284–287. doi:10.1038/3099
38. Stumvoll M, Häring H. The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism. Diabetes. 2002;51(8):2341–2347. doi:10.2337/diabetes.51.8.2341
39. Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004;43(2):297–305. doi:10.1161/01.HYP.0000113626.76571.5b
40. Xu W, Xu J, Sun B, et al. The effect of PPARG gene polymorphisms on the risk of coronary heart disease: a meta-analysis. Mol Biol Rep. 2013;40(2):875–884. doi:10.1007/s11033-012-2128-4
41. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi:10.1016/j.cell.2012.01.035
42. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi:10.2337/db07-1403
43. Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi:10.4161/gmic.19897
44. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016;28(7):979.
45. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi:10.1073/pnas.0407076101
46. Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A. 2011;108(52):21075–21080. doi:10.1073/pnas.1118922109
47. Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127(3):777–791. doi:10.1053/j.gastro.2004.06.049
48. Abaj F, Sotoudeh G, Karimi E, Rafiee M, Koohdani F. Interaction between the dietary indices and PPAR-γ Pro12Ala gene variants on cardiovascular risk factors in patients with type 2 diabetes mellitus. Int J Clin Pract. 2021;75(8):e14307. doi:10.1111/ijcp.14307
49. Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi:10.2337/db08-1637
50. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi:10.2337/db06-1491
51. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi:10.1038/nature04330
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
